Abstract
Biofilm formation by the pathogenic bacteria generates a serious threat to the public health as it can increase the virulence potential, resistance to drugs, and escape from the host immune response mechanisms. Among the environmental factors that influence the biofilm formation, there are only limited reports available on the role of antimicrobial agents. During the antimicrobial drug administration or application for any purpose, the microbial population can expect to get exposed to the sub-minimum inhibitory concentration (sub-MIC) of the drug which will have an unprecedented impact on microbial responses. Hence, the study has been conducted to investigate the effects of sub-MIC levels of zinc oxide nanoparticles (ZnO NPs) on the biofilm formation of Klebsiella pneumoniae and Staphylococcus aureus. Here, the selected bacteria were primarily screened for the biofilm formation by using the Congo red agar method, and their susceptibility to ZnO NPs was also evaluated. Quantitative difference in biofilm formation by the selected organisms in the presence of ZnO NPs at the sub-MIC level was further carried out by using the microtiter plate-crystal violet assay. Further, the samples were subjected to atomic force microscopy (AFM) analysis to evaluate the properties and pattern of the biofilm modulated under the experimental conditions used. From these, the organisms treated with sub-MIC levels of ZnO NPs were found to have enhanced biofilm formation when compared with the untreated sample. Also, no microbial growth could be observed for the samples treated with the minimum inhibitory concentration (MIC) of ZnO NPs. The results observed in the study provide key insights into the impact of nanomaterials on clinically important microorganisms which demands critical thinking on the antimicrobial use of nanomaterials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc oxide nanoparticles (ZnO NPs) have been studied widely during the last few years for various clinical and commercial applications due to their antimicrobial properties. This has made ZnO NPs to have applications in the medical industry for wound dressing, development of surgical instruments, bone prosthetics production, and also in other industries for textile production, food packaging, cutting board materials, wastewater treatment, etc. (Jin et al. 2019; Rambabu et al. 2021; Huang et al. 2021; Saddik et al. 2022). Medical devices like urinary catheters have also been fabricated with ZnO NPs for the improvement of their clinical performance (Ivanova et al. 2021). ZnO NPs exhibit multi-mechanistic antimicrobial properties through the membrane-damaging abrasiveness, generation of reactive oxygen species, and also by the release of antibacterial zinc ions from the nanoparticle surface with impact on the bacterial glycolysis, and transmembrane proton translocation (Mendes et al. 2022). In most of the cases, the microbial populations can get exposed to the sub-MIC levels of ZnO NPs either due to their slow release or the dilution that may happen at the sites apart from its point of administration. The impact of such a phenomenon is greatly unexplored which makes the current study an important initiative on the same. When present at levels below the minimum inhibitory concentration (MIC), the antimicrobial agents can have an inducible effect on the microbial antibiotic resistance and virulence.
At the sub-MIC concentrations (SMC), the antimicrobial agents can expect to act as key signalling molecules to alter the biochemistry and structure of bacteria thereby leading to increased resistance. These global changes can also modulate their pathogenicity, physicochemical properties, and crucial bacterial cellular functions including adhesion, surface hydrophobicity, fimbriation, motility, and host-bacterial interactions (Yuan et al. 2023). Exposure to the SMCs of drugs might also be linked to an enhanced formation of biofilm. This is a visible and easily detectable change in the bacterial physiology and metabolism activated by the low concentration of the applied antimicrobial agent.
The formation of biofilm is a major adaptative strategy frequently used by bacteria, and the mechanistic insight into the same has already been described in various pathogenic microorganisms (Kelly et al. 2020). Bacterial contact with any surface can lead to biofilm formation especially when the surface is immersed in water or slightly moist, and also in an environment with abundant or limited nutrients. Diverse factors have already been described to regulate the formation and structural complexity of the biofilm. In the clinical context, biofilm can serve as a pathogen reservoir and is linked to the severity of conditions like bloodstream and urinary tract infections (Pinto et al. 2021). As the biofilm-associated bacteria are highly resistant to antimicrobial treatments and host immune response mechanisms, factors favouring the biofilm formation among the clinically important bacteria generate much concern.
Many previous studies have already reported the ability of antibiotics such as chlorhexidine, tetracycline, kanamycin, spectinomycin, streptomycin, and neomycin to have an inducible effect on the bacterial biofilm at SMC (Omer and Aka 2022). Here, the sub-inhibitory doses can be considered to execute selective pressure among bacteria for the predominant growth and subsequently, the biofilm formation to result in drug resistance. This promotion of biofilm development could ultimately be due to the transcriptional activation of genes involved in virulence and bacterial homeostasis. Various nanomaterials have already been investigated widely for their antimicrobial applications even by projecting them as the superior solution to manage the drug-resistant microorganisms (Allahverdiyev et al. 2011; Zhao et al. 2022). However, at the SMC levels, the nanoparticles can also have an inducible effect on bacterial biofilm formation. As ZnO NPs are one of the most commonly used antimicrobial agents, the current study has been designed to identify the impact of ZnO NPs at SMC on the biofilm formation of Klebsiella pneumoniae and Staphylococcus aureus. Here, the microtiter plate-crystal violet assay was carried out for the direct quantification of the biofilm formation by the selected organisms at different SMCs of ZnO NPs. Atomic force microscopy analysis was also carried out to study the pattern and morphological features of biofilm modulated in the presence of SMC of ZnO NPs. Results of the study provide novel insights into the role of nanomaterials as inducers of biofilm in clinically important organisms at sub-MIC concentrations.
Materials and methods
Materials
Congo red (RM927-25G) used in this study was procured from Hi-Media (India). Mueller–Hinton agar (MHA), Mueller–Hinton broth (MHB), and nutrient agar (NA) were purchased from Hi-Media Laboratories Pvt Ltd. ZnO nanoparticles (721077-100G) 20% (w/w) in water were purchased from Sigma Aldrich. Staphylococcus aureus (ATCC 25923) and Klebsiella pneumoniae (ATCC 25955) were obtained from the culture collection of the Microbiology lab, School of Biosciences, Mahatma Gandhi University.
Identification and characterisation of the selected bacteria
The selected strains of K. pneumoniae and S. aureus used in this study were characterised by using the Biomerieux VITEK 2 Compact system through biochemical identification and antibiotic susceptibility testing analyses. For K. pneumoniae, susceptibility against gentamicin, trimethoprim-sulfamethoxazole, piperacillin-tazobactam, ciprofloxacin, cefoperazone-sulbactam, amikacin, imipenem, cefepime, meropenem, tigecycline, colistin, amoxycillin-clavulanate, cefuroxime, ceftriaxone, ertapenem, and cefuroxime-axetil was assessed. For S. aureus, susceptibility against benzylpenicillin, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, nitrofurantoin, gentamicin, ciprofloxacin, vancomycin, teicoplanin, linezolid, tetracycline, daptomycin, levofloxacin, tigecycline, and oxacillin was examined (Nakasone et al. 2007).
Screening of the selected organisms for biofilm production
Congo red agar (CRA) medium was prepared by supplementing sucrose (50 g/L), agar (10 g/L), and Congo red indicator dye (0.8 g/L) to the brain heart infusion (BHI) broth (37 g/L). Congo red indicator was prepared as a concentrated aqueous solution and autoclaved at 121 °C for 15 min, separately from other medium constituents, and was then added into the sterile BHI agar media when it was cooled to 55 °C after autoclaving. Prepared CRA plates were then inoculated with K. pneumoniae and S. aureus by the streak plate method and incubated for 48 h at 37 °C (Harika et al. 2020).
Activity of ZnO NPs against selected bacteria
The antibacterial activity of the ZnO NPs against S. aureus and K. pneumoniae was evaluated by using the standard agar well diffusion method on Mueller–Hinton agar (MHA) by following the CLSI guidelines. For this, bacterial cell suspensions with a turbidity of 0.5 McFarland (equivalent to 1.5 × 108 CFU/mL) were prepared in Mueller–Hinton broth. The lawn culture method was then used to inoculate the organisms onto the surface of MHA plates. Then, 60 µL of ZnO NP (20% w/w) suspension was added into the wells followed by incubation at 37 °C for 24 h. The zone of inhibition of ZnO NPs against the test organisms was then measured using a standard zone measuring scale and recorded (Ashitha et al. 2020).
Minimum inhibitory concentration of ZnO NPs
The minimum inhibitory concentration (MIC) of ZnO NPs against S. aureus and K. pneumoniae was determined by the standard broth microdilution method using sterile 96-well microtiter plates by following the CLSI guidelines. Here, 0.015% resazurin dye solution was used as the indicator for bacterial growth. The MIC value of ZnO NPs against each organism was then identified based on the colour change observed with the addition of resazurin after incubating the organism in the presence of different concentrations of ZnO NPs (in the concentration range of 2 × 10−6 to 5 mg/mL). From the obtained results, SMC of ZnO NPs was identified for further experiments (Jayakumar et al. 2019).
Biofilm formation by the selected bacteria treated with sub-MIC levels of ZnO NPs
The tissue culture plate method (TCP) was used for the quantification and comparison of biofilm formed by the selected organisms in the presence of ZnO NPs at SMC. For this, ZnO NP suspension in TSB was prepared at different concentrations below the MIC level (sub-MICs). Here, five different sub-MIC levels (0.7, 0.6, 0.5, 0.4, and 0.3 mg/mL) of ZnO NP solutions were prepared. Test inoculums were prepared by incubating the selected microorganisms overnight at 37 °C in trypticase soy broth (TSB) supplemented with 1% glucose. The turbidity of the overnight incubated bacterial culture was adjusted to 0.5 McFarland using sterile TSB, and 100 µL from this was added to each well of the tissue culture plate. Further, 100 µL of the prepared ZnO NP suspension was also added into the wells and kept for incubation at 37 °C for 48 h under static conditions. After the incubation, the contents were aspirated, and the plates were washed twice with phosphate-buffered saline (PBS). The wells were further stained with 0.1% crystal violet for 30 min at room temperature. Wells were further washed, dried, and treated with 95% ethanol. Biofilms formed were further quantified by measuring the absorbance at 595 nm using a multiplate reader (Perkin Elmer Lamda 650). For each of the organisms tested, biofilm assays were performed in triplicates to determine the mean biofilm absorbance value. Biofilm formation under each treatment was also graded into strong, moderate, and non/weak based on the absorbance observed (Neethu et al. 2020).
AFM analysis of the biofilm formed by selected organisms at sub-MIC levels of ZnO NPs
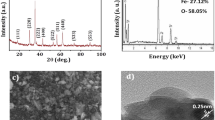
Biofilm formed by K. pneumoniae and S. aureus in the presence of ZnO NPs at SMC was further studied by the AFM analysis. For this, the selected organisms were cultured in the presence of selected SMC levels (0.7, 0.6, 0.5, 0.4, 0.3, and 0 mg/mL) of ZnO NPs by keeping a small, sterile glass slide inside the media to allow the biofilm formation on its surface. Further, the developed biofilm was fixed with 2.5% glutaraldehyde, and the dried glass pieces were further observed under a confocal Raman microscope with AFM (WITec Alpha300 RA, Germany) to study the thickness, surface morphology, and other properties of the formed biofilm (Panichikkal et al. 2022).
Antibiotic susceptibility of biofilms treated with ZnO nanoparticles
Antibiotic susceptibility of the biofilms formed by the selected organisms (K. pneumoniae and S. aureus) treated with the SMC of ZnO NPs was evaluated by the MIC determination using micro-broth dilution. For this, bacterial inoculum was prepared by incubating both K. pneumoniae and S. aureus in sterile tryptone soya broth (TSB) at 37 °C for 24 h. After the incubation, the cell concentration was adjusted to 1.5 × 108 CFU/mL (0.5 McFarland) using the sterile TSB. Subsequently, 100 µL of the prepared inoculum was transferred into 10 mL of TSB supplemented with 5% sucrose. ZnO NPs were then added into the inoculated broth, with the final concentration of ZnO NPs being 0.04% for K. pneumoniae and 0.05% for S. aureus, followed by incubation at 37 °C for 48 h. Untreated bacteria were maintained as controls, and three replicates were carried out for each set of treatments. After the incubation, the broth containing planktonic cells was discarded, and the biofilm was washed twice with sterile PBS. Antibiotic susceptibility of biofilm was further evaluated by using the automated MIC determination of the Biomerieux VITEK 2 Compact system. For K. pneumoniae, the susceptibility against gentamicin, trimethoprim-sulfamethoxazole, piperacillin-tazobactam, ciprofloxacin, cefoperazone-sulbactam, amikacin, imipenem, cefepime, meropenem, tigecycline, colistin, amoxycillin-clavulanate, cefuroxime, ceftriaxone, ertapenem, and cefuroxime-axetil was evaluated. For S. aureus, susceptibility against benzylpenicillin, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, nitrofurantoin, gentamicin, ciprofloxacin, vancomycin, teicoplanin, linezolid, tetracycline, daptomycin, levofloxacin, tigecycline, and oxacillin was evaluated.
Statistical analysis
A completely randomised experimental design was used for all the analyses carried out in this study. Data were expressed as mean ± standard error, and the significance level was set at p < 0.05. The biofilm formation assay was analysed using the one-way ANOVA followed by the post-hoc analysis to compare the absorbance values among different treatment groups. The results of AFM analysis, including the biofilm thickness and surface morphology, were also analysed using the paired t-test to compare the differences between the biofilms formed under the treatment using ZnO NPs at the SMC level. For the evaluation of antibiotic susceptibility in biofilms treated with ZnO NPs, the MIC values obtained for each of the antibiotics were compared with untreated groups using a t-test for K. pneumoniae and S. aureus separately. All the statistical analyses were conducted using the software, IBM SPSS Statistics.
Results
Identification and characterisation of the selected bacteria
Both the strains of bacteria used in this study were characterised by using the Biomerieux VITEK 2 Compact system. From the obtained results, the bacteria were confirmed as S. aureus and K. pneumoniae. Both the tested strains were also found to be sensitive to all the tested antibiotics. The observed antibiotic susceptibility and MIC values are provided in Table S1.
Screening the selected bacteria for biofilm production
The test organisms were screened for their ability for biofilm production using Congo red agar. Here, the colonies of K. pneumoniae were observed as black-coloured after the incubation, and hence, it could be considered as a strong biofilm producer. Whereas, the colonies of S. aureus were observed as dark red or brown coloured due to the moderate biofilm production (Fig. 1).
Antibacterial activity analysis of ZnO NPs against selected bacteria
The antibacterial activity analysis of ZnO NPs was carried out against S. aureus and K. pneumoniae by using the agar well diffusion method. Here, the zone of inhibition observed was 18 mm against S. aureus and 16 mm against K. pneumoniae (Fig. S1).
Minimum inhibitory concentration of ZnO NPs against selected bacteria
The MIC of ZnO NPs against S. aureus and K. pneumoniae was determined by using the standard microtiter plate assay. Here, the MIC value of ZnO NPs against K. pneumoniae and S. aureus was found to be 0.78 mg/mL (Fig. 2).
Tissue culture plate-based analysis of biofilm production by the selected organisms
The biofilm produced by K. pneumoniae and S. aureus in the presence of sub-MIC concentrations of ZnO NPs was quantified by tissue culture plate assay by using crystal violet staining. Here, the organisms were incubated at five different SMCs (0.7, 0.6, 0.5, 0.4, and 0.3 mg/mL) of ZnO NPs. In the presence of all the selected SMCs of ZnO NPs, both the test organisms were found to have an enhancement in the biofilm production (Fig. 3). In the case of K. pneumoniae, maximum biofilm production was observed in the presence of 0.4 mg/mL (0.04%) of ZnO NPs, and for S. aureus, it was at 0.5 mg/mL (0.05%) of ZnO NPs.
Atomic force microscopy analysis of biofilm formed by selected bacteria
Atomic force microscopic analysis was carried out to determine the planar (2D) and cubic (3D) surface morphologies and topographies of the biofilm formed by K. pneumoniae and S. aureus when incubated with ZnO NPs at the SMC level. Significant differences were observed in the surface topographies of the biofilms formed by both test organisms when incubated with ZnO NPs (Fig. 4). AFM analysis also revealed the irregularity and surface roughness of the biofilms formed (Fig. 5). Here, the biofilms formed at SMCs of ZnO NPs showed increased thickness than the untreated bacterial samples. The surface roughness of the biofilms obtained from the AFM analysis was used to compare and analyse the effect of SMC levels of ZnO NPs on biofilm formation. For K. pneumoniae, the surface parameters showed a maximum value for the biofilm formed in the presence of 0.4 mg/mL (0.04%) of ZnO NPs, and for S. aureus, the same was observed at 0.5 mg/mL (0.05%) (Fig. 5).
Antibiotic susceptibility of biofilms treated with ZnO nanoparticles
In the case of K. pneumoniae, the untreated cells were found to have susceptibility against amoxycillin-clavulanate at 2 µg/mL, whereas treated cells showed susceptibility at a higher concentration of 4 µg/mL (Table S2). For the remaining antibiotics, no change in susceptibility was observed. Similarly, in the case of S. aureus, the treated cells showed susceptibility at higher concentrations against benzylpenicillin, vancomycin, and levofloxacin, which were respectively at 0.12, 1.00, and 0.25 µg/mL, whereas the untreated cells showed susceptibility at 0.06, 0.5, and 0.12 µg/mL of benzylpenicillin, vancomycin, and levofloxacin, respectively. In contrast, for daptomycin, the treated cells showed susceptibility at 1 µg/mL, while the same for untreated cells was 8 µg/mL, and no change in susceptibility was observed for the remaining antibiotics (Table S3).
Discussion
Biofilm plays a major role in the dissemination of infections, especially those related to implants or medical devices. Any implanted material is susceptible to colonisation by microorganisms from diverse sources (Yu et al. 2021). Bacteria present inside the biofilm are much more resistant to the antimicrobial agents than their planktonic forms (Martins et al. 2019). Nowadays, several antimicrobial agents including nanoparticles are used for the fabrication of medical devices for diverse applications. In most of the cases, the slow release of nanomaterials can happen from the nanoengineered devices. Even though antimicrobial effects are expected from these released materials, the outcome could be concentration dependent, because when released at SMCs, the nanomaterial-induced stress may favour the genetic selection of more resistant bacteria with enhanced ability to develop the biofilm (Bernardi et al. 2021). A detailed insight into the same has not been investigated, which makes the results of the current study to have a high significance. Even though there is evidence from the previous studies on the SMC levels of antibacterial drugs to have the ability to induce biofilm formation in certain bacterial species, the current study is important as the focus here is to identify the impact of SMC levels of ZnO NPs on the quantity of biofilm formed by K. pneumoniae and S. aureus. This has been carried out by the microtiter-plate biofilm assay and AFM analysis of the developed biofilms. Here, K. pneumoniae and S. aureus were selected as the test organisms due to the clinical and medical relevance of the biofilm formed by them. K. pneumoniae is one of the most commonly recovered clinical pathogens from nosocomial infections and has the ability to develop a thick layer of biofilm. At the same time, most of the S. aureus strains including the methicillin-resistant S. aureus (MRSA) have the ability to form biofilm which can even lead to patient mortality.
In the current study, both K. pneumoniae and S. aureus were screened for their ability to form the biofilm by using the CRA method. Here, K. pneumoniae was found to form black-coloured colonies which confirms its strong biofilm production potential. Also, S. aureus formed dark brown coloured colonies typical of moderate biofilm formation. In a recent study on S. aureus from the food samples, 69% of the isolates were reported to be moderate biofilm producers, 20% were strong producers, and 11% were non-biofilm producers (Ballah et al. 2022). At the same time, among the K. pneumoniae strains isolated from the clinical samples, 62.5% have been reported to be strong biofilm producers (Chu et al. 2020). Further, the antibacterial activity analysis of ZnO NPs was found to result in a 16 mm zone of inhibition for K. pneumoniae and 18 mm for S. aureus. The minimum concentration of the ZnO NPs required for inhibiting the growth of selected organisms was also estimated by using the microdilution method, and the MIC of ZnO NPs against both K. pneumoniae and S. aureus was found to be 1.78 mg/mL. Several studies have previously reported the antimicrobial properties of ZnO NPs to be mainly due to the production of reactive oxygen species which can result in increased oxidative stress and disruption of the bacterial cell membrane (Karami et al. 2020). The concentrations of the ZnO NPs at 0.07, 0.06, 0.05, 0.04, and 0.03% were further selected as the SMCs for biofilm formation assays. Here, the biofilm formed by K. pneumoniae and S. aureus in the presence of selected SMCs of ZnO NPs was compared with the biofilm of untreated organisms by the crystal violet-microtiter plate assay. In a previous study, the crystal violet assay method was reported to be effective in quantifying the biofilm formation by S. aureus when exposed to the SMC levels of antibiotics. From the results of the same study, biofilm formation was observed to be induced in the presence of SMC levels of acetylisovaleryltylosin tartrate (Yang et al. 2017). At the same time, biofilm production by Streptococcus mutans in the presence of enzyme ficin has also been investigated by the crystal violet assay method. Here, a dose-dependent suppression of biofilm formation of S. mutans was observed in the presence of ficin (Sun et al. 2021). In the current study, biofilm formation was quantified based on the absorbance at 595 nm for each of the treatments by using a microtiter plate reader. For both the selected organisms, all the selected SMC treatments showed enhanced absorbance when compared with the untreated control which indicated the biofilm-inducible nature of selected SMC levels of ZnO NPs. In K. pneumoniae, the maximum mean absorbance of 1.3393 was observed for the samples treated with 0.04% ZnO NPs, and the minimum mean absorbance of 0.594 was observed for 0.07%, whereas the untreated samples showed a mean absorbance of only 0.4443. In the case of S. aureus, the maximum mean absorbance of 0.438 was observed for the samples treated with 0.05% ZnO NPs, and the minimum mean absorbance of 0.2511 was observed for 0.07% ZnO NPs, whereas the untreated samples showed a mean absorbance of only 0.2176. The AFM analysis of the developed biofilm was also carried out using the confocal Raman microscope with AFM. Here, “Gwyddion 2.53” software was used to study and plot the graphical representation of the average roughness of biofilm formed at different treatments conducted in this study.
The AFM profile of the biofilm showed an enhancement in its surface roughness when cultured in the presence of SMCs of ZnO NPs in comparison with the untreated controls. K. pneumoniae was found to have a maximum surface roughness (183.17091 nm) at 0.04% concentration of ZnO NPs and the same for S. aureus (560.99291 nm) was observed at 0.05% of ZnO NPs. Here, the biofilm formation by bacteria might have enhanced due to the exposure to ZnO NPs at SMCs as a part of their stress management. Several other studies have previously reported the biofilm-inducible nature of antimicrobial agents at SMC levels. When Sara et al. studied the effects of subinhibitory concentrations of different antibiotics on the biofilm formation of various clinical isolates, they noticed an increase in the OD value. This could be due to the formation of a higher percentage of adhered cells, indicating an increase in the biofilm formation. The subinhibitory concentration of each of the antibiotics tested was reported to be able to increase the biofilm formation of clinically isolated Enterococcus faecalis (Bernardi et al. 2021).
In the current study, significant results were obtained while analysing the antibiotic susceptibility of biofilms formed by K. pneumoniae and S. aureus formed in response to SMC of ZnO NPs. The susceptibility of K. pneumoniae to amoxycillin-clavulanate was found to be influenced by treatment with the ZnO NPs, because the treated cells here exhibited antibiotic susceptibility at a higher concentration (4 µg/mL) when compared to that of untreated cells (2 µg/mL). Similarly, for S. aureus, the treated cells showed antibiotic susceptibility at a higher concentration of benzylpenicillin, vancomycin, and levofloxacin, respectively, at 0.12, 1.00, and 0.25 µg/mL, while untreated cells showed susceptibility at lower concentrations of 0.06, 0.5, and 0.12 µg/mL. In contrast, for daptomycin, the treated cells showed susceptibility at 1 µg/mL, while the untreated cells showed susceptibility at 8 µg/mL. These results suggest that the susceptibility of some antibiotics to K. pneumoniae and S. aureus biofilms could be affected due to the treatment with ZnO NPs. Also, these findings indicate the complex interactions between ZnO NPs and the biofilm-associated pathogens, resulting in unprecedented effects on their antibiotic susceptibility. However, further studies are needed to understand the underlying mechanisms. Possible mechanisms acted here could be the stress responses in bacteria induced as a result of changes in gene expression and cellular processes. The interaction of ZnO NPs with the bacterial membranes might have triggered the signalling cascades that promoted resistance development in response to the stress or damage caused by the nanoparticles. Interaction with ZnO NPs has been previously reported to induce efflux pump activity in bacteria, leading to the decreased accumulation of antibiotics (Fadwa et al. 2021). Also, the biofilm matrix made of thicker extracellular polymeric substances has been reported to serve as a barrier against the effects of antibiotics (Singh et al. 2016). Additionally, continuous exposure to the sub-MIC concentrations of nanoparticles could lead to the development of more resistance mechanisms in bacteria and thereby the evolution of antimicrobial resistance. This acquired resistance could potentially result in cross-resistance to other antibiotics through resistance-sharing mechanisms and genetic adaptations. Also, it may lead to selective pressure on bacterial populations, and thereby the evolution of resistant phenotypes.
Conclusion
Antimicrobial exposure at the sub-MIC concentrations is one of the stress factors that could influence the genotype and phenotype of resistant microorganisms. This might have increased the resistance of K. pneumoniae and S. aureus as evidenced by the enhanced biofilm formation observed in the study. So, the usage of ZnO NPs as an antimicrobial agent in an improper way could be a serious concern for the resistance development among pathogens. The biofilm analysis as conducted in the study showed the ability of ZnO NPs to enhance the biofilm formation at sub-MIC levels in K. pneumoniae and S. aureus. Conscious and correct use of antibacterial compounds, together with the functionalization and conditioning of the surfaces, will be necessary to decrease the risk of the evolution of resistant bacteria.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M (2011) Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 6:933–940. https://doi.org/10.2217/FMB.11.78
Ashitha A, Radhakrishnan EK, Mathew J (2020) Characterization of biosurfactant produced by the endophyte Burkholderia sp. WYAT7 and evaluation of its antibacterial and antibiofilm potentials. J Biotechnol 313:1–10. https://doi.org/10.1016/J.JBIOTEC.2020.03.005
Ballah FM, Islam MS, Rana ML (2022) Phenotypic and genotypic detection of biofilm-forming Staphylococcus aureus from different food sources in Bangladesh. Biology 11:949. https://doi.org/10.3390/BIOLOGY11070949
Bernardi S, Anderson A, Macchiarelli G (2021) Subinhibitory antibiotic concentrations enhance biofilm formation of clinical Enterococcus faecalis isolates. Antibiotics 10:874. https://doi.org/10.3390/ANTIBIOTICS10070874
Chu L, Zhou X, Shen Y, Yu Y (2020) Inhibitory effect of trisodium citrate on biofilms formed by Klebsiella pneumoniae. J Glob Antimicrob Resist 22:452–456. https://doi.org/10.1016/J.JGAR.2020.04.025
Fadwa AO, Alkoblan DK, Mateen A, Albarag AM (2021) Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J Biol Sci 28:928. https://doi.org/10.1016/J.SJBS.2020.09.064
Harika K, Shenoy V, Narasimhaswamy N, Chawla K (2020) Detection of biofilm production and its impact on antibiotic resistance profile of bacterial isolates from chronic wound infections. J Glob Infect Dis 12:129–134. https://doi.org/10.4103/JGID.JGID_150_19
Huang R, Zhang S, Zhang W, Yang X (2021) Progress of zinc oxide-based nanocomposites in the textile industry. IET Collaborative Intelligent Manufacturing 3:281–289. https://doi.org/10.1049/CIM2.12029
Ivanova A, Ivanova K, Perelshtein I et al (2021) Sonochemically engineered nano-enabled zinc oxide/amylase coatings prevent the occurrence of catheter-associated urinary tract infections. Mater Sci Eng C Mater Biol Appl. https://doi.org/10.1016/J.MSEC.2021.112518
Jayakumar A, Heera KV, Sumi TS et al (2019) Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int J Biol Macromol 136:395–403. https://doi.org/10.1016/J.IJBIOMAC.2019.06.018
Jin S-E, Eon Jin J, Hwang W et al (2019) Photocatalytic antibacterial application of zinc oxide nanoparticles and self-assembled networks under dual UV irradiation for enhanced disinfection. Int J Nanomedicine 14:1737–1751. https://doi.org/10.2147/IJN.S192277
Karami A, Xie Z, Zhang J et al (2020) Insights into the antimicrobial mechanism of Ag and I incorporated ZnO nanoparticle derivatives under visible light. Mater Sci Eng, C 107:110220. https://doi.org/10.1016/J.MSEC.2019.110220
Kelly S, Lanigan N, O’Neill I et al (2020) Bifidobacterial biofilm formation is a multifactorial adaptive phenomenon in response to bile exposure. Sci Rep. https://www.nature.com/articles/s41598-020-68179-9
Martins KB, Ferreira AM, Pereira VC et al (2019) In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus saprophyticus isolated from patients with urinary tract infections. Front Microbiol. https://doi.org/10.3389/FMICB.2019.00040
Mendes CR, Dilarri G, Forsan CF et al (2022) Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci Rep 12:1–10. https://doi.org/10.1038/s41598-022-06657-y
Nakasone I, Kinjo T, Yamane N et al (2007) Laboratory-based evaluation of the colorimetric VITEK-2 compact system for species identification and of the advanced expert system for detection of antimicrobial resistances: VITEK-2 compact system identification and antimicrobial susceptibility testing. Diagn Microbiol Infect Dis 58:191–198. https://doi.org/10.1016/J.DIAGMICROBIO.2006.12.008
Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M (2020) Surface functionalization of central venous catheter with mycofabricated silver nanoparticles and its antibiofilm activity on multidrug resistant Acinetobacter baumannii. Microb Pathog 138:103832. https://doi.org/10.1016/J.MICPATH.2019.103832
Omer HS, Aka ST (2022) Effects of subminimal inhibitory concentrations of chlorhexidine on the chlorhexidine resistance and biofilm formation in clinical drug-resistant Acinetobacter baumannii isolates. Polytechnic J 12:85–91. https://doi.org/10.25156/PTJ.V12N2Y2022.PP85-91
Panichikkal J, Jose A, Sreekumaran S et al (2022) Biofilm and biocontrol modulation of Paenibacillus sp. CCB36 by supplementation with zinc oxide nanoparticles and chitosan nanoparticles. Appl Biochem Biotechnol 194:1606–1620. https://doi.org/10.1007/S12010-021-03710-W
Pinto H, Simões M, Borges A (2021) Prevalence and impact of biofilms on bloodstream and urinary tract infections: a systematic review and meta-analysis. Antibiotics (Basel). https://doi.org/10.3390/ANTIBIOTICS10070825
Rambabu K, Bharath G, Banat F, Show PL (2021) Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. https://doi.org/10.1016/J.JHAZMAT.2020.123560
Saddik MS, Elsayed MMA, El-Mokhtar MA et al (2022) Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics. https://doi.org/10.3390/PHARMACEUTICS14010111
Singh R, Sahore S, Kaur P et al (2016) Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog Dis. https://doi.org/10.1093/FEMSPD/FTW056
Sun Y, Jiang W, Zhang M et al (2021) The inhibitory effects of ficin on Streptococcus mutans biofilm formation. Biomed Res Int. https://doi.org/10.1155/2021/6692328
Yang B, Lei Z, Zhao Y et al (2017) Combination susceptibility testing of common antimicrobials in vitro and the effects of sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front Microbiol 8:2125. https://doi.org/10.3389/FMICB.2017.02125/BIBTEX
Yu J, Jiang F, Zhang F et al (2021) Thermonucleases contribute to Staphylococcus aureus biofilm formation in implant-associated infections–a redundant and complementary story. Front Microbiol. https://doi.org/10.3389/FMICB.2021.687888/FULL
Yuan L, Dai H, He G et al (2023) Multi-omics reveals the increased biofilm formation of Salmonella Typhimurium M3 by the induction of tetracycline at sub-inhibitory concentrations. SSRN Electron J. https://doi.org/10.2139/SSRN.4363616
Zhao X, Tang H, Jiang X (2022) Deploying gold nanomaterials in combating multi-drug-resistant bacteria. ACS Nano. https://doi.org/10.1021/ACSNANO.2C02269
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
K., S., Nechikkadan, S., Theresa, M. et al. ZnO nanoparticles induced biofilm formation in Klebsiella pneumoniae and Staphylococcus aureus at sub-inhibitory concentrations. Folia Microbiol (2024). https://doi.org/10.1007/s12223-024-01158-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12223-024-01158-z