Abstract
In hydroponics, nutrient management is the limiting factor to obtaining optimal production, and nitrogen (N) is the key component to consider when optimizing nutrient management in these types of systems. The objective of this study is to evaluate different combinations of N fertilizer concentrations in order to optimize the yield of basic seed potato minitubers in a three-phase hydroponic system. Treatments consisted of five combinations of N concentrations, applied before and after 21 days after plant transplant as follows, respectively: 1) 13 and 13, 2) 13 and 0, 3) 13 and 7.8, 4) 13 and 16, and 5) 13 and 26 mmol L−1. Propagation was performed by transplanting 3–4 cm potato plantlets cv. Agata from sprouts. There were significant effects of N treatments on all measured variables (root, leaf, stem, and plant dry weight and minituber number and weight). To obtain the maximum minituber number yield, 9.51 minitubers/plant, corresponding to 67 minitubers/m2, post 21-day adjusted N concentration was 18.4 mmol/L. Treatment 4 promoted higher basic seed potato minituber yield in a three-phase hydroponic system.
Resumen
En hidroponía, el manejo de los nutrientes es el factor limitante para la obtención de producción óptima, y el nitrógeno (N) es el componente clave a considerar cuando se optimiza el manejo de nutrientes en estos tipos de sistemas. El objetivo de este estudio fue evaluar las diferentes combinaciones de concentraciones de fertilizante nitrogenado a fin de optimizar el rendimiento de minitubérculos de semilla básica de papa en un sistema hidropónico trifásico. Los tratamientos consistieron de cinco combinaciones de concentraciones de N, aplicados antes y después de 21 días del trasplante, de la siguiente manera, respectivamente: 1) 13 y 13, 2) 13 y 0, 3) 13 y 7.8, 4) 13 y 16, y 5) 13 y 26 mmol L-1. Se efectuó la propagación mediante el trasplante de plántulas de papa de 3–4 cm cv. Agata de brotes. Hubo efectos significativos de los tratamientos de N en todas las variables medidas (peso seco de raíz, hoja, tallo y planta, y número y peso de minitubérculos). Para obtener el número máximo de minitubérculos de rendimiento, 9.51 minis/planta, correspondiendo a 67 minis/m2, la concentración ajustada de N posterior a los 21 días fue de18.4 mmol/l. El tratamiento 4 promovió el más alto rendimiento de minitubérculos de semilla básica de papa en un sistema hidropónico trifásico.
Resumo
Na hidroponia, o gerenciamento de nutrientes é o fator limitante para a obtenção de produção ótima sendo o nitrogênio (N) o componente chave a ser considerado ao otimizar o gerenciamento de nutrientes nesses sistemas. Assim, o objetivo desse trabalho foi avaliar diferentes combinações de concentrações de nitrogênio para a otimização da produtividade de tubérculos de batata-semente básica em sistema hidropônico de três fases. Os tratamentos consistiram de cinco combinações de concentrações de N até 21 dias após o transplante (DAT) e após 21 DAT (13 e 13 - controle; 13 e 0; 13 e 7,8; 13 e 16; 13 e 26 mmol L−1). O material de propagação utilizado foi broto da cultivar Ágata, com 3 a 4 cm de comprimento, em média. Houve efeito significativo dos tratamentos com N sobre todas as variáveis avaliadas (matérias de raiz, folha, caule e planta secas e número e massa de minituberculos). Para obter o número máximo de minitubérculos na colheita, 9,51 minitubérculos/planta, correspondendo a 67 minitubérculos/m2, a concentração de N ajustada foi de 18,4 mmol/L. O tratamento 4 propiciou a mais alta colheita de minituberculos da classe básica de batata-semente em um sistema hidropônico trifásico.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditionally, potatoes are propagated vegetatively and can be produced in several categories: Pre-Nuclear – PNU, Nuclear – NU, and Generations 1–5 (G1 - G5). The PNU is derived from tubers originating from plant material free of rod-shaped viruses, spindle tuber viroids, Clavibacter michiganense subsp. sepedonicus and E. carotovora. The Nuclear category is derived from the Pre-Nuclear material. The category G1 is derived from the category NU; G2 is derived from G1 or NU seedstock; G3 is derived from G2; G4 is derived from G3; and G5 is derived from G4 (OSCS 2018). The different categories of seed tubers correlates to their degree of health since the closer to Pre-Nuclear category the tubers are, the better their health, because pathogens are typically carried across generations in vegetative propagation. These ratings impact availability and costs.
It is generally not desirable to use higher categories (G1 and G2) to produce commercial potato tubers, although they are considered healthier, due to their expensive and limited supply. However, the high productivity rate of seed tubers G1 and G2 results in lowering the overall cost of seed tubers produced.
Production of basic seed potatoes can be made with micropropagation in tissue culture (in vitro plantlets or microtubers). Some of the difficulties when using this tool are the need for greater knowledge, training of specialized personnel, high operational cost, and assembly of laboratory structure with specific equipment and reagents for its development. Consequently, the final price per in vitro plantlet is high. Additionally, the plants obtained by tissue culture are more sensitive to transport stress and require acclimatization of the environment and can be easily damaged (Buckseth et al. 2016).
An alternative to using tubers for the propagation of potatoes is to use the sprouts derived from early generation tubers (Rahman and Akanda 2009; Davidson and Xie 2014). The use of sprouts from early generation tubers as vegetative propagating material can result in important advantages, both in the production process of the basic category seed potatoes and reducing planting material costs (Ho et al. 1988). Sprouts removed from early generation tubers are planted in greenhouses in various systems, such as vases, flower beds, or hydroponically.
Although hydroponic systems will be evaluated in the production of basic seed potatoes, there are several parameters under controlled growing conditions that can be manipulated to optimize the systems, especially across three phases. With respect to using sprouts as starting material, studies in three-phase hydroponic systems are rare or nonexistent. The three-phase system is comprised of a solid (sand), liquid (nutrient solution), and gas phase (air in the pore space). Nitrogen combination studies using sprouts as propagating material in a three-phase hydroponic system were not found in the literature for the production of basic seed potatoes.
Previous research by Factor et al. (2007) to produce seed potato minitubers using hydroponics evaluated the Deep Flow Technique (DFT), the Nutrient Film Technique (NFT), and aeroponics with ‘Agata’ and ‘Monalisa’ cultivar potato plants. The authors noted that the aeroponic system showed the best results and the number of minitubers per plant and per square meter had significantly higher average values in this system compared to NFT and DFT systems.
In hydroponic systems, the management of nutrients ultimately determines the success of the enterprise. This study focuses on determining the appropriate N supply to optimize growth and yield of potatoes propagated by sprout in hydroponic systems. Nitrogen management is critical to optimizing basic seed potato production (Souza et al. 2012; Buckseth et al. 2016; Fontes et al. 2016). The capability to maintain the N concentration at target level in the nutrient solution, to a large extent, determines the high rate of potato tuber growth (Chang and Lee 2016).
Moreover, in any system, the low efficiency of nitrogen use leads to environmental hazards such as water pollution from nitrates and atmosphere pollution by nitrous oxides (Hakeem et al. 2016). This environmental hazard is reduced by using a recirculation system.
The objective of this study is to evaluate combinations of nitrogen fertilizer concentrations to optimize the yield of minitubers of seed potatoes in a three-phase hydroponic system.
Material and Methods
The experiment was conducted from February to May 2011 in an enclosed chapel-type unheated greenhouse. The flat coordinates are UTM N = 7,703,227.057 m, E = 721,773.150 m, referenced to the geodetic system WGS84, zone 23S with an average altitude of 649 m.
The treatments consisted of five concentrations of nitrogen, 0.0, 7.8, 13.0, 16.0 and 26.0 mmol L−1 in the following combinations: T1 = 13 and 13 mmol L−1; T2 = 13 and 0 mmol L−1; T3 = 13 and 7.8 mmol L−1; T4 = 13 and 16 mmol L−1 and T5 = 13 and 26 mmol L−1, where the first value in each treatment corresponds to the N concentration that was used up to 21 days after transplantation (DAT). The second value corresponds to the N concentration after 21 DAT that was used up to the final harvest period, at 55 DAT. Treatment T1 (13 and 13 mmol L−1) was considered the standard treatment and the control for the experiment. The experimental setup consisted of a randomized block design with six replications, one per replication totaling 30 plants. The propagation material was sprouts from cv. Agata, 3 to 4 cm in length, on average. The sprouts were removed from basic seed stock tubers.
Essentially, the system consisted of conical pots, where the plants were contained and received daily nutrient solution applications, the excess of which was drained to the nutrient solution tank. This reservoir was equipped with an electric pump that drained the solutions back to the conical pots, as shown in Fig. 1.
In our experimental system, the solid phase was composed of expanded clay pebbles and sand (substrate). The liquid nutrient solution, as shown in Fig. 2, was placed in the expanded clay pebbles layer, washed with running tap water and then with deionized water three times.
Preparation of Pots
Black polyethylene pots 28 cm diameter at the mouth × 27 cm height, with a capacity of 8.5 L were drilled in the center of the bottom with a 12 mm “glass” type drill. A 10 mm (PN40) black polyethylene pipe was placed in this hole for drainage. The internal and external surfaces of the pot near the hole were sanded. The inside of the pot was sealed with hot glue while the outside was sealed with caulking material.
After the drains were installed, each pot received a layer of approximately 7 cm of expanded clay pebbles, followed by a cylindrical shaped clarité screen with a diameter of 23 cm. Washed sand was then used to fill the remainder of the pots above the clay layer.
Irrigation System
The irrigation system was engineered for installation convenience and flexibility to allow for easy adjustment of the hydroponics in conical pots. The system consisted of electric pumps for drainage (washing machine pumps), reservoirs with 50 L of nutrient solution, and a multi-voltage digital timer. The electric pumps were installed at a lower altimeter level in relation to the pickup point of the nutrient solution. The nutrient solution passed from the reservoir to the pump pipe in a 40 mm black polyethylene pipe connected to a 40 mm PVC coupling (Fig. 3b). The output for irrigation was made with a 20 mm PVC pipe supported on the experiment bench (Fig. 3c). From the main line PVC irrigation pipe, 16 mm black polyethylene (PN30) threaded adapters were connected. The lateral tubes were then connected to the adapters (barbed couplings) and fed into each pot individually (Fig. 3a).
Each pot received two lateral tubes (wells) fixed on two pieces of 0.1 m, 10 mm black polyethylene microtube (PN40) inserted in the sand (Fig. 3a).
The irrigation time was controlled by a digital timer with the following daily schedule of irrigation system: on from 8:00 to 8:01 am, on from 8:11 to 8:12 am; on from 12:00 to 12:01 pm and 12:11 to 12:12 pm; on from 4:00 to 4:01 pm and 4:11 to 4:12 pm. At night, the irrigation system was only triggered once, from 12:00 to12:01 am.
Irrigation Nutrient Solution Return System
The nutrient solution return system was comprised of a 40 mm PVC pipe located below the drain lines of the pots with a 10% slope toward the nutrient solution tank. The PVC pipe was pierced in places where each pot drained down perpendicularly. At the end of each drain PVC pipe (40 mm) contained a 90° PVC elbow (Fig. 3d).
Management of Nutrient Solution
The nutrient solution used contained 11.7, 1.3, 1.5, 6.5, 4.0, 1.25, and 2.0 mmol L−1 of N-NO3−, N-NH4+, P-H2PO4−, K+, Ca2+, Mg2+ and S-SO42− respectively. Micronutrients were provided at concentrations of 12.6, 46, 1.3, 0.1, 0.3, and 80 mmol L−1 of Mn, B, Zn, Mo, Cu and Fe respectively, adapted from Andriolo (2006). This solution is composed of the following salts: KH2PO4; MgSO4.7H2O; NH4NO3; Ca(NO3)2.4H2O; KNO3; NaNO3; KCl; CaCl2.2H2O; CuSO4.5H20; ZnSO4.7H2O; MnCl2.4H2O; H3BO3; (NH4)6Mo7O24.4H2O; FeCl3.6H2O; and C10H14N2O8Na2.2H2O (EDTA).
The nutrient solution was monitored every other day by measuring the pH and electric conductivity (EC). Every two days, nutrient solution pH and EC measurements were taken. Solution pH was measured using a digital pH meter and maintained at 5.5 through the addition of sodium hydroxide (1 M NaOH) or hydrochloric acid (1 M HCl). EC was monitored with a conductivity meter. Nutrient solutions were renewed to their initial levels when EC values of a reservoir reached 30% depletion in one of the treatments, as recommended by Martinez and Silva Filho (2006). The general care of the potato crop was carried out following the recommendations detailed in Fontes (2005).
Characteristics Evaluated
At harvest time (55 DAT), the following parameters were evaluated: minituber numbers; fresh weight and dry weight of roots, leaves, stems, minitubers, and total biomass. Dry weight partitioning was determined from the dry mass of individual plant parts as a percentage of total plant dry weight.
Statistical Analysis
Data were subjected to analysis of variance and treatment means were compared using the Fisher LSD test (p < 0.05). Data collected after 21 DAT were submitted to analysis of regression. The significance of the regression coefficients was determined using the Student’s t-test, adopting the p < 0.10 and the coefficient of determination\( \left({R}^2=\frac{SS_{Regression}}{SS_{Treatment}}\right) \).
Results and Discussion
There was a significant effect of treatments on the observed mean values of evaluated variables, minituber number (TN) and fresh weight (TFW), dry weight of root (RDW), stem (SDW), leaf (LDW), minituber (TUDW) and total plant dry weight (PDW) as shown in Table 1.
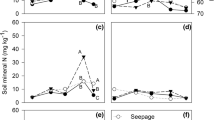
Treatment 4 (13 and 16 mmol L−1) led to higher average values of number of minitubers (TN), fresh weight of minitubers (TFW), and minituber dry weight (TUDW). The results shown that low (0, 7.8, and 13 mmol L−1) or high N concentration (26 mmol L−1) applied after 21 days was not appropriated to meet the plant N demands for growth. The combination 13 and 16 mmol L−1 of N (T4) led to higher number of minitubers per plant (TN) and led to an increase in 27.7% in TN compared with the standard treatment T1 (13 and 13 mmol L−1). In T4, minitubers accounted for 49% of the total plant dry weight (PDW) (Fig. 4d).
The percentage of plant dry weight partitioning to the root was not affected by treatments (Fig. 4a). There was an effect of N treatment on dry weight partitioning among the plant organs (Figs. 4 b-d). The preferred sink was the minituber (Fig. 4d). These results are according to Silva et al. (2013) who studied three potato cultivars (‘Agata’, ‘Atlantic’, and ‘Bintje’) in a hydroponic system, supplied with two different nitrogen sources (calcium nitrate and urea), to determine the effect on biomass accumulation and partitioning. The authors found that, as an average, from the total dry weight, around 73 and 50% was partitioning in the tuber.
There was a smaller percentage of dry weight partitioning in the stem at N concentration of 13 and 0 mmol L−1 (T2) than the N concentration of 13 and 7.8 mmol L−1 (T3) (Fig. 4b). Treatment 3 allocated significantly more resources to leaf growth and less to minituber growth than the other treatments (Figs. 4 c, d). The production of minitubers is more linked to the stolon number available for tuber initiation, which is determined by the degree of stolon branching (Patrick and Colyvas 2014) than the allocation of photoassimilates in the potato plant shoot at the final stage of the crop cycle. When potato plants have a limited supply of N, nutritional stress can occur, as well as the inhibition of plant growth, and reduction in the amount of leaves, altering the partitioning of dry weight (Oliveira 2000).
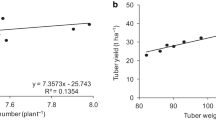
The adjusted equations between N concentration after 21 DAT and minituber number (Fig. 5a), minituber fresh weight (Fig. 5b), root and stem dry weight (Fig. 6a) and minituber, leaf, and total dry weight (Fig. 6b) are presented. The maximum values for all those variables are attained with N higher than 13 mmol L−1, considered as the normal concentration Andriolo (2006). Thus, 18.4 mmol/L was the N concentration associated with the maximum value of minituber number obtained (9.51 tubers/plant). In other experimental conditions, values of 4.8 to 26.8 minitubers/plant were obtained (Wang et al. 2018). The corresponding values for N concentrations in the nutrient solution to obtain maximum value of minituber fresh weight (Fig. 5b) and dry weight of stem, root, minituber, leaf and total (Fig. 6) were: 19.3, 18.4, 19.3, 18.8, 19.1, and 18.3 mmol/L, respectively. The N concentration in the nutrient solution affects several plant processes, as the stomatal opening and the photosynthetic efficiency, determining dry weight yield and assimilates partitioning among plant organs. The decrease of N concentration in a closed hydroponic system promoted the reduction of photosynthesis, plant growth, water use and growth of potato tubers (Chang and Lee 2016).
Using propagating material produced in vitro (cv. Atlantic), in hydroponic systems with fiber cement tiles, Diaz and Medeiros (2005) reported that the reducing the concentration of N at the beginning of minituber growth from 12 to 9 mmol L−1 produced a positive effect on the number of minitubers formed. The results observed in the present study corroborate those of Kelling et al. (2015), which indicate that there is a need for an appropriate amount at the beginning of the crop and a higher supply in the field experimentation. The N concentration in the T4 treatment (13 and 16 mmol L−1) led to higher productivity and a greater minituber number than other treatments (Table 1). Contrary to what was observed in this experiment, Krauss and Marschner (1982), cited by Diaz and Medeiros (2005), states that the decrease in the supply of N to plants causes an increase in the production of minitubers, which was not observed in our treatments T2 and T3.
Potato plant growth and tuber yield response to nutrient solution concentration (EC used to estimate) in a closed hydroponic system depended on the season which indicates that nutrient uptake is controlled by carbon assimilation in the plant (Muller et al. 2007). In an opened hydroponic system, using potato sprouts, the maximum mini tubers yield (178 g/pot) was obtained with 12,6 mmol L−1 of N but no effect of N concentration on the minituber number (5.87 tubers/pot) was observed (Sampaio et al. 2009).
The final yield of minitubers depends on, to a large extent, biotic and abiotic factors, such as potato plant canopy health, molecular and hormonal regulations, gene expression, genotype, sucrose and other photoassimilates (Klossterman and Bachem 2014). In addition, the hydroponic system used (aeroponics, NFT, deep-flow technique, and beds), nutrient solution, plant nitrogen status, air temperature, humidity (day/night), temperature of the nutrient solution, day length, radiation incidence, and number of harvest intervals can all have an impact on minituber production (Kooman et al. 1996; Goffart et al. 2008; Pereira and Villa Nova 2008; Otazú 2010; Mori et al. 2015; Oraby et al. 2015; Virtanen and Tuomisto 2017).
During the potato growth stages, photoassimilates and dry weight distribution is constantly taking place at various plant organs (Fig. 5a, e and b). In the initial plant development stage, there is more partitioning towards shoot growth, while in the tuber production stage, there is greater partitioning of photoassimilates towards the production of stolons, and consequently, minitubers. This occurs at appropriate nutritional levels and under environmentally favorable conditions (Klossterman and Bachem 2014). With nitrogen levels extend below or above the recommended level, the initiation of stolons may be impaired (Sonnewald and Sonnewald 2014). Mäck and Schjoerring (2002) report that under low N availability, C and N allocation occurs predominantly in tubers. Additionally, competition between tubers seems less necessary than in high N availability. Low sucrose may also induce low tuber formation and tuber yield (Sonnewald and Sonnewald 2014). The transport of photoassimilates, in the form of sucrose, is one of the driving forces for tuber growth (Klossterman and Bachem 2014; Paul et al. 2017). Carbon metabolism is linked to nitrogen metabolism, and any change in nitrogen availability may affect carbon metabolism (Stitt et al. 2002). The use of sucrose to form tubers also depends on the supply of amino acids. Muttucumaru et al. (2014) concluded in their studies that glutamine, glutamate, and serine are the main amino acids of leaf transport products for tubers in potato plants. The assimilation of nitrogen to produce amino acids requires adenosine triphosphate (ATP), reducing power, and a carbon skeleton from reactions that occur in photosynthesis, glycolysis, and respiration (Wang et al. 2014).
In terms of productivity, ease of assembly, affordability (especially with respect to electric pumps), and versatility for mounting, this system is useful for the production of basic seed potatoes.
Conclusion
The combination of 13 and 18.4 mmol L−1 N (T4) before and after 21 DAT, respectively, was found to be the optimal N fertilization regimen for the production of basic seed potato minitubers in this three-phase hydroponic system.
References
Andriolo, J.L. 2006. Sistema hidropônico fechado com subirrigação para produção de minitubérculos de batata. In: Simepósio de Melhoramento Genético e Previsão de Epifitias em Batata, Santa Maria. Anais, UFSM.
Buckseth, T., A.K. Sharma, K.K. Pandey, B.P. Singh, and R. Muthuraj. 2016. Methods of pre-basic seed potato production with special reference to aeroponics – A review. Scientia Horticulturae 204: 79–87. https://doi.org/10.1016/j.scienta.2016.03.041.
Chang, D.C., and Y.B. Lee. 2016. Response of potatoes to different nutrient solution management in a closed hydroponic system. Response of potatoes to different nutrient solution management in a closed hydroponic system. Journal of Plant Nutrition 39 (11): 1547–1555. https://doi.org/10.1080/01904167.2015.1109120.
Davidson, R.D. and K. Xie. 2014. Seed potato production. In: Navarre, R. and Pavek, M.J. (eds.). Chapter 8. Wallingford; Oxfordshire, UK; Boston, MA: CAB International. pp. 115-132. ISBN: 978-1-78064-280-2.
Diaz, B.C. and C.A. Medeiros. 2005. Produção hidropônica de sementes pré-básicas de batata em diferentes concentrações de nitrogênio na solução nutritiva. http://www.abhorticultura.com.br/biblioteca/arquivos/Download/Biblioteca/45_0464.pdf (Accessed in July 4, 2011).
Factor, T.L., J.A.C. Araujo, F.P.C. Kawakami, and V. Iunck. 2007. Produção de minitubérculos de batatasemente em sistemas hidropônicos NFT, DFT e Aeroponia. Horticultura Brasileira 25: 082–087.
Fontes, P.C.R. 2005. Cultura da batata. In Olericultura: teoria e prática, ed. P.C.R. Fontes. Suprema: Viçosa.
Fontes, P.C.R., H. Braun, M.C.C. Silva, F.S. Coelho, P.R. Cecon, and F.L. Partelli. 2016. Tuber yield prognosis model and agronomic nitrogen use efficiency of potato cultivars. Australian Journal of Crop Science 10:933-939. https://doi.org/10.21475/ajcs.2016.10.07.p7404.
Goffart, J.P., M. Olivier, and M. Frankinet. 2008. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: Past-present-future. Potato Research 51: 355–383.
Hakeem, K.R., M. Sabir, M. Ozturk, M.S. Akhtar, and F.H. Ibrahim. 2016. Nitrate and nitrogen oxides: Sources, health effects and their remediation. In: de Voogt P. (eds) Reviews of environmental contamination and toxicology, volume 242. Reviews of environmental contamination and toxicology (continuation of residue reviews), vol 242. Springer, Cham. https://doi.org/10.1007/398_2016_11.
Ho, T.V., N.T. Hoa, T.T. Loan, L.T. Tuyet, and P. Vander Zaag. 1988. Techniques for using sprouts for potato production in the tropics. Potato Research 31: 379–383. https://doi.org/10.1007/BF02357872.
Kelling, K.A., R.F. Hensler, and P.E. Speth. 2015. Importance of early-season nitrogen rate and placement to russet Burbank potatoes. American Journal of Potato Research 92: 502–510. https://doi.org/10.1007/s12230-015-9464-6.
Klossterman, B. and B. Bachem. 2014. The potato botany, production and uses. In: Navarre, R. and Pavek, M.J. (eds.). Chapter 4. Wallingford; Oxfordshire, UK; Boston, MA: CAB International. pp. 45-63. ISBN: 978-1-78064-280-2.
Kooman, P.L., M. Fahem, P. Tegera, and A.J. Haverkort. 1996. Effects of climate on different potato genotypes 1. Radiation interception, total and tuber dry matter production. European Journal of Agronomy 5: 193–205.
Krauss, A., and H. Marschner. 1982. Influence of nitrogen nutrition, daylength and temperature on contents of gibberellic and abscisic acid and on tuberization in potato plants. Potato Research 25: 13–21.
Mäck, G., and J.K. Schjoerring. 2002. Effect of NO3 − supply on N metabolism of potato plants (Solanum tuberosum L.) with special focus on the tubers. Plant. Cell and Environment 25: 999–1009. https://doi.org/10.1046/j.1365-3040.2002.00892.x.
Martinez, H.E.P., and J.B. Silva Filho. 2006. Introdução ao cultivo hidropônico de plantas. 3ª ed. Editora UFV: Viçosa.
Mori, K., K. Asano, S. Tamiya, T. Nakao, and M. Mori. 2015. Challenges of breeding potato cultivars to grow in various environments and to meet different demands. Breeding Science 65: 3–16. https://doi.org/10.1270/jsbbs.65.3.
Muller, D.R., D.A. Bisognin, J.L. Andriolo, J. Dellai, and F. Copetti. 2007. Produção hidropônica de batata em diferentes concentrações de solução nutritiva e épocas de cultivo. Pesquisa Agropecuária Brasileira 42: 647–653.
Muttucumaru, N., A.J. Keys, M.A. Parry, S.J. Powers, and N.G. Halford. 2014. Photosynthetic assimilation of 14C into amino acids in potato (Solanum tuberosum) and asparagine in the tubers. Planta 239: 161–170. https://doi.org/10.1007/s00425-013-1967-0.
Oliveira, C.A.S. 2000. Potato crop growth as affect by nitrogen and plant density. Pesquisa Agropecuária Brasileira 35: 939–950. https://doi.org/10.1590/S0100-204X2000000500011.
Oraby, H., A. Lachance, and Y. Desjardins. 2015. A low nutrient solution temperature and the application of stress treatments increase potato mini-tubers production in an aeroponic system. American Journal of Potato Research 92: 387–397. https://doi.org/10.1007/s12230-015-9444-x.
OSCS – Oregon Seed Certification Service. 2018. 2018 Oregon potato seed certification standards. Corvallis: Oregon State University.
Otazú, V. 2010. Manual on quality seed potato production using aeroponics. Peru: International Potato Center (CIP), Lima.
Patrick, J.W., and K. Colyvas. 2014. Crop yield components – Photoassimilate supply- or utilisation limited-organ development? Functional Plant Biology 41: 893–913. https://doi.org/10.1071/FP14048.
Paul, S., M.K. Das, P. Baishya, A. Ramteke, M. Farooq, B. Baroowa, R. Sunkar, and N. Gogoi. 2017. Effect of high temperature on yield associated parameters and vascular bundle development in five potato cultivars. Scientia Horticulturae 225: 134–140. https://doi.org/10.1016/j.scienta.2017.06.061.
Pereira, A.B., and N.A. Villa Nova. 2008. Potato maximum yield as affected by crop parameters and climatic factors in Brazil. HortScience 43 (5): 1611–1614.
Rahman, M.S., and A.M. Akanda. 2009. Performance of seed potato produced from sprout cutting, stem cutting and conventional tuber against PVY and PLRV. Bangladesh Journal of Agricultural Research 34 (4): 609–622.
Sampaio, J.F., Jr., P.C.R. Fontes, M.A. Guimarães, and M.A. Moreira. 2009. Produção de mini-tubérculos de batata propagada por brotos, em função de doses de nitrogênio aplicadas ao substrato. Bioscience Journal 25: 75–82.
Silva, J.G., M.G.C. França, F.T.F. Gomide, and J.R. Magalhães. 2013. Different nitrogen sources affect biomass partitioning and quality of potato production in a hydroponic system. American Journal of Potato Research 90: 179–185. https://doi.org/10.1007/s12230-012-9297-5.
Sonnewald, S., and U. Sonnewald. 2014. Regulation of potato tuber sprouting. Planta 239: 27–38. https://doi.org/10.1007/s00425-013-1968-z.
Souza, C.B.S., P.C.R. Fontes, M.A. Moreira, M. Puiatti, H.E.P. Martinez, and R.F. Araújo. 2012. Production of basic potato seed minitubers in substrate and different nitrogen rates. Revista Ceres 59: 850–858. https://doi.org/10.1590/S0034-737X2012000600016.
Stitt, M., C. Müller, P. Matt, Y. Gibon, P. Carillo, R. Morcuende, W.R. Scheible, and A. Krapp. 2002. Steps towards an integrated view of nitrogen metabolism. Journal of Experimental Botany 53: 959–970. https://doi.org/10.1093/jexbot/53.370.959.
Virtanen, E., and J. Tuomisto. 2017. Hydroponic minituber production in growth room conditions and carry-over effects of the technique on produced minitubers. Journal of Agricultural Science 9: 41–48. https://doi.org/10.5539/jas.v9n1p41.
Wang, M., Q. Shen, G. Xu, and S. Guo. 2014. Chapter one - new insight into the strategy for nitrogen metabolism in plant cells. In: International review of cell and molecular biology. Jeon, K.W. (ed.). Academic Press 310:1–37. https://doi.org/10.1016/B978-0-12-800180-6.00001-3.
Wang, C.C., X.Y. Wang, K.X. Wang, J.J. Hu, M.X. Tang, W. He, and P. Vander Zaag. 2018. Manipulating aeroponically grown potatoes with gibberellins and calcium nitrate. American Journal of Potato Research 59: 1–11. https://doi.org/10.1007/s12230-018-9635-3.
Acknowledgements
We thank the Brazilian National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), and Foundation of Support Research of the State of Minas Gerais, Brazil (FAPEMIG) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Filho, J.B., Fontes, P.C.R., Martinez, H.E.P. et al. Varying Nitrogen Concentrations to Optimize Basic Seed Potato Minitubers Production in a Three-Phase Hydroponic System. Am. J. Potato Res. 95, 687–695 (2018). https://doi.org/10.1007/s12230-018-9676-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-018-9676-7