Abstract
The availability and use of crop protectants including azoxystrobin in combination with fumigation has extended the Central Wisconsin effective growing season by 2 to 4 weeks. This study, evaluating the influence of these crop protection practices on the optimum nitrogen rate and time of application for Russet Burbank potato (Solanum tuberosum L.), was established as two 3-year field trials designed as split-split plot experiments. Both experiments used metam sodium as the main plots and fungicide treatment (chlorothalonil Zn alone or chlorothalonil Zn alternated with azoxystrobin for the first six sprays) as the first split. In-season fertilizer N rate (179, 224, 269, or 313 kg N ha−1) or in-season N timing (N split into two, three, or four applications at 269 kg N ha−1) was the second split. Not fumigating resulted in significantly higher verticillium ratings and severely repressed crop yield and tuber quality responses to both fungicide treatment and N rate. On average, fumigation increased total yield 13.6 Mg ha−1yr−1, U.S. No. 1 tubers by 9 % and U.S. No. 1 tubers >170 g by 5 % over where fumigation was not used. In 2 of the 3 years when azoxystrobin was included in the fungicide program early blight severity was reduced by about 50 %, and on fumigated areas yields were increased 4.8 Mg ha−1, whereas no yield increase was seen from this fungicide treatment on the non-fumigated plots. In these same years, fumigation increased optimum N rate by about 50 kg ha−1; however, there was no apparent interaction with fungicide treatment. Although fumigation, fungicide treatment, and time of N application each influenced tuber yield or tuber quality in some years, in the two more responsive years some interactions between these factors were statistically significant, with benefits generally only seen where plots were fumigated.
Resumen
La disponibilidad y uso de protectores del cultivo, incluyendo azoxistrobina en combinación con fumigación, ha extendido efectivamente el ciclo de cultivo por 2 a 4 semanas en el centro de Wisconsin. Este estudio, evaluando la influencia de estas prácticas de protección del cultivo en el nivel óptimo de nitrógeno y tiempo de aplicación para la papa Russet Burbank (Solanum tuberosum L.), se estableció como dos ensayos de campo de tres años diseñados como experimentos de parcelas subdivididas. En ambos experimentos se usó metam-sodio como la parcela principal, y el tratamiento con el fungicida (clorotalonil-Zn solo o clorotalonil-Zn alternado con azoxistrobina en las primeras seis aspersiones) como la primera división. En el ciclo el nivel de fertilización nitrogenada (179, 224, 269, o 313 kg N ha-1), o tiempos de aplicación (el N dividido en dos, tres o cuatro aplicaciones de 269 kg N ha-1) como la segunda división. La no fumigación dio como resultado niveles significativamente altos de Verticillium y rendimiento del cultivo severamente disminuido, así como en las respuestas en la calidad de tubérculo a ambos tratamientos de fungicida y de nivel de N. En promedio, la fumigación aumentó el rendimiento total 13.6 Mg ha-1 año 1, tubérculos US 1 en 9 %, y tubérculos US 1 > 170 g en 5 % sobre aquellos en donde no se usó fumigación. En dos de los tres años, cuando se incluyó azoxistrobina en el programa de fungicidas, la severidad del tizón temprano se redujo en carca del 50 %, y en las áreas fumigadas los rendimientos aumentaron 4.8 Mg ha-1, mientras que no se observó aumento en el rendimiento de este tratamiento de fungicida en los lotes no fumigados. En estos mismos años, la fumigación aumentó el nivel óptimo de N en cerca de 50 kg ha-1; no obstante, no hubo interacción aparente con el tratamiento del fungicida. Aun cuando la fumigación, el tratamiento con fungicida, y el tiempo de aplicación del N cada uno influenció el rendimiento y la calidad de tubérculo en algunos años, en los dos años de mayor respuesta, algunas de las interacciones entre estos factores fueron estadísticamente significativas, con beneficios generalmente observados solamente donde se fumigaron los lotes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen management is a critical component of potato (Solanum tuberosum L.) production systems. In addition to being the primary nutrient affecting crop yield and quality, nitrogen management has also been shown to influence other aspects of crop health, including severity of verticillium wilt (Verticillium dahdiae Kleb.) (Huber and Watson 1974; Lazarovits et al. 1997; Lambert et al. 2005) and early blight (Alternaria solani, Jones and Grout) (Barclay et al. 1973; Marshall-Farrar et al. 1998; Franc and Christ 2001). Literature suggests that fertilizer N may affect both the plant resistance to verticillium and the pathogen survival in the soil, and several studies show that N rate and form can affect disease incidence and severity (Davis 1985; Davis and Everson 1986; Pennypacker 1989; Davis et al. 1994; Lazarovits et al. 1997; Lazarovits 2010). Reviews by Pennypacker (1989), Lambert et al. (2005), and Lazarovits (2010) discuss various possible modes of action, including conversion of ammonium to ammonia, other nitrogenous conversions, and the influence of volatile fatty acids and soil pH.
Although high rates of N fertilizer have also been shown to suppress early blight (Davis 1985; Miller and Rosen 2005), the rate needed to achieve control is typically higher than that required for maximum yield (Barclay et al. 1973; MacKenzie 1981). Because a majority of potato cultivars currently grown in North America are susceptible to both pathogens, growers commonly employ a variety of control tactics, including fumigation, crop rotation, fertility adjustment, and fungicide application (Rowe and Powelson 2002; Lambert et al. 2005). Soil fumigation with metam sodium, while shown to be generally effective for verticillium wilt control (Powelson and Carter 1973; Tsror et al. 2005; Molina et al. 2014) significantly influences the soil biota, including the microbes affecting nitrogen transformations (Macalady et al. 1998; Ibekwe et al. 2001; Collins et al. 2006). For example, Collins et al. (2006) showed a significant decrease in soil N mineralization on fumigated areas and both Wolcott et al. (1960) and Koike (1961) determined that fumigation delayed the conversion of ammonium to nitrate-N up to 8 weeks. Where ammonical N sources are used, this could potentially negatively impact potato production as several studies have shown reduced tuber yield and/or quality where potatoes have been supplied all ammonium N along with a nitrification inhibitor that slows the conversion of NH4 + to NO3 – (Polizotto et al. 1975; Hendrickson et al. 1978; Kelling et al. 2011). However, Davis et al. (1986) reported no yield differences between all ammonium or mixed ammonium-nitrate fertilizers following fumigation at one location and an advantage to the all NH4 + source at the other. It is also apparent that fumigation results in more vigorous vegetative growth later in the season. This has caused growers to ask if they should be altering their N fertilizer rate or time of application to try to capture this late-season growth.
Similarly, the use of the protectant fungicide azoxystrobin for early blight control has resulted in the observation that potato vines remain green longer compared to potatoes treated with traditional fungicides (Stevenson et al. 1999; Stevenson 2001; Miller and Rosen 2005). This appears to be especially true with late-maturing or indeterminant varieties such as Russet Burbank. This observation also led to speculation that azoxystrobin may be interacting with other diseases including Verticillium (Miller and Rosen 2005). Researchers generally agree that N management can influence the observed severity of early blight (Soltanpour and Harrison 1974; MacKenzie 1981; Marshall-Farrar et al. 1998). However, although a Minnesota study saw better disease control at higher N rates even where fungicides were used, there were conflicting data regarding the N rate/fungicide interaction (Miller and Rosen 2005). In year one, yields responded to the highest N rate used (340 kg N ha−1) and yields and disease control were highest where azoxystrobin and chlorothalonil were alternated. In year two, there was no yield response to N rate where this fungicide program was used. The authors concluded that using azoxystrobin did not reduce the recommended N rate nor did keeping plants green longer increase the need for post-hilling N treatments (Miller and Rosen 2005).
Although the benefits of soil fumigation and the use of improved fungicide programs have been well established (Stevenson 2001; Rowe and Powelson 2002), there is little published research that evaluated the effect of both of these practices on optimal fertilizer N rates or time of application. Our primary objective was to determine if the use of metam sodium fumigant and/or azoxystrobin as a part of the weekly fungicide program, and therefore contributing to a longer effective growing season, resulted in increasing or decreasing the optimum N rate and fertilizer N use efficiency. In addition, the second experiment evaluated if the use of these crop protectants increased the need for N applications later in the season.
Methods and Materials
From 2000 through 2002, two split-split plot, field experiments were conducted at the University of Wisconsin Hancock Agricultural Research Station (44o7′N, 89o31′W) on Plainfield loamy sand soils (sandy, mixed, mesic, Typic Udipsamments) evaluating the interaction of fumigation, fungicide program and nitrogen rate or time of application on potato crop performance. The two experiments included fumigant as the main plot, fungicide program as the first split with nitrogen fertilizer rate or time of application as the second split. The two replications of fumigant treatments were with and without fall applications of 346 L ha−1 Vapam (metam sodium) providing 176 kg ha−1 a.i. from AmVac Chemical Corp. The fungicide programs were weekly sprays of chlorothalonil Zn at 2.48 L ha−1 (1.24 kg ha−1 a.i., Syngenta Crop Protection LLC), starting at row closure or chlorothalonil alternated with azoxystrobin at 45 mL ha−1 (11.2 g ha−1 a.i., Syngenta Crop Protection, LLC) for a total of three azoxystrobin sprays using four replications. Fertilizer N rates were 179, 234, 269, or 314 kg N ha−1, applied 33 % at emergence as ammonium sulfate, 50 % at tuberization as ammonium nitrate, and 17 % at tuberization + 20 days as ammonium nitrate also using four replications. The nitrogen timing experiment used one rate of N (269 kg N ha−1) split into two (33 % at emergence and 67 % at tuberization), three (described above), or four (33 % at emergence, 33 % at tuberization, 17 % at tuberization + 20 days, and 17 % at tuberization + 40 days) applications with the emergence application as ammonium sulfate and the others as ammonium nitrate. Some plots were used for both experiments. In all cases, the in-season N fertilizer was hand-applied in approximately a 10-cm band along the row. The emergence and tuberization treatments were followed by a light hilling. All plots received 34 kg N ha−1 as a part of the starter fertilizer, placed 5 cm to each side of the seed furrow.
Russet Burbank seed pieces were machine-planted on 22 to 24 April each year with 30-cm in-row spacing. Individual plots were four rows wide (92 cm between rows) by 6.1 m long and were arranged in randomized blocks. A new field that did not have potato for the previous 2 years was used each year and field corn was the immediately preceding crop. Other fertilization, pest management, and irrigation practices were performed by research station personnel and were according to UW recommendations, and common to grower practices used in the region.
Starting at about 40 days after emergence (dae), 40 of the most recently matured petioles (fourth or fifth from the top of the plant) were sampled from each plot and samplings continued every 8 to 12 days for four samplings. Petioles were dried at 65 °C and ground to pass a 0.63-mm screen. Samples were extracted with distilled water with analyses performed using a Lachat autoanalyzer (Lachat Instruments 1996).
Mid-season disease evaluations were conducted twice each year for early blight and verticillium wilt by visually examining four 1.0-m2 areas from the center two rows of each plot for symptoms of each disease. Each area was rated separately for severity of wilt and early blight on a modified Horsfall-Barratt scale (Horsfall and Barratt 1945), where 0 is no disease, 1 = 1 to 3 %, 2 = 4 to 6 %, 3 = 7 to 12 %, 4 = 13 to 24 %, 5 = 25 to 50 %, 6 = 51 to 76 %, 7 = 77 to 88 %, 8 = 89 to 94 %, 9 = 95 to 97 %, 10 = 98 to 99 %, and 11 = 100 % foliage exhibiting disease symptoms. The four disease symptom ratings in each plot were converted to disease severity ( %).
Potato tubers from the two center rows of each plot were mechanically harvested in late-September each year. The tubers were graded into U.S. No. 1, undersize (not retained on a 5.1-cm screen), and cull (off-shape, green, diseased or blemished). The U.S. No. 1 tubers were electronically size graded into <113, 114 to 170, 171 to 284, 285 to 370, 371 to 454, and >454 g categories. Tuber specific gravity was determined by weighing about 3.6 kg of washed U.S. No. 1 tubers in air and again suspended in water (Kleinschmidt et al. 1984) and specific gravity values converted to tuber dry matter (USDA 1964). Fifteen of the largest tubers were evaluated for internal defects. Tuber total N content was measured after drying (60 °C) an 800-g subsample of U.S. No. 1 tubers, grinding (<1 mm), and Kjeldahl digestion (Nelson and Sommers 1973) using a Lachat autoanalyzer (Lachat Instruments 1992).
Data (tuber total yield, quality parameters, petiole NO3-N, disease evaluations, tuber N concentration) were statistically analyzed using PROC ANOVA for a three factor split-split plot design with fumigation as the main plot, fungicide at the first split, and N rate or N timing as the final split in randomized blocks (SAS Institute 1999). Data were analyzed for each year separately since rainfall and insect pressure were very different in 2002. Optimum N rates were determined by regression of total yield for each fumigation/fungicide combination and year and subsequently choosing the best fit model (SAS Institute 1999).
Results and Discussion
Nitrogen Rate Study
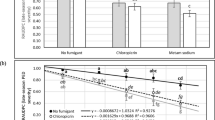
As shown in Table 1, significant tuber yield and quality increases were seen when the plots were fumigated. Total yield increased an average of 13.6 Mg ha−1 with fumigation across the 3 years, while percent grade U.S. No. 1 increased 9 % and percent of the U.S. No. 1 tubers greater than 170 g increased 5 %. These are about the same magnitude of increases seen for fumigation by other researchers (O’Sullivan and Reyes 1980; Ben-Yephet et al. 1983; Davis et al. 1986; Tsror et al. 2005). In addition, in 2000 and 2001, both N rate and fungicide program at times also positively increased yield and/or quality. However, due to the sometimes overriding influence of the fumigant, the benefits of higher N rates or using azoxystrobin as a part of the fungicide program were only seen or were larger when fumigant was used. For example, averaged across 2000 and 2001, the use of azoxystrobin without fumigant resulted in no change in total yield (34.9 Mg ha−1 without versus 34.1 Mg ha−1 with azoxystrobin), whereas when fumigant was used and azoxystrobin included in the fungicide program total tuber yields increased from 47.0 to 51.8 Mg ha−1.
Similarly, the yield increase from higher N rates was much more evident where fumigant was applied in 2001. In that year, average yields for the four N rates without fumigant were 31.6, 34.9, 34.4, and 33.6 Mg ha−1, respectively, and 47.6, 49.2, 52.4, and 54.2 Mg ha−1 with fumigant. Although yields were increased with N rate in 2000, the fumigant x N rate interaction was not significant. O’Sullivan and Reyes (1980), using the variety Kennebec, concluded that response to N was independent of fumigation. Regression of yield against N rate for 2000 and 2001 (Fig. 1) shows no yield increase from more than 220 kg N ha−1 where the plots were not fumigated even when azoxystrobin was included in the fungicide program. However, where fumigant was used, yields continued to increase to at least 275 kg N ha−1 for both fungicide programs. In addition, azoxystrobin provided about a 4.8 Mg ha−1 yield advantage across all N rates in these years. These data are similar to the Russet Burbank responses seen by Miller and Rosen (2005), where in year 1 of their 2-year Minnesota study, they saw no interaction between N rate and fungicide program, with yield increasing to the highest N rate and about the same advantage to using azoxystrobin as was seen in this experiment. However, in year 2 of their work, N provided a yield increase where chlorothanonil was used but no N response where azoxystrobin was included.
As is apparent from the tuber yield and quality data in Table 1, in 2002 the crop was negatively impacted by factors other than the imposed treatments. In addition to a 5.8 cm rain on 10 June, a severe storm of 33.5 cm fell over the 3-day period of 20 to 22 June. This was after the tuberization (T) fertilizer N treatment, but before the T + 20 day treatment. Since this soil only holds about 2.5 cm of water in the top 30 cm of soil (Starr et al. 2005) and this is the zone that contains most of the potato roots (Tanner et al. 1982), it is likely that a significant amount of the previously applied N leached out of the root zone in that year. Furthermore, the 2002 plots were bordered by a Colorado potato beetle experiment. In spite of multiple diverse insecticide treatments, it was not possible to keep the beetle population in check after mid-July. For these reasons, treatment effects were much less apparent in that year than in 2000 and 2001.
Table 1 also shows that fumigation and N had a significant effect on the yield of U.S. No. 1 tubers and those greater than 170 g. Averaged over all three experiment years, fumigation increased the percent U.S. No. 1 tubers by 9 % per year, resulting in an average yield increase of U.S. No. 1 tubers of 10.4 Mg ha−1. Results of increasing the N rate on yield of U.S. No. 1 tubers were inconsistent as an increase was seen in 2000, but there was no significant effect of N in 2001 or 2002. Fumigation increased the yield of the U.S. No. 1 tubers >170 g from 9.4 to 15.8 Mg ha−1in 2000 and 2.0 to 5.7 Mg ha−1 in 2002, but this parameter was not affected in 2001. Increasing N rate, however, resulted in more large tubers in all years, averaging 4.7, 7.5, 8.0, and 8.2 Mg ha−1 U.S. No. 1 > 170 g for the four respective N rates. Like the Miller and Rosen (2005) experiment, this study showed that either fungicide program resulted in about the same proportion of tubers >170 g; however, the Minnesota work showed that where no fungicide was applied this percentage was lower.
Fumigation consistently increased tuber dry matter, averaging 17.8 versus 18.6 % without and with fumigant, respectively (data not shown). Miller and Rosen (2005) also observed an increase in tuber specific gravity where azoxystrobin was included, whereas we did not. Soltanpour and Harrison (1974) also saw an increase in specific gravity where a fungicide was used. Examination of tubers for internal defects (hollow heart or internal brown spot) showed low occurrence (<2 %) and no measurable differences between treatments (data not shown).
In general disease pressure from both diseases was considered moderate in 2000 and 2001 and more severe in 2002 because of the very wet weather in that year. Although disease ratings were done twice each year, only the later rating is shown for 2000 and 2001 as values for the first were generally low and non-discriminating. At the second evaluation, as shown in Table 2, early blight severity ratings were significantly reduced by including azoxystrobin in the fungicide program, in both 2000 and 2001. Early blight severity averaged 2.9 % in 2000 and 15.8 % in 2001 where chlorothalonil was used compared to 1.8 and 7.1 % in those years where azoxystrobin was included. Interestingly, although the difference was relatively small, fumigation also affected the early blight evaluation in 2000 and 2001, averaging 8.3 % without and 5.5 % with fumigation. As expected, fumigation also significantly decreased Verticillium ratings in 2001 and 2002 (the plots were not rated for Verticillium in 2000). For example, average ratings on 31 July 2001 without fumigation were 46.6 versus 9.8 % for the fumigated plots. By the time of the final evaluation in 2002 (13 August), the vegetation was in such poor condition that it was not possible to distinguish between early blight or Verticillium symptoms. Similar to the data of O’Sullivan and Reyes (1980) and Platt and Arsenault (2001), N rate did not consistently affect either the early blight or verticillium disease ratings except for the overall rating in 2002 where there was a trend (p = 0.11) for less severe symptoms with increasing N rates (93.3, 92.6, 89.9, and 83.4 % for the 179 to 314 kg ha−1 N rates). Miller and Rosen (2005) noted reduced levels of early blight at higher N rates even when azoxystrobin was used, and other researchers saw a suppression of early blight at higher N rates (Barclay et al. 1973; Soltanpour and Harrison 1974; MacKenzie 1981; Davis 1985). Also, unlike the data from our study and those of O’Sullivan and Reyes (1980), several researchers reported a suppression of Verticillium at higher N rates (Davis 1985; Pennypacker 1989; Davis et al. 1990; Davis and Huisman 2001).
Table 3 shows mid-season and late-season petiole NO3-N concentrations and tuber N concentration were influenced by fumigation/fungicide program, and fertilizer N rate. Mid-season petiole NO3-N levels were significantly affected by N rate in all years, with average concentrations of 1.05, 1.23, 1.42, and 1.55 % NO3-N for the respective 179 to 314 kg ha−1 N rates; however, these levels, although sampled at about the same stage of growth, varied substantially from year-to-year. For example, in 2000 only the highest N rate showed a sufficient amount of N in the petioles, whereas in 2001 all rates were above the sufficiency level of 1.2 to 1.6 % NO3-N for Russet Burbank at 50 dae as defined by Kelling (2000). This variation was likely due to the amount of early season nitrogen leaching that had occurred in the respective seasons on this very sandy soil, as there were four rainfall events greater than 2.5 cm between emergence and this petiole sampling in 2000 versus only one in 2001. In 2002, treatment variables other than N rate also affected petiole NO3-N concentrations, with sufficient amounts present only occurring at the high N rates where crop growth and perhaps tuber bulking, were slowed by no fumigation and not using azoxystrobin. Clearly, the major storm event of 33.4 cm of rainfall on 20 to 22 June 2002 significantly affected crop growth. In addition, there were two other events greater than 2.5 cm earlier in June of that year.
By the late-season petiole sampling in late July/early August, petiole NO3-N concentrations averaged across all years for the various N rates were 0.50, 0.74, 0.75, and 0.92 %, respectively. However, sufficient N rates (defined as 0.5 to 0.8 % NO3-N for Russet Burbank at 70 dae; Kelling 2000) were present in all treatments in 2001 but only the three higher N rate treatments without fumigant in 2002. Although the fungicide x N rate interaction term was significant at this sampling time in both 2000 and 2001, the results were inconsistent as NO3-N concentrations generally increased with increasing N rates, but tended to be higher where azoxystrobin was used in 2000 and lower for these treatments in 2001. Miller and Rosen (2005) also saw no effect of fungicide on petiole NO3-N concentrations, although they concluded their sampling occurred before early blight pressure was severe. Fumigation generally resulted in lower petiole NO3-N levels in 2002 for this later sampling, but was interactive with fungicide and N rate. Similarly, O’Sullivan and Reyes (1980) also reported lower petiole NO3-N levels from fumigated plots. It is likely the fumigation-stimulated increased growth resulted in some dilution of tissue nitrate.
Fumigation significantly increased tuber N uptake (total yield x percent dry matter x tuber N concentration) in all 3 years of the study, mostly because of the effect of fumigation on tuber yield as fumigation had less effect on tuber N concentrations (1.38, 1.72, and 1.69 % N without fumigant and 1.37, 1.57, and 1.52 % N with fumigant for each of the 3 years). Nitrogen rate only increased tuber N concentration in one of three years (Table 3), and the 3-year tuber N averages were 1.31, 1.53, 1.58, and 1.66 % N for the four respective N rates.
Nitrogen Timing Study
The influence of splitting a single rate of in-season fertilizer N (269 kg ha−1) into two, three, or four applications on crop performance is shown in Table 4. As with the N rate experiment, these data show that fumigation significantly increased tuber yield and quality in all 3 years, and in one of the years using azoxystrobin increased yields on the fumigated plots. Where N timing effects were significant, splitting the N especially into four applications tended to reduce yields (2000 p = 0.07, or in 2001 on fumigated areas) or U.S. No. 1 (2002) and large tubers (2000 or 2001 where azoxystrobin was used). Apparently at this rate of N, the last fertilizer N application at tuberization plus 40 days was too late to maintain yield or quality in spite of the significant amount of leaching that occurred in 2000 and 2002. It is also likely that the Colorado beetle problem in 2002 kept the crop from being able to effectively utilize this mid-July application. Other studies on this soil have also seen lower tuber yields and/or quality where N applications are after mid-July (Kelling and Speth 1998; Kelling et al. 2015).
Since the emergence application of N was made as ammonium sulfate and since potatoes tend to perform better with nitrate or a mixture of forms (Hendrickson et al. 1978; Cao and Tibbitts 1993; Kelling et al. 2011), these data provide some indirect evidence that fall fumigation does not impede potato use of early-season applied ammonium-N. Other researchers also observed no difference in yield when various N forms were used following fumigation (Davis et al. 1986). Reviews by Pennypacker (1989) and Lazarovits et al. (1997) report several studies where plants fertilized with ammonium sulfate showed a delay in Verticillium symptoms and senescence. In the experiment reported here, the timing of the N applications had no measurable effects on any of the disease evaluations in any of the years (data not shown). Miller and Rosen (2005) also concluded that post hilling N applications were not effective in managing disease or improving yield; however Davis et al. (1994) observed reduced verticillium severity where N was split versus a single application.
In general, the treatments that increased yield (fumigant and azoxystrobin in some years) resulted in decreased tuber N by dilutions with higher yields in 2000 and 2002, whereas application of N later in the season resulted in higher tuber N concentrations (Table 4). Apparently this later applied N moved into the tuber, but was not in place in time to increase total yield.
Conclusions
These experiments showed that fumigation substantially increased tuber yield by an average of over 13 Mg ha−1, and this treatment also increased tuber grade, size, and dry matter. In 2 of the 3 study years where fumigant was not used, little response was seen to better early blight control with azoxystrobin or using higher rates of N fertilizer; however, with fumigation, including azoxystrobin in the fungicide program increased yields by 4.8 Mg ha−1 and yields responded to an additional 50 kg N ha−1. Most of these improvements could be attributed to an increase in tuber size. In the third year, severe N leaching and incomplete late-season Colorado potato beetle control restricted these responses. It is clear that the benefits of extra N and more effective blight control can only be expressed when other yield-limiting factors such as Verticillium or insects are adequately controlled. Disease evaluations showed the effectiveness of the various fumigation and fungicide treatments, but unlike other studies, additional N did not reduce Verticillium on Alternaria symptom expression. Nitrogen rate distinctly increased petiole NO3-N and in 1 year tuber N concentrations; however, fumigation and/or azoxystrobin treatment tended to reduce plant N concentrations due to dilution by more growth. As has been seen in other studies on this soil, excessive splitting of the N (especially in non-leaching years) reduced yield and quality, suggesting that most of the N should be applied by early bulking.
These experiments confirm that fumigation and the use of azoxystrobin can increase yields substantially and this increase in yield potential necessitates increased levels of fertilizer N. Application of the majority of the in-season N should be made by early bulking even where crop protectants are used to extend the growing season.
References
Barclay, G.M., H.J. Murphy, F.E. Manzer, and F.E. Hutchinson. 1973. Effects of differential rates of nitrogen and phosphorus on early blight in potatoes. American Potato Journal 60: 42–48.
Ben-Yephet, Y., E. Siti, and Z. Frank. 1983. Control of Verticillium dahliae by metam sodium in loessial soil and effect on potato tuber yields. Plant Disease 67: 1223–1225.
Cao, W., and T.W. Tibbitts. 1993. Study of various NH4 +/NO3 – mixtures for enhancing growth of potatoes. Journal of Plant Nutrition 16: 1691–1704.
Collins, H.P., A. Alva, R.A. Boydston, R.L. Cochran, P.B. Hamm, A. McGuire, and E. Riga. 2006. Soil microbial, fungal, and nematode responses to soil fumigation and cover crops under potato production. Biology and Fertility of Soils 42: 247–257.
Davis, J.M., W.H. Loescher, M.W. Hammond, and R.E. Thornton. 1986. Response of Russet Burbank potatoes to soil fumigation and nitrogen fertilizers. American Potato Journal 63: 71–79.
Davis, J.R. 1985. Approaches to control of potato early dying caused by Verticillium dahliae. American Potato Journal 62: 177–185.
Davis, J.R., and D.O. Everson. 1986. Relationship of Verticillium dahliae in soil and plant tissue, irrigation method, and N fertility to verticillium wilt of potato. Phytopathology 76: 730–736.
Davis, J.R., and O.C. Huisman. 2001. Verticillium wilt. In Compendium of potato diseases, 2nd ed, ed. W.R. Stevenson, R. Loria, G.D. Franc, and D.P. Weingartner, 45–46. St. Paul: APS Press.
Davis, J.R., L.H. Sorenson, J.C. Stark, and D.T. Westermann. 1990. Fertility and management practices to control verticillium wilt of the Russet Burbank potato. American Potato Journal 67: 55–65.
Davis, J.R., J.C. Stark, L.H. Sorenson, and A.T. Schneider. 1994. Interactive effects of nitrogen and phosphorus on verticillium wilt of Russet Burbank potato. American Potato Journal 71: 467–481.
Franc, G.D., and B.J. Christ. 2001. Early blight. In Compendium of potato diseases, 2nd ed, ed. W.R. Stevenson, R. Loria, G.D. Franc, and D.P. Weingartner, 22–23. St. Paul: APS Press.
Hendrickson, L.L., D.R. Keeney, L.M. Walsh, and E.A. Liegel. 1978. Evaluation of nitrapyrin as a means of improving N efficiency in irrigated sands. Agronomy Journal 70: 699–703.
Horsfall, J.G., and R.W. Barratt. 1945. An improved grading system for measuring plant diseases. Phytopathology 35: 655.
Huber, D.M., and R.D. Watson. 1974. Nitrogen form and plant disease. Annual Review of Phytopathology 12: 139–165.
Ibekwe, A.M., S.K. Papiernick, J. Gan, S.R. Yates, and D.E. Crowley. 2001. Impact of soil fumigants on soil microbial communities. Applied Environmental Microbiology 67: 3245–3257.
Kelling, K.A. 2000. Research observations on petiole nitrate testing. Proceedings of the Annual Wisconsin Potato Meetings 13: 175–184.
Kelling, K.A., and P.E. Speth. 1998. Timing of nitrogen application on potatoes. Proceedings of the Annual Wisconsin Potato Meetings 11: 61–72.
Kelling, K.A., R.P. Wolkowski, and M.D. Ruark. 2011. Potato response to nitrogen form and nitrification inhibitors. American Journal of Potato Research 88: 459–469.
Kelling, K.A., F.J. Arriaga, B. Lowery, M.O. Jordan, and P.E. Speth. 2015. Use of hill shape with various nitrogen timing splits to improve fertilizer use efficiency. American Journal of Potato Research 92: 71–78.
Kleinschmidt, G.D., G.E. Kleinkopf, D.T. Westermann, and J.C. Zalewski. 1984. Specific gravity of potatoes. Current Information Series No. 609, University of Idaho.
Koike, H. 1961. The effects of fumigants on nitrate production in soil. Proceedings of the Soil Science Society of America 25: 204–206.
Lachat Instruments. 1992. Total Kjeldahl nitrogen in soil/plants. Quikchem method 13-107-06-02-D. User Manual, Lachat Instruments, Mequon, Wisconsin.
Lachat Instruments. 1996. Nitrate in water extracts. QuikChem method 12-101-04-1-B. User Manual, Lachat Instruments, Mequon, Wisconsin.
Lambert, D.H., M.L. Powelson, and W.R. Stevenson. 2005. Nutritional interactions influencing diseases of potato. American Journal of Potato Research 82: 309–319.
Lazarovits, G. 2010. Managing soil borne disease of potatoes using ecologically based approaches. American Journal of Potato Research 87: 401–411.
Lazarovits, G., K. Cohn, and M. Tenuta. 1997. Control of Verticillium dahliae with soil amendments: Efficacy and mode of action. In Advances in Verticillium research and disease, ed. E.C. Tjamos, R.C. Rowe, J.B. Heale, and D.R. Fravel, 274–291. Athens, Greece: Proceedings of the Seventh International Verticillium Symposium.
Macalady, J.L., M.E. Fuller, and K.M. Scow. 1998. Effects of metam sodium fumigation on microbial activity and community structure. Journal of Environmental Quality 27: 54–63.
MacKenzie, D.R. 1981. Association of potato early blight, nitrogen fertilizer rate, and potato yield. Plant Diseases 65: 575–577.
Marshall-Farrar, K.D., M. McGrath, R.V. James, and W.R. Stevenson. 1998. Characterization of Phytophthora infestans in Wisconsin from 1993 to 1995. Plant Disease 82: 434–436.
Miller, J.S., and C.J. Rosen. 2005. Interactive effects of fungicide programs and nitrogen management on potato yield and quality. American Journal of Potato Research 82: 399–409.
Molina, O.I., M. Tenuta, A. El-Hadrami, K. Buckley, C. Cavers, and F. Daayf. 2014. Potato early dying and yield responses to compost, green manures, seed meal and chemical treatments. American Journal of Potato Research 91: 414–428.
Nelson, D.W., and L.E. Sommers. 1973. Determination of total nitrogen in plant material. Agronomy Journal 65: 109–112.
O’Sullivan, J.O., and A.A. Reyes. 1980. Effects of soil fumigation, rotation and nitrogen on yield, petiole NO3-N and verticillium wilt of potatoes. Journal of the American Society for Horticultural Science 105(6): 809–812.
Pennypacker, B.W. 1989. The role of mineral nutrition on the control of verticillium wilt. In Soil borne plant pathogens: Management of diseases with macro- and micro-elements, ed. A.W. Engelhard, 33–45. St. Paul: APS Press.
Platt, H.W., and W.J. Arsenault. 2001. Management of nitrogen and phosphorus rates does not suppress verticillium wilt in Yukon Gold. American Journal of Potato Research 78: 215–219.
Polizotto, K.R., G.E. Wilcox, and E.J. Jones. 1975. Response of growth and mineral composition of potato to nitrate and ammonium nitrogen. Journal of the American Society for Horticultural Science 100: 165–168.
Powelson, R.L., and G.E. Carter. 1973. Efficacy of soil fumigants for control of verticillium wilt of potatoes. American Potato Journal 50: 162–167.
Rowe, R.C., and M.L. Powelson. 2002. Potato early dying: Management challenges in a changing production environment. Plant Disease 86: 1184–1193.
SAS (Statistical Analysis System Institute). 1999. SAS User’s Guide, Version 8.0. Statistical Analysis Systems Institute, Cary, North Carolina.
Soltanpour, P.N., and M.D. Harrison. 1974. Interrelations between nitrogen and phosphorus fertilization and early blight control of potatoes. American Potato Journal 51: 1–7.
Starr, G.C., E.T. Cooley, B. Lowery, and K.A. Kelling. 2005. Soil water fluctuations in loamy sand under irrigated potato. Soil Science 170: 77–89.
Stevenson, W.R. 2001. Potato disease management – one step forward and two steps back. Proceedings of the Annual Wisconsin Potato Meetings 14: 175–213.
Stevenson, W.R., R.V. James, and R.E. Rand. 1999. When and how to use early blight and late blight fungicides. Proceedings of the Annual Wisconsin Potato Meetings 12: 227–280.
Tanner, C.B., G.G. Weis, and D. Curwen. 1982. Russet Burbank rooting in sandy soils with pans following deep plowing. American Potato Journal 59: 107–112.
Tsror, L., E. Shlevin, and I. Peretz-Alon. 2005. Efficacy of metam-sodium for controlling Verticillium dahliae prior to potato production in sandy soils. American Journal of Potato Research 82: 419–423.
United States Department of Agriculture. 1964. Determining solids in potatoes. Agricultural Handbook no. 267. U.S. Government Printing Office, Washington, DC.
Wolcott, A.R., F. Maciak, L.N. Shepherd, and R.E. Lucas. 1960. Effects of Telone on nitrogen transformations and on growth of celery in organic soil. Down to Earth 16: 10–14.
Acknowledgments
Support for portions of this research was provided by the Wisconsin Potato and Vegetable Growers Association Potato Industry Board, the Wisconsin Fertilizer Research Council, and UW College of Agricultural and Life Sciences, and is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelling, K.A., Stevenson, W.R., Speth, P.E. et al. Interactive Effects of Fumigation and Fungicides on Potato Response to Nitrogen Rate or Timing. Am. J. Potato Res. 93, 533–542 (2016). https://doi.org/10.1007/s12230-016-9532-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-016-9532-6