Abstract
Endophytic bacteria with multi-trait plant beneficial features have applications to enhance agricultural productivity by supporting the plant growth, yield, and disease resistance. In this study, Paenibacillus sp. CCB36 was isolated from the rhizome of Curcuma caesia Roxb., and its biofilm formation and antifungal properties have been evaluated in the presence of nanoparticles. Chitosan nanoparticles (CNPs) were synthesized and characterized by UV–visible spectrophotometry, Fourier transform infrared (FTIR) spectroscopy, high-resolution-transmission electron microscopic (HR-TEM) analysis, scanning electron microscopic (SEM) analysis, and dynamic light scattering (DLS). The effect of zinc oxide nanoparticles (ZnONPs) and CNPs on biofilm formation of Paenibacillus sp. CCB36 was evaluated by tissue culture plate assay. ZnONPs reduced its biofilm formation and was found to get modulated in the presence of CNPs as revealed by atomic force microscopy (AFM). Hence, CNPs were selected for further studies. Interestingly, biocontrol property of Paenibacillus sp. CCB36 against Rhizoctonia solani was also found to get enhanced when supplemented with chitosan nanoparticles. The results of the study indicate application of nanoparticles to improve colonization and active functioning of endophytic bacteria which can have significant application in agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has the potential to positively influence all the sectors including agriculture, food, and pharmaceutical industries and has also been utilized to reduce the harmful effects of chemical inputs on environment and human health [11, 21, 28]. In agriculture, the unique physicochemical properties of nanomaterials such as catalytic reactivity, high surface area, size, and shape make them to have promising applications [23]. Nanotechnology has been explored very much in modern agriculture to develop sustainable methods to enhance the crop yield and also to control plant diseases [21]. Nanoparticles can have promising role in the control of phytopathogens and pests due to its enhanced solubility, specificity, permeability, and stability [32]. Biofertilizers and biopesticides formulated with nanosized particles have already been described to enhance its performance in an environment friendly manner [4].
Plant growth-promoting microorganisms have been widely used to enhance plant productivity and disease management [5, 10, 26]. Hence, nanotechnological engineering of plant beneficial microorganisms can ultimately improve the yield and resistance of plants, and the approach is comparatively novel. The plant-associated bacteria directly promote the plant growth by nitrogen fixation and phosphate solubilization and also through the synthesis of phytohormones and siderophores [8]. Additionally, these bacteria have diverse mechanisms to protect the plant from pathogens [29]. Hence, improving these functioning of endophytes through nano-supplementation will have significant field applications.
Zinc oxide nanoparticles (ZnONPs) and chitosan nanoparticles (CNPs) have already been studied to promote the plant growth and disease resistance. Even though ZnONPs are known to improve the seed germination and yield, at higher concentrations, these could be detrimental [33]. But, zinc (Zn) has a vital role in carbohydrate and protein metabolism, and it regulates the synthesis of plant growth hormones like the indole-3-acetic acid (IAA). Zn is also an essential component of dehydrogenase, proteinase, and peptide enzymes and promotes starch formation, seed maturation, and production [24]. Chitosan has already been recognized to have the potential to control plant diseases by suppressing the growth, sporulation, and germination of pathogens and also by disrupting pathogens while inducing different defense responses in plant [14].
In the current study, endophytic Paenibacillus sp. CCB36 was isolated from the rhizome of Curcuma caesia Roxb. (Zingiberaceae), which has been previously demonstrated to have anti-oxidant [7], antimicrobial [19], and anti-ulcerogenic [25] activities and hence traditionally used as medicine [13]. The current study has been focused on the effect of ZnONPs and CNPs on the biofilm and biocontrol properties of isolated Paenibacillus sp. CCB36.
Materials and Methods
Isolation of Endophytic Bacteria from C. caesia Roxb.
The rhizomes of C. caesia Roxb. collected from Indian Council of Agricultural Research (ICAR)-Indian Institute of Spices Research, Kozhikode, Kerala, India, were used for the isolation of endophytic bacteria. The sterilization of surface of rhizome and the bacterial isolation were carried out as per the previously described methods [16]. For this, the rhizomes were washed with running tap water to remove the soil particles, and scales were also removed. These were further washed several times with distilled water. The samples were then dipped in 70% ethanol for 1 min and washed with sterile distilled water. The rhizome pieces were then treated with 2% sodium hypochlorite for 10 min followed by washed with sterile distilled water for eight times. For the isolation of endophytic bacteria, the processed rhizome was cut into pieces of 1 cm diameter and 1–2 cm length and placed on nutrient agar plates followed by incubation for 48 h at 28 ℃.
Screening of Antiphytopathogenic Property of Endophytic Isolates
Antifungal activity of all the isolated endophytes was done by dual culture method [27] against phytopathogenic fungi like Pythium myriotylum and Rhizoctonia solani. For this, the isolates were streaked on one side of the nutrient agar plates and incubated for 2 days at 28 ℃. Mycelial discs of phytopathogenic fungi were further inoculated on the other side of nutrient agar plates, and media with phytopathogens alone were served as the control. All the plates were then incubated at 28 ℃ for 3–5 days and were observed for fungal growth inhibition. Among these, the isolate that showed strong antiphytopathogenic activity was further evaluated for activity against Fusarium oxysporum, Sclerotium rolfsii, Phytophthora infestans, Rhizoctonia solani, and Colletotrichum acutatum.
Molecular Identification of Selected Isolate
Here, 16S rDNA sequence similarity based identification of the selected isolate CCB36 was carried out as it showed inhibition to all the selected phytopathogens. Genomic DNA isolated was used as the template for PCR amplification of 16S rDNA using the primers 16SF (5′-GAG TTT GAT CCT GGC TCA G-3′) and 16SR (5′-GAT ATT ACC GCG GCG CCT G-3′). Polymerase chain reaction (PCR) was carried out in a MycyclerTM (BIORAD, USA) with 50 μL reaction volume containing 5 μL of genomic DNA, 2 μL of both forward and reverse primers (10 pM), and 25 μL of mastermix (Takara), and it was made up to 50 μL with Milli-Q water. PCR reactions were cycled 35 times with initial denaturation at 94 ℃ for 3 min and the denaturation at 94 ℃ for 30 s, annealing at 58 ℃ for 30 s, and extension at 72 ℃ for 2 min were used cyclically with a final extension at 72 ℃ for 7 min. Then, the amplified product was confirmed by agarose gel electrophoresis [16]. The product was further sequenced at AgriGenome Labs Pvt. Ltd., Cochin, Kerala, India. The obtained sequence data was subjected to BLAST (Basic Local Alignment Search Tool) analysis.
Synthesis and Characterization of CNPs
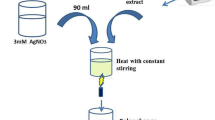
Chitosan from shrimp shells (Cat. No: PCT0817) with molecular weight 800–20,000 Daltons and degree of deacetylation (DDA) > = 75.0% was procured from the HiMedia Laboratories, Mumbai, India. For the synthesis of CNPs, 0.3 g chitosan was dissolved in 100 mL of 1% acetic acid solution and stirred at 1000 rpm for 25 min at 28 °C until the solution became clear. An opalescent suspension was further formed upon the drop by drop addition of 10 mL of 1% sodium tripolyphosphate (TPP) aqueous solution to the chitosan solution under stirring at 28 °C. Then, the CNPs were purified by centrifuging the suspension at 10,000 rpm for 30 min at 4 °C [6, 12].
The purified CNPs were characterized by using UV–visible spectrophotometry (SHIMADZU UV-2600 series), Fourier transform infrared (FTIR) (Schimadzu IR Prestige 21 FTIR) spectroscopy, High-resolution-transmission electron microscopy (HR-TEM) (JEOL, JEM-2100), Scanning electron microscopy (SEM) (JEOL JSM-7610 FPlus), and Dynamic light scattering (DLS). UV–visible spectrum of CNPs was recorded in the range of 200 to 800 nm, and FTIR spectrum was recorded in the range of 4500 to 700 cm−1. CNPs for the TEM analysis were prepared as a film on carbon-coated copper TEM grids and were allowed to stand for 2 min. The extra solution was removed by a blotting paper, and the grid was allowed to dry before analysis.
For the SEM analysis, mounting of dried CNPs was carried out on specimen stubs and coated with gold for 60 s using DII 29030SCTR smart coater [36]. The particle size of CNPs was analyzed by DLS (HORIBA SZ-100) at a sample holder temperature of 25 ℃.
Evaluation of Effect of ZnONPs and CNPs on Endophytic Isolate CCB36
ZnONPs (1.7 g/mL ± 0.1 g/mL density at 25 °C and ≤ 40 nm average particle size) used were purchased from Sigma-Aldrich, USA (Cat. No.: 721077), and the CNPs were synthesized as described above. The endophytic isolate CCB36 was inoculated into 150 mL of nutrient broth and was incubated at 28 °C for 24 h with constant shaking at 200 rpm (ELTEK OS-3). After this, CCB36 was swabbed on nutrient agar plates and 2, 4, and 6 mg of ZnONPs, and CNPs were added into the respective wells made on the swabbed nutrient agar plates. The plates were further incubated at 28 °C for 24 h [18].
Evaluation of Biofilm Formation of CCB36 in the Presence of ZnONPs and CNPs
The evaluation of biofilm formation was carried out by tissue culture plate assay. For this, overnight grown Luria Bertani (LB) broth (pH 7.5 ± 0.2) (Cat. No: M1245, HiMedia Laboratories, Mumbai, India) culture of isolate CCB36 was centrifuged at 5000 rpm for 5 min. The collected cells were washed with sterile phosphate-buffered saline (PBS), resuspended in LB broth, and made into the final optical density of 1 at 600 nm. Then, 200 µL bacterial suspension, bacterial suspension with 5 mg/mL CNPs, bacterial suspension with 20 µg/mL ZnONPs, and bacterial suspension with both 5 mg/mL CNPs and 20 µg/mL ZnONPs were added into the wells of microtiter plate (96 wells). LB broth, LB broth with 5 mg/mL CNPs, LB broth with 20 µg/mL ZnONPs, and LB broth with both 5 mg/mL CNPs and 20 µg/mL ZnONPs were used as control, and the experiment was repeated for three times. After the incubation at 28 °C for 18–20 h, the culture suspension was removed and the wells were washed with sterile PBS twice to remove the non-adhered cells. The plates were air dried and stained with 0.1% crystal violet for 30 min at 28 °C. Excess stain was removed, and wells were washed twice with PBS. The wells were air dried, and 200 µL ethanol was added to the stained wells and kept for 10 min. Then, absorbance was recorded at 570 nm using Varioskan Flash spectral scanning multimode reader (Thermo Scientific).
Statistical analysis
Data obtained from three replications of the experiment were statistically analyzed by using the software Origin Pro7 SRO (Northampton, MA, USA) with the Tukey’s post hoc multiple comparison tests to determine the significant difference in various groups at 5% level of significance [2].
Atomic Force Microscopy (AFM) for Biofilm Imaging
Here, overnight grown culture of CCB36 was centrifuged at 5000 rpm for 5 min at 4 °C. The collected cells were washed with sterile phosphate-buffered saline (PBS), and cells were resuspended in LB broth and diluted to the final optical density of 1 at 600 nm. After that, 200 µL bacterial suspension, bacterial suspension with 5 mg/mL CNPs, bacterial suspension with 20 µg/mL ZnONPs, and bacterial suspension with both 5 mg/mL CNPs and 20 µg/mL ZnONPs were added into the wells of a microtiter plate (96 wells) with control as described before. Sterile glass slide pieces (1 × 1 cm) were aseptically transferred to the bottom of each well, and after 18–20 h of incubation at 28 °C under static condition, the glass pieces were removed from wells with sterile forceps and gently washed with sterile PBS. The glass pieces were fixed with 2.5% glutaraldehyde for 1–2 h and dehydrated with 95% ethanol. Then, the fixed dried glass pieces were observed under Confocal Raman microscope with AFM (WITec Alpha300 RA, Germany) [15]. Here, the probe of the microscope connected to a cantilever is scanned over the surface of the glass piece, with a small repulsive force between the glass piece and the probe. The average roughness of biofilm under different treatments was measured by using the software “Gwyddion 2.53.”
Evaluation of Biofilm Forming Property of CCB36 in the Presence of Different Concentrations of CNPs
Here, CCB36 cell suspension was prepared as described before. Then, 200 µL of the bacterial suspension and bacterial suspension with 1, 2.5, 5, 7.5, and 10, mg/mL of CNPs were separately added into the wells of a microtiter plate (96 well). Also LB broth and LB broth with 1, 2.5, 5, 7.5, and 10 mg/mL of CNPs were used as control. After the incubation at 28 °C for 18–20 h, the culture suspension was removed from the microtiter plate and processed as described above for analyzing biofilm [38].
Evaluation of Biocontrol Property of CCB36 Against Rhizoctonia solani
Here, potato tuber pieces (2–3 cm in diameter) were surface sterilized by treating with 2% sodium hypochlorite solution for 2–5 min and washed thoroughly with sterile distilled water. Then, 30 mL of nutrient broth, overnight grown culture of CCB36, nutrient broth with CNPs (5 mg/mL), and CCB36 cultured with CNPs (5 mg/mL) were centrifuged at 5000 rpm for 5 min at 4 °C. The surface-sterilized potato pieces were further dipped in collected supernatant for 2–3 min and were then kept in sterilized Petri plates. Then, the pathogen Rhizoctonia solani was inoculated on each of the potato pieces and incubated for 3–5 days at 28 °C [17].
Microscopic Analysis of Potato Tubers Using Lactophenol Cotton Blue (LPCB) Staining
A drop of lactophenol cotton blue stain was placed on the center of a clean slide, and tissues of potato pieces treated with R. solani as per described above were placed in the drop of stain and teased gently. A coverslip was kept over the stain, and the slides were observed under the microscope with 100 × (oil immersion) magnification (Olympus-CX43) [34].
Results
Plants provide vast and diverse niche for the endophytic microorganisms which could promote plant growth and disease resistance. The plant-associated microbial communities perform many vital functions for the optimal adaptation of the plant to the habitat.
Isolation of Endophytes from C. caesia Roxb.
In the study, rhizome of Curcuma caesia Roxb. was used for the endophytic bacterial isolation, and it resulted in the purification of 36 different bacterial isolates named as CCB1 to CCB36.
Antiphytopathogenic Property of Endophytic Isolates
All the bacterial isolates were screened for antiphytopathogenic property against P. myriotylum and R. solani (Table S1). Among these, CCB36 was found to have remarkable inhibitory effect against both the pathogens. Upon further analysis, CCB36 was also found to inhibit F. oxysporum, S. rolfsii, P. infestans, and C. acutatum (Fig. 1). Hence, this was selected for further studies.
Molecular Identification of Endophytic Isolate CCB36
The PCR amplification product of 16S rDNA of CCB36 was found to have 1500 bp size which was further purified and sequenced (Fig. S1). Sequence similarity search by BLAST analysis showed the 16S rDNA of CCB36 to have 100% identity with Paenibacillus polymyxa.
Synthesis and Characterization of CNPs
The clear chitosan solution was changed to an opalescent suspension upon the addition of aqueous solution of sodium tripolyphosphate (TPP), which indicated the formation of chitosan nanoparticles. This was further characterized by UV–visible spectroscopy, FTIR, TEM, SEM, and DLS analysis. The UV–visible spectrum of CNPs exhibited corresponding peak at 250 nm. Functional groups of CNPs were studied by FTIR analysis. The FTIR spectrum of the CNPs showed major absorption peaks at 3453.69, 3356.28, 3197.15, 3181.72, 1662.71, 1538.3, and 1329.98 cm−1, respectively. The spectral values of the CNPs between 3200 and 3550 cm−1 could be due to the -O–H bonding. The surface morphological structure was further examined by SEM and TEM analysis, which clearly showed spherical shaped chitosan nanoparticles. The particle size distribution of CNPs was measured by DLS, which showed the CNPs with average particle size of 105.7 nm (Fig. 2).
Effect of ZnONPs and CNPs on Endophytic CCB36
Three concentrations (2, 4, and 6 mg) of ZnONPs and CNPs used were not found to have inhibitory effect on CCB36. Hence, these nanoparticles were used for further studies related to biofilm formation and biocontrol activity of CCB36.
Biofilm Formation of CCB36 in the Presence of Supplemented ZnONPs and CNPs
Microtiter plate assay was used to quantify the biofilm formed by the selected endophytic isolate. Here, CCB36 exhibited variation in biofilm forming property in the presence of supplemented ZnONPs and CNPs. Significant increase in the formation of biofilm was observed for CCB36 when supplemented with 5 mg/mL chitosan nanoparticles when compared with ZnONPs (20 µg/mL) treatment and control (Fig. 3). In the presence of both ZnONPs and CNPs supplementation, CCB36 was found to form biofilm which was statistically comparable to unsupplemented and ZnONPs supplemented treatment.
Biofilm formation by Paenibacillus sp. CCB36 in the presence of chitosan nanoparticles and ZnO nanoparticles (LB + P, Luria Bertani broth with Paenibacillus sp. CCB36; LB + CNPs + P, Luria Bertani broth with chitosan nanoparticles (5 mg/mL) and Paenibacillus sp. CCB36; LB + ZnONPs + P, Luria Bertani broth with zinc oxide nanoparticles (20 µg/mL) and Paenibacillus sp. CCB36; LB + CNPs + ZnONPs + P, Luria Bertani broth with chitosan nanoparticles (5 mg/mL), zinc oxide nanoparticles (20 µg/mL), and Paenibacillus sp. CCB36)
Atomic Force Microscopy (AFM) Analysis on Biofilm Changes of CCB36 due to Nanoparticle Supplementation
AFM analysis showed the morphological features and 3D topography of biofilm formed by CCB36 in the presence of supplemented ZnONPs and CNPs (Fig. 4). Here, increased thickness of biofilm of CCB36 could be observed by AFM in the presence of 5 mg/mL CNPs supplementation when compared with other treatments. However, the biofilm forming property of CCB36 was decreased when 20 µg/mL of ZnONPs was added. This observation could also be reflected in the evaluation of its roughness value (Fig. 5). The roughness value of the CCB36 surface treated with CNPs showed the highest value among all the treatments used. This indicated enhanced biofilm formation by CCB36 in the presence of CNPs and was supportive to the result obtained in the microtiter plate assay.
AFM analysis of biofilm formed by Paenibacillus sp. CCB36 in the presence of zinc oxide nanoparticles and chitosan nanoparticles: a LB control, b LB with chitosan nanoparticles control, c LB with ZnO nanoparticles control, d LB with chitosan nanoparticles and ZnO nanoparticles control, e Paenibacillus sp. CCB36 inoculated LB broth, f Paenibacillus sp. CCB36 inoculated LB broth with chitosan nanoparticles, g Paenibacillus sp. CCB36 inoculated LB broth with ZnO nanoparticles, h Paenibacillus sp. CCB36 inoculated LB broth with chitosan nanoparticles and ZnO nanoparticles (LB- Luria Bertani broth and ZnO- zinc oxide)
Surface profile of Paenibacillus sp. CCB36 biofilm formed under different treatments exhibiting variation in roughness (Rq) (LB, Luria Bertani broth; LB + CNPs, Luria Bertani broth with chitosan nanoparticles; LB + ZnONPs, Luria Bertani broth with zinc oxide nanoparticles; LB + CNPs + ZnONPs, Luria Bertani broth with zinc oxide nanoparticles and chitosan nanoparticles; LB + P, Luria Bertani broth with Paenibacillus sp. CCB36; LB + CNPs + P, Luria Bertani broth with chitosan nanoparticles and Paenibacillus sp. CCB36; LB + ZnONPs + P, Luria Bertani broth with zinc oxide nanoparticles and Paenibacillus sp. CCB36; LB + CNPs + ZnONPs + P, Luria Bertani broth with zinc oxide nanoparticles, chitosan nanoparticles, and Paenibacillus sp. CCB36)
Biofilm Formation of CCB36 in the Presence of CNPs
The CCB36 was observed to show difference in the thickness of biofilm produced when supplemented with different concentrations of chitosan nanoparticles. Specifically, increased biofilm was found to be formed by CCB36 with the supplementation of 2.5 mg/mL chitosan nanoparticles (Fig. 6).
Biofilm formation of Paenibacillus sp. CCB36 in the presence of different concentrations of supplemented chitosan nanoparticles (P, Paenibacillus sp. CCB36; P + 1C, Paenibacillus sp. CCB36 with 1 mg/mL chitosan nanoparticles; P + 2.5C, Paenibacillus sp. CCB36 with 2.5 mg/mL chitosan nanoparticles; P + 5C, Paenibacillus sp. CCB36 with 5 mg/mL chitosan nanoparticles; P + 7.5C, Paenibacillus sp. CCB36 with 7.5 mg/mL chitosan nanoparticles; P + 10C, Paenibacillus sp. CCB36sp. CCB36with 10 mg/mL chitosan nanoparticles)
Biocontrol Property of CCB36 Supplemented with CNPs Against R. solani on Potato
Surface-sterilized potato tuber pieces were used for this study. An enhanced growth of mycelia of R. solani was observed on the potato pieces which were treated with distilled water and sterile nutrient broth. Treatment with CCB36 was observed to provide protection to potato when compared with the control. Significantly reduced growth of R. solani was observed for the potato pieces treated with CCB36 cultured in the presence of CNPs. This revealed the enhanced antiphytopathogenic effect of CCB36 due to supplementation of CNPs (Fig. 7).
Biocontrol activity of Paenibacillus sp. CCB36 supplemented with chitosan nanoparticles against Rhizoctonia solani infection on potato: A distilled water, B nutrient broth control, C chitosan nanoparticles treated, D Paenibacillus sp. CCB36 treated, E Paenibacillus sp. CCB36 cultured with supplemented chitosan nanoparticles treated
Microscopic Analysis of Potato
Microscopic evaluation of antiphytopathogenic activity of CCB36 against R. solani on potato was also conducted. Samples from potato pieces treated with distilled water, nutrient broth, and CNPs exhibited the presence of R. solani hyphae abundantly near the starch grains of potato. The treatment with suspension of CCB36 slightly reduced the number of R. solani hyphae. The samples from potato pieces treated with suspension of CCB36 cultured with CNPs showed minimum hyphae of R. solani which confirmed the enhanced biocontrol effect of CCB36 when cultured with CNPs supplementation (Fig. 8).
Microscopic images showing biocontrol effect of Paenibacillus sp. CCB36 against Rhizoctonia solani infection on potato: a Rhizoctonia solani, b potato treated with distilled water, c potato treated with nutrient broth, d potato treated with chitosan nanoparticles, e potato treated with Paenibacillus sp. CCB36, and f potato treated with Paenibacillus sp. CCB36 cultures with supplemented chitosan nanoparticles (red arrows indicate the presence of Rhizoctonia solani hyphae)
Discussion
Plants have been documented as meta-organisms, holding a diverse microbiome with mutual symbiotic relationship. Each plant is associated with various species of microbial strains and majority of them perform significant ecological functions beneficial to the plant [3]. These groups of organisms encourage the plant growth and also provide resistance to both biotic and abiotic stress conditions [20]. The beneficial microbiome of medicinal plants are also known to secrete distinctive bioactive secondary compounds having broad range of applications [9].
In the study, rhizome of Curcuma caesia Roxb. was selected for the isolation of endophytic bacteria as it was traditionally used as a medicine due to rich content of bioactive molecules. Hence, its associated microbiome might also expect to have remarkable application. Among all the endophytic bacteria isolated, CCB36 identified as Paenibacillus sp. exhibited strong antifungal activity against phytopathogens. Paenibacillus spp. are often considered antimicrobial factories due to its ability to synthesize biologically active molecules which are inhibitory to phytopathogens. Various strains of P. polymyxa have formerly been found to antagonize Phytophthora sp., Rhizoctonia solani, Pseudomonas syringae, and Xanthomonas campestris. The chemical basis of the same has also been considered as antimicrobial compounds such as polymyxin, fusaricidin A, gavaserin, and saltavalin. These metabolites can mechanistically cause the cell lysis and leakage of ions, disruption of the structural integrity of membranes, inhibition of mycelial growth, inhibition of spore germination, and protein synthesis [37, 39].
The endophytic CCB36 with antiphytopathogenic property was used as the candidate organism in the study to demonstrate its colonization through biofilm and enhanced antagonism with the supplementation of CNPs and ZnONPs. Even though colonization on plant tissue is the primary requirement for the functional establishment of endophytes, most of them cannot colonize and establish biofilm formation on non-host plant tissues. In the screening for biofilm formation, the CCB36 was found to form increased biofilm in the presence of chitosan nanoparticles when compared to the control. However, ZnONPs suppressed the formation of biofilm. The optimum biofilm was formed in the presence of 2.5 mg/mL CNPs and then declined when concentration was increased. Previous study has already reported the coupling of chitosan with plant growth-promoting rhizobacteria to have enhancement effect on its plant beneficial traits [1]. But the augmented biofilm forming property of plant beneficial microorganism due to coupling with chitosan nanoparticles has not yet been studied in detail. This indicates the novelty of the study and can have significant applications in delivery and field performance of plant beneficial microorganisms.
Atomic force microscopy (AFM) was used to visualize the biofilm forming property of CCB36 on a non-living surface. AFM has been used in the study for the direct nanoscale observation on the surface morphology of CCB36 supplemented with nanosized particles and bacterial biofilms formed. More aggregated bacterial cells were observed in the AFM topography of CCB36 treated with chitosan nanoparticles. The average roughness value of surface of the same treatment was higher than other treatments and control. In the presence of chitosan nanoparticles, the bacteria exhibited intense adhesion and aggregation. This suggests that it may be utilized to boost the colonization of selected bacteria on plants.
The biocontrol property of CCB36 was also evaluated in the presence of chitosan nanoparticles on potato against R. solani. Potato pieces treated with the cell-free supernatant of CNP-treated CCB36 were found to have enhanced protection from the infection caused by R. solani when compared with CCB36 alone treatment. Many previous studies have already demonstrated the antimicrobial applications of chitosan in agriculture, food, and pharmaceutical industries [22, 30, 31, 35, 40]. As the CNP-treated tubers were not protected from the infection by R. solani, the chitosan nanoparticles alone used in the present study could not provide any direct antagonism to R. solani. But, these CNPs functioned effectively to boost up the biocontrol property of CCB36. Here, the used CNPs might have induced the CCB36 as a pathogen, because chitin is a major component of cell wall of most of the fungal phytopathogens.
The finding was further substantiated by a microscopic analysis of samples collected from potato pieces treated with CCB36 supernatant where CCB36 was cultured in the presence of CNPs. This demonstrated the suppression of mycelia growth on potato samples. This might be due to the activity of induced metabolite production by CCB36 in response to the supplemented chitosan nanoparticles. Paenibacillus spp. have already been reported to offer protection against diseases affecting a wide variety of crops [29]. Hence, the results obtained in the current study can have application to improve the functioning of other Paenibacillus spp. also. The isolate CCB36 with CNPs supplementation can have significant applications in the agricultural field.
Conclusions
Endophytic bacteria ubiquitously colonize the internal tissues of plants and provide resistance from drought, heavy metals, salt, and phytopathogens and also promote the host growth through various mechanisms. In the present study, Paenibacillus sp. CCB36 with potential antifungal activity was isolated and found to have an increased ability to form biofilm in the presence of chitosan nanoparticles. Biofilm formation of plant beneficial bacteria is a crucial primary phase for the colonization on plant tissues. In addition to the biofilm formation, CNPs enhanced the biocontrol property of CCB36 which provided better protection to the potato pieces against Rhizoctonia solani. Microscopic analysis confirmed the presence of abundant fungal mycelia in potato pieces treated with control and enhanced suppression of pathogens on potato treated with CCB36 cultivated with supplemented CNPs. These results indicate the modulating effect of chitosan nanoparticles on plant beneficial traits of CCB36 such as biofilm formation and antagonism against R. solani. Hence, the coupling of CCB36 with chitosan nanoparticles will be an effective eco-friendly means to control crop plant disease in the agricultural field.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Agbodjato, N., Noumavo, P. A., Adjanohoun, A., Agbessi, L., & Baba-Moussa, L. (2016). Biotechnology Research International, 2016, 11.
Ansari, F. A., & Ahmad, I. (2019). Fluorescent Pseudomonas -FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes. Scientific Reports, 9.
Berg, G., Mahnert, A., & Moissl-Eichinger, C. (2014). Frontiers in Microbiology, 5, 15.
Bhattacharyya, A., Duraisamy, P., Govindarajan, M., Buhroo, A. A., & Prasad, R. (2016). Nano-biofungicides: Emerging trend in insect pest control. 307–319.
Compant, S., Duffy, B., Nowak, J., Clement, C., & Barka, E. A. (2005). Applied and Environment Microbiology, 71, 4951–4959.
Deng, Q.-Y., Zhou, C.-R., & Luo, B.-H. (2008). Pharmaceutical Biology, 44, 336–342.
Devi, H. P., Mazumder, P. B., & Devi, L. P. (2015). Toxicology Reports, 2, 423–428.
Egamberdieva, D., Wirth, S. J., Alqarawi, A. A., Abd_Allah, E. F., & Hashem, A. (2017). Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Frontiers in Microbiology, 8.
Ek-Ramos, M. J., Gomez-Flores, R., Orozco-Flores, A. A., Rodríguez-Padilla, C., González-Ochoa, G., & Tamez-Guerra, P. (2019). Bioactive products from plant-endophytic gram-positive bacteria. Frontiers in Microbiology, 10.
Fadiji, A. E., & Babalola, O. O. (2020). Frontiers in Bioengineering and Biotechnology, 8, 467.
Fraceto, L. F., Grillo, R., de Medeiros, G. A., Scognamiglio, V., Rea, G., & Bartolucci, C. (2016). Nanotechnology in agriculture: Which innovation potential does it have? Frontiers in Environmental Science, 4.
Ghadi, A., Mahjoub, S., Tabandeh, F., & Talebnia, F. (2014). Caspian Journal of Internal Medicine, 5, 156–161.
Grover, M., Shah, K., Khullar, G., Gupta, J., & Behl, T. (2019). Journal of Pharmacy and Pharmacology, 71, 725–732.
Hassan, O., & Chang, T. (2017). Asian Journal of Plant Pathology, 11, 53–70.
James, S. A., Powell, L. C., & Wright, C. J. (2016). In D. Dhanasekaran & N. Thajuddin (Eds.), Microbial biofilms - importance and applications. IntechOpen.
Jasim, B., Joseph, A. A., John, C. J., Mathew, J., & Radhakrishnan, E. K. (2013). 3 Biotech, 4, 197–204.
Jimtha, J. C., Jishma, P., Arathy, G. B., Anisha, C., & Radhakrishnan, E. K. (2016). Journal of Soil Science and Plant Nutrition, 16, 578–590.
Kaur, R., Kaur, B., Suttee, A., & Kalsi, V. (2018). Asian Journal of Pharmaceutical and Clinical Research, 11, 94.
Khare, E., Mishra, J., & Arora, N. K. (2018). Multifaceted interactions between endophytes and plant: Developments and prospects. Frontiers in Microbiology, 9.
Lahiri, D., Nag, M., Sheikh, H. I., Sarkar, T., Edinur, H. A., Pati, S., & Ray, R. R. (2021). Frontiers in Microbiology, 12, 636588.
Lee, D.-S., & Je, J.-Y. (2013). Journal of Agricultural and Food Chemistry, 61, 6574–6579.
Marchiol, L. (2018). New visions in plant science. IntechOpen.
Mazaherinia, S., Astaraei, A. R., Fotovat, A., & Monshi, A. (2010). Nano iron oxide particles efficiency on Fe, Mn, Zn and Cu concentrations in wheat plant. World Applied Sciences Journal.
Mazumder, P., & Devi, H. (2016). Pharmacognosy Research, 8, 43.
Mburu, S. W., Koskey, G., Njeru, E. M., & Maingi, J. M. (2021). AIMS Agriculture and Food, 6, 496–524.
Mohamad, O. A. A., Li, L., Ma, J.-B., Hatab, S., Xu, L., Guo, J.-W., Rasulov, B. A., Liu, Y.-H., Hedlund, B. P,. & Li, W.-J. (2018). Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Frontiers in Microbiology, 9.
Nag, M., Lahiri, D., Sarkar, T., Ghosh, S., Dey, A., Edinur, H. A., Pati, S., & Ray, R. R. (2021). Frontiers in Chemistry, 9, 690590.
Padda, K. P., Puri, A., & Chanway, C. P. (2017). Paenibacillus polymyxa: A prominent biofertilizer and biocontrol agent for sustainable agriculture, 165–191.
Pati, S., Chatterji, A., Dash, B. P., Raveen Nelson, B., Sarkar, T., Shahimi, S., AtanEdinur, H., BintiAbd Manan, T. S., Jena, P., Mohanta, Y. K., & Acharya, D. (2020). Polymers, 12, 2361.
Pati, S., Sarkar, T., Sheikh, H. I., Bharadwaj, K. K., Mohapatra, P. K., Chatterji, A., Dash, B. P., Edinur, H. A., & Nelson, B. R. (2021). Frontiers in Marine Science, 8, 1–12.
Prasad, R., Bhattacharyya, A., & Nguyen, Q. D. (2017). Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Frontiers in Microbiology, 8.
Prasad, T. N. V. K. V., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V., Reddy, K. R., Sreeprasad, T. S., Sajanlal, P. R., & Pradeep, T. (2012). Journal of Plant Nutrition, 35, 905–927.
Raja, S., Subhashini, P., & Thangaradjou, T. (2016). Mycology, 7, 112–123.
Ray, S. D. (2011). Acta Poloniae Pharmaceutica, 68, 619–622.
Roshmi, T., Anju, J., Rintu, T. V., Soniya, E. V., Jyothis, M., & Radhakrishnan, E. K. (2014). Brazilian Journal of Microbiology, 45, 1221–1227.
Ryu, C.-M., Kim, J., Choi, O., Kim, S. H., & Park, C. S. (2006). Biological Control, 39, 282–289.
Walker, D. I., Keevil, C. W., & Hatti Kaul, R. (2015). Low-concentration diffusible molecules affect the formation of biofilms by mixed marine communities. Cogent Biology, 1.
Weselowski, B., Nathoo, N., Eastman, A. W., MacDonald, J., & Yuan, Z.-C. (2016). Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiology, 16.
Xing, K., Zhu, X., Peng, X., & Qin, S. (2014). Agronomy for Sustainable Development, 35, 569–588.
Zhang, F., Li, X.-L., Zhu, S.-J., Ojaghian, M. R., & Zhang, J.-Z. (2018). Biological Control, 127, 70–77.
Acknowledgements
The authors acknowledge the Kerala State Council for Science, Technology and Environment (KSCSTE) under the KSCSTE-SRS, KSCSTE-KBC-YIPB, and also JAIVAM Project. The authors also acknowledge the Department of Science and Technology (DST) under DST PURSEII and Kerala State Plan Fund Project for the instrumentation facility. The authors also acknowledge the Director, Sophisticated Analytical Instrument Facility (DST-SAIF), School of Environmental Sciences, Mahatma Gandhi University, Kottayam, India, for providing atomic force microscope (AFM) facility and also acknowledge the Director, International and Interuniversity Centre for Nanoscience and Nanotechnology for the help and support for transmission electron microscopic (TEM) analysis and dynamic light scattering (DLS) facility and the Director, Center for Nanosciences, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Kochi, Kerala, India, for the scanning electron microscopic (SEM) analysis facility.
Author information
Authors and Affiliations
Contributions
Radhakrishnan Edayileveetil Krishnankutty contributed to the study conception and design. Experiments and analysis were performed by Jishma Panichikkal, Sreejith Sreekumaran, Ashitha Jose, Anju Kanjirakandi Ashokan, and Cimmiya Susan Baby. The first draft of the manuscript was written by Jishma Panichikkal. The manuscript was revised, and corrections were included by Radhakrishnan Edayileveetil Krishnankutty, Jishma Panichikkal, and Ashitha Jose. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panichikkal, J., Jose, A., Sreekumaran, S. et al. Biofilm and Biocontrol Modulation of Paenibacillus sp. CCB36 by Supplementation with Zinc Oxide Nanoparticles and Chitosan Nanoparticles. Appl Biochem Biotechnol 194, 1606–1620 (2022). https://doi.org/10.1007/s12010-021-03710-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03710-w