Abstract
Diethyl phthalate (DEP) is one of the extensively used plasticizers which has been considered a priority hazardous pollutant due to its carcinogenic, endocrine disrupter, and multi-toxic effects on humans. The identification of DEP in different parts of the ecosphere has increased the global community’s attention to the elimination of this pollutant in a bio-eco-friendly way. In this research, a novel aerobic bacterial strain nominates as ShA (GenBank accession number: MN298858) capable of consuming DEP as carbon and energy sources, was isolated from the upper phase (0–10 cm) of Anzali international wetland sediments by enrichment culture method. Morphological characteristics and 16S rRNA gene sequence analysis demonstrated that strain ShA belonged to Pseudomonas putida. The substrate utilization test demonstrated that strain ShA was able to grow in mineral salt medium containing dimethyl phthalate (DMP) and phthalic acid (PA) isomers including terephthalic and isophthalic acid. Degradation assay showed strain ShA completely degraded 200 mg/L DEP within 22 h (pH 7.0, 30 °C). Surprisingly, PA as the main intermediate of DEP biodegradation was identified by GC-FID. Moreover, the rapid degradation of 2000 mg/L PA to CO2 and H2O was viewed in 22 h by strain ShA. The possible route of DEP degradation was DEP directly to PA and then PA consumption for growth. This study obtained results that provide a great contribution to applying strain ShA in the biodegradation of low molecular weight of PAEs and PA isomers in natural ecosystems. This is the first report of a P. putida strain able to degrade DEP and PA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates or phthalic acid esters (PAEs) are human-made and endocrine disrupter (ECD) pollutants that are broadly produced to make different plastics more flexible and soft (Ahuactzin-Pérez et al. 2018; Carstens et al. 2020; Mahto et al. 2022a; Shariati et al. 2022b). Due to the faint chemical bond between phthalates and consumer products, these pollutants arrive in various parts of the ecosystem (Kim et al. 2020; Lee et al. 2019) continually through the processes of leaching, adsorption, volatilization, diffusion, and deposition (Gao and Wen 2016; Mahto et al. 2021). Diethyl phthalate (IUPAC name: diethyl benzene-1,2-dicarboxylate) (DEP) is one of the prevailing plasticizers in the world that is often applied in personal care products such as cosmetics and fragrances (Guo and Kannan 2013; Boll et al. 2020). Humans are directly exposed to DEP through inhalation, derm, and ingestion (Zhang et al. 2013; Weschler et al. 2015; Tran and Kannan 2015; Andersen et al. 2018), and an indirect way DEP can be absorbed by fishes, plants, and other living organisms, therewith entering the human food chain decreased the food security (Sun et al. 2015; Hu et al. 2016; Chen et al. 2017; Tang et al. 2020; Song et al. 2020). DEP and its intermediates have multi-toxic, mutagenic, and carcinogenic effects on living organisms and the human body (Demirtaş et al. 2020; Seyoum and Pradhan 2019; Tsai et al. 2021; Sicińska et al. 2021) and can cause a variety of diseases such as cancer (Weaver et al. 2020), asthma and allergies (Braun et al. 2013), overweight (Hatch et al. 2008; Buser et al. 2014), and reproductive health (Upson et al. 2013; Zhang et al. 2021), etc. DEP metabolites (monoethyl phthalate, MEP) have been detected in diverse body fluids, including urine (Schwedler et al. 2017; Kolena et al. 2017), serum (Wang et al. 2019), semen (Chang et al. 2017), breast milk (Kim et al. 2015; Fan et al. 2019), and saliva (Silva et al. 2005). Hence, DEP is listed as one of the priorities of hazardous contaminants in the ecosphere by the US Environmental Protection Agency (USEPA), European Union (EU), and the China National Environmental Monitoring Center (Huang et al. 2021). Several studies showed that DEP is a stable and persistent pollutant in sediment and can accumulate in the aquatic ecosystem, on the other hand, the degradation of this compound under natural processes in sediments is slow (Net et al. 2015; Selvaraj et al. 2015; Gao and Wen 2016; Tao et al. 2019). Due to the undeniable performance of bacteria in the biodegradation of PAEs (Boll et al. 2020; Shariati et al. 2021, 2022a), the identification of new native bacterial species with the ability to break down DEP in an eco-friendly way can be a great help in removing these pollutants from the aquatic environment. The biodegradation of PAEs takes place in two primary and ultimate stages. In the primary stage, DEP biodegradation is usually performed under the process of de-esterification. During the de-esterification, DEP is hydrolyzed to monoethyl phthalates (IUPAC name: 2-(ethoxycarbonyl) benzoic acid) (MEP) and then to phthalic acid (PA; ortho-positioned) by esterase enzymes. Also, DEP is transformed to PA through the trans-esterification pathway by substituting an ethyl group with a methyl group in each step and producing MEP. In some exceptions, DEP can be hydrolyzed directly to PA without any intermediate. In the ultimate stage, PA is degraded to CO2 and H2O by phthalate dioxygenase enzymes (Fig. 1) (Tao et al. 2019; Hu et al. 2021; Mahto et al. 2022b). Some DEP-degrading bacteria from the genera Rhodococcus sp. (Lu et al. 2009), Camelimonas (Chen et al. 2015), Pseudomonas (Kumar et al. 2017; Tao et al. 2019), and Gordonia (Li et al. 2017) have been isolated from a diverse ecosystem. In a study, it was reported that Rhodococcus sp. strain L4 isolated from activated sludge could degrade 100 mg/L DEP after 6 d (Lu et al. 2009). The results of a study showed that Pseudomonas sp. strain DNE-S1 isolated from landfill soil had the ability to consume DEP. They have proven the trans-esterification way as the main route of DEP biodegradation by strain DNE-S1 (Tao et al. 2019). Singh et al. (2017) stated that three strains of Pseudomonas sp. PKDM2, PKDE1, and PKDE2 were able to degrade 75% of 500 mg/L DEP after 44 h. Anzali international wetland adjacent to the Caspian Sea is one of the most significant ecosystems in the world that was registered in the Ramsar convention 1979. This wetland is of great importance in environmental, economic, and tourism aspects. The catchment area of this wetland is 3610 km2 and 30 rivers plus industrial, agricultural, hospital and domestic wastes flow into this wetland without any pretreatment (Jamshidi and Bastami 2016; Shariati et al. 2019b). Results of recent projects showed that this international wetland is heavily polluted by phthalate. Researchers stated that the concentration of phthalate in the Anzali international wetland is 20–40 times more than the environmental risk limit. In addition to the risk of contamination of this pollutant on the health of plants, animals, and wetland microorganisms and interference in biochemical and environmental processes, the pollution could be easily transferred to human bodies as residents in this region widely consume caught fish and birds of this wetland (Hassanzadeh et al. 2014; Li et al. 2017; Shariati et al. 2019a). Given the important role of this wetland in ensuring the food security of indigenous people, finding ways to reduce phthalate pollution is very important. In the present research, in an attempt to find a bacterial strain with the capability to degrade DEP, for the first time, a novel native strain ShA with the ability to utilize the low molecular weight of PAEs and PA isomers was isolated from Anzali international wetland. Our research also aimed to provide the basic requirements for refining and bioremediation of DEP or other organic pollutants in natural ecosystems under aerobic conditions.
Material and methods
Chemicals
Di-isodecyl phthalate (IUPAC name: bis (8-methylnonyl) benzene-1,2-dicarboxylate) (DIDP), di-(2- ethyl hexyl) phthalate (IUPAC name: bis (2-ethylhexyl) benzene-1,2-dicarboxylate) (DEHP), dimethyl phthalate (IUPAC name: Dimethyl benzene-1,2-dicarboxylate) (DMP), DEP, di-n-butyl phthalate (IUPAC name: dibutyl benzene-1,2-dicarboxylate), Mono (2-ethylhexyl) phthalate (IUPAC name: 2-(2-ethylhexoxycarbonyl) benzoate) (MEHP), mono-butyl-phthalate (IUPAC name: 2-(butoxycarbonyl)benzoic acid) (MBP), 2-ethyl hexanol (IUPAC name: 2-ethylhexan-1-ol), phthalic acid, terephthalic acid (TPA; para-positioned), and isophthalic acid (IPA; meta-positioned) were bought from Sigma Aldrich Chemie GmBH (Hamburg, Germany) with purity > 95%. Ethyl acetate (HPLC grade) as a solvent was provided by Carl Roth (Karlsruhe, Germany).
Sampling site
Anzali international wetland (latitude = 363,201, longitude = 4,144,076) is located in the north of Iran, Anzali City, adjacent to the Caspian Sea in an area of 193 km2 with a geographic coordinate of 37°25′ to 37°30′N, 49°15′ to 49°30′E. The mean annual rainfall is 1280 mm and the average annual evapotranspiration is 980 mm. Anzali Wetland is one of the first internationally registered wetlands of Iran in the Ramsar Convention International Wetlands List in 1975. Certainly, Anzali international wetland is of great ecological, environmental, and economic importance, and tourist for the people and the government. This wetland is one of the freshwater wetlands which is located on the way of the rivers that flow into the Caspian Sea and plays the role of a treatment plant for running water in these rivers. It supports an extremely diverse wetland flora and fauna. Anzali Wetland is home to a variety of animal species and species of birds. It is also a good place for migratory birds and the most important source for the breeding and reproduction of Caviar fishes. Flowing more than 30 main and secondary rivers containing agricultural waste municipal wastewater, agricultural runoff, industrial effluent, and mineral waste into Anzali international wetland along with intense human activities (shipping, farming, and eco-tourism exploitation) have led to phthalates pollution and other hazardous contaminants in this lagoon (Jamshidi and Bastami 2016; Shariati et al. 2021, 2022b).
Isolation and identification of DEP-degrading bacterium
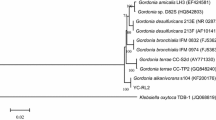
A DEP-degrading bacterium was isolated from a PAE-enriched consortium in mineral salt medium (MSM: CaCl2 0.01 g/L, MgSO4 0.1 g/L, (NH)2 SO4 1 g/L, KH2PO4 4.5 g/L, K2HPO4 5.8 g/L, NaCl 1 g/L, and 1 mL trace element) as described below. A microbial consortium An6 related to Sorkhankol station (the most polluted station of Anzali international wetland) (Hassanzadeh et al. 2014; Shariati et al. 2019a) was enriched during 2 months incubation period. Then, MSM agar plates containing 500 mg/L DEP were prepared in aseptic conditions. Plates comprising MSM agar without any carbon source were considered as control. Afterward, 100 μL of the consortium was added to the plates containing 500 mg/L DEP by spread culture method and kept in an incubator at 30 °C for 5 days. After the growth of the bacterium in the plates containing DEP and comparison with the control plates (without any carbon source), the sub-culturing was continued for 2 months until reaching a single colony with the ability to the degradation of DEP (Wu et al. 2011c; Jin et al. 2012; Wang et al. 2019). This strain was selected as a DEP-degrading bacterium and nominated as ShA. The identification of strain ShA was conducted based on its morphological and biochemical characteristics (MacFadden 1980; Krieg and Holt 1984; Wu et al. 2011a; Edison et al. 2012) and phylogenic analysis by the PCR Colony method as described below (Kai et al. 2019). Twenty-four hours of cultivation of this strain in LB medium was produced. The particular general primers R1492 (5′GGTTACCTTGTTACGACTT-3′) and F27 (5′-AGAGTTTGATCCTGGCTCAG-3′) were applied for amplifying the 16S rRNA gene of the strain ShA. The PCR reaction was performed in a 50-μL tube containing 20 µL sterile distilled water, 2.5 µL forward primer, 2.5 µL reverse primers, 25 µL Red Tag DNA polymerase (GENAXXON bioscience), and a small amount of suspension of a pure culture of strain ShA. A Flex cycler2 Analytik Jena PCR system with thermocycler programming, 5 min at 95 °C, 34 cycles of 30 s at 95 °C, 30 s at 55 °C, and 90 s at 72 °C along with 5 min at 72 °C was used for 16S rRNA gene amplification. One percent agarose gel electrophoresis and Fast Gene Gel/PCR Extraction Kit (Nippon Genetic Co, Ltd.) were used for PCR product qualification and purification, respectively. The purified 16S rRNA fragment was sequenced by the Sanger sequencing method (Eurofins Genomics, Germany). After sequencing, the forward and the reverse sequences were edited and combined by using the BioEdit 6. The outcome combined 16S rRNA sequence was submitted to NCBI and then compared with recognized 16S rRNA sequences using BLAST and the most similar sequences were used as a reference for drawing a phylogenetic tree. A phylogenetic tree was created using the neighbor-joining method and validated through bootstrap analysis (1000 replicates) with MEGA 7.0 software (Kumar and Maitra 2016).
Inoculum procurement
To prepare the inoculum of strain ShA for the following test, a single colony of ShA was inoculated to 50 mL MSM containing 200 mg/L DEP and incubated in a rotary shaker at 30 °C and 180 rpm for 4 days in the dark. After growing the bacteria in the suspension, it was applied as inoculum. For all tests, the size of the inoculum (Absorbance578nm = 0.4, measured by a UV-Ultrospec 3000 pro spectrophotometer) was 1% (v/v).
PAEs utilization assessment
Determination of PAE profile utilization by newly isolated strain ShA was conducted through inoculation of 1% of the ShA inoculum into MSM medium (50 mL) supplemented with DMP, DEP, DBP, DEHP, DOP, DIDP, MEHP at 200 mg/L, orthophthalic acid, terephthalic acid, isophthalic acid at 1000 mg/L. Suspensions (MSM containing 1% inoculum of bacteria) without any carbon source were set as biotic control. All treatments were kept in the incubator at 30 °C and 180 rpm for 7 days in dark conditions. The growth of bacteria in the different substrates was determined by measuring A578 using a UV-Ultrospec 3000 pro spectrophotometer with intervals of 24 h (Jin et al. 2015; Ebenau-Jehle et al. 2017; Shariati et al. 2022a).
DEP degradation evaluation by strain ShA
Due to the low solubility of DEP in water and the problems of preparing a uniform culture medium containing this ester, it was necessary to extract the entire culture medium with ethyl acetate at each sampling time. Therefore, to overcome these problems, three biological repetitions were considered for each sampling time. Fifty-milliliter flasks containing 10 mL of MSM culture medium supplemented with 200 mg/L DEP were prepared and then 100 μL of bacterial inoculation (A578 = 0.4) was added to each flask. At each sampling time (0, 11, 23, 26, 30, 36, 48, and 55 h), the growth of bacteria in the presence of DEP was measured using a UV-Ultrospec 3000 pro spectrophotometer at an absorbance of 578 nm. To measure the residual DEP at each sampling time, the DEP in all flasks was extracted separately using 10 mL of ethyl acetate at 12-h intervals and measured by a gas chromatography coupled flame ionization detector. For this purpose, the whole suspension was mixed with 10 mL of ethyl acetate by shaking vigorously for 4 min. The aqueous phase was separated from the organic phase by centrifuging at 6000 rpm for 10 min. Then, 1 µL of the organic phase was injected into the split/splitless injection port (split ratio: 10) of an Agilent 7890A GC-FID instrument (USA). The carrier gas was high-purity helium (99.9999%). A DB-5 fused silica capillary column (30 m × 0.25 mm × 0.25 µm) was applied for separations. For each separation analysis, the detector and injector temperatures were set at 340 and 300 °C, respectively. The primary temperature of the oven was adjusted at 70 °C (2 min), increased to 150 °C at 30 °C /min (without stop), ramped to 270 °C by 8 °C /min (without stop), and finally increased to 300 °C by 30 °C /min (for 3 min) (Wu et al. 2011c; Zhao et al. 2016).
Ortho phthalic acid (PA) degradation by strain ShA
PA is the main intermediate of PAEs degradation. Due to this fact, the breakdown of PA by strain ShA was investigated. To measure the growth of strain ShA in the presence of PA, 50 mL of MSM medium containing 2000 mg/L PA was prepared. Then 500 μL of ShA inoculum (1%) was added to the media and incubated in a rotary shaker (180 rpm and 30 °C). During the incubation, the growth of bacteria and the remaining PA were determined by measuring the absorbance of 578 nm and 276 nm, respectively, by a UV-Ultrospec 3000 pro spectrophotometer with intervals of 3 h (Ebenau-Jehle et al. 2017).
Statistical analysis
Each test was performed in 3 triplicates; control experiments without inoculation and/or without phthalate were conducted under the same conditions. Growth curves, chromatograms, and error bars were designed by Prism 8.0.
Results
Identification and classification of ShA
From the PAE consortium cultivation on the agar + MSM containing DEP during incubation at 30 °C and sub-culturing for 2 months in dark, one pure aerobic, flagellated, rod shape, non-spore-forming, and the gram-negative isolate was grown and named under the codes ShA (Fig. 2).
The Blast result of the aligned sequence of the ShA strain (1410 bp) on the NCBI showed that this strain has a high similarity (99.79%) to the Pseudomonas putida strain BN-st. Based on the morphological and biochemical properties of strain ShA (Table 1), the blast results, and the phylogenetic tree (Fig. 3), this strain was identified as Pseudomonas putida strain ShA. Afterward, its sequence was recorded in NCBI Gene bank with accession number MN298858.
Biodegradation of DEP by Pseudomonas putida strain ShA
As shown in Fig. 4, strain ShA was able to grow in all isomers of phthalic acid including in orthophthalic acid, terephthalic acid, and isophthalic acid, and low molecular weight of phthalates DMP, DEP, and DBP. DEP is one of the most widely used plasticizers in the world and was included in the EPA list of 100 dangerous substances. Therefore, it is important to identify native bacteria that degrade DEP rapidly. As it is shown in Fig. 5, strain ShA was able to completely degrade approximately 200 mg/L of DEP without any starter or amendment in less than 30 h. Interestingly, in our study PA which is the main metabolite of PAEs degradation was identified with a high concentration by the GC equipped with the FID detector (Fig. 6).
Biodegradation of PA by Pseudomonas putida strain ShA
Considering the importance of PA breakdown in progressing the complete degradation reaction of phthalic acid esters and the production of carbon dioxide and water as the final output, the ability of the new strain ShA to degrade high concentrations (2000 mg/L) of orthophthalic acid was investigated. Results demonstrated that 2000 mg/L of PA was degraded entirely by Pseudomonas putida strain ShA in less than 22 h (A578 = 0.4, inoculum size 1%) (Fig. 7). It can be concluded that this strain is excellently effective in the degradation of high concentrations of PA.
Discussion
The entrance of DEP into the environment and then organisms and the human body causes reduced food security and various diseases (Tsai et al. 2021; Sicińska et al. 2021). Also, the half-life of DEP in soil and sediment with a low level of oxygen could increase to several months or even years (Boll et al. 2020; Zhang et al. 2021). Today, the use of helpful microorganisms with the ability to degrade DEP is a key and ideal method for the remediation of this pollutant from the different parts of the ecosphere (Tao et al. 2019; Hu et al. 2021). To reach this goal, the isolation and identification of new native strains with different physiological, genetic, biochemical, and ecological characteristics are very urgent (Barrett et al. 2020; Hu et al. 2021).
Pseudomonas putida has a significant role in the environment and is a helpful and versatile bacteria for its ability as an aromatic or aliphatic hydrocarbons degrader and as a plant growth-promoting bacteria (Dutta et al. 2017; Molina et al. 2020; Costa-Gutierrez et al. 2020). It is a well-known bacteria in the degradation of styrene (Ward et al. 2005), PAHs (Khan et al. 2014; Dutta et al. 2017), oil (Maia et al. 2019), bisphenol A (Eltoukhy et al. 2020), and pentachlorophenol (Ammeri et al. 2021); however, there has been no report about the biodegradation of phthalates and phthalic acid isomers by Pseudomonas putida strains. Although in previous studies bacterial strains were isolated from soil, sludge, constructed wetlands, river sediments, sewage sludge, activated sludge, soil, etc. (Wang et al. 2020; Eltoukhy et al. 2020; Hu et al. 2021), for the first time in our study, Pseudomonas putida strain ShA was isolated from sediment of a natural wetland that is heavily polluted by phthalate (Hassanzadeh et al. 2014; Shariati et al. 2019a).
PAE consumption test showed that Pseudomonas putida strain ShA could biodegrade DEP, DBP, DMP, PA, isophthalic acid, and terephthalic acid. The results of a study showed that Pseudomonas sp. P136 isolated from the soil was able to completely degrade the phthalic acid isomers (iso, ter, and ortho) at 2000 mg/L within 50–60 h (Nozawa and Maruyama 1988). In a project, Pseudomonas sp. strain PPD, Pseudomonas aeruginosa strain PP4, and Acinetobacter lwoffii strain ISP4 were isolated from the soil (Krishna et al. 2006). The strain ISP4 was only able to consume isophthalic acid, but the other two Pseudomonas strains were able to degrade all the PA isomers (iso, ortho, and ter). Researchers expressed that very few strains including Comamonas testosteroni strain E6, Pseudomonas testosteroni EN 5a, strain PP4, Pseudomonas strain PPD, and Pseudomonas aeruginosa can degrade all phthalate isomers (Krishna and Phale 2008). As a first report, in addition to the strains listed above, Pseudomonas putida strain ShA isolated from a natural wetland was able to consume all phthalic acid isomers.
Our results revealed that Pseudomonas putida strain ShA could degrade 200 mg/L of DEP in less than 30 h (A578 = 0.4, 1%). Also, PA was identified as a metabolite from DEP degradation using GC-FID, which confirmed the ability of this bacterium to break down DEP. According to the literature review, although there is no report on the biodegradation of PAEs by the pseudomonas putida strains, there has been some research on other species of the genus Pseudomonas in PAEs degradation. Pseudomonas fluorescence strain FS1 was able to break down 99% of 100 mg/L DMP and DEP within 3 d (Zeng et al. 2004). The results of a study stated Pseudomonas fluorescens strain B-1 was able to degrade DBP (Xu et al. 2005). In an investigation, the ability of Pseudomonas stutzeri in DBP biodegradation has been confirmed (Liao et al. 2010). The research of Singh et al. (2017) showed that Pseudomonas sp. strain PKDM2, Pseudomonas sp. strain PKDE1, and Pseudomonas sp. PKDE2 could biodegrade 75% of 500 mg/L DEP after 44 h. Some researchers isolated Pseudomonas sp. strain DNE-S1 with the ability to consume DEP from landfill soil (Tao et al. 2019). Compared to other DEP-degrading strains (Table 2), the ShA strain isolated in this study had a higher ability and speed in DEP degradation than other strains, except for Pseudomonas sp. strain DNE-S1 (Tao et al. 2019). Besides, studies showed that every Pseudomonas putida strain cannot degrade DEP. Eltoukhy et al. (2020) isolated a Pseudomonas putida YC-AE1 from the soil with the ability to degrade (BPA, BPB, BPF, and BPS), slightly DEHP, and DBP (20%). Nevertheless, it could not degrade DEP at a concentration of 100 mg/L for 72 h. Based on the literature review, the isolation source and species of our strain differ from other typic DEP-degrading strains.
PA is the well-known central metabolite in the degradation of PAEs and biodegradation of DEP by strain ShA. PA as a central metabolite of PAE biodegradation is a kind of endocrine disrupter pollutant in the environment, especially in the aquatic ecosystem. Also, PA is considered a secondary pollutant in the aquatic ecosystem (Boll et al. 2020). Studies have shown that some bacteria can break down PAEs but could not degrade PA (Chatterjee and Dutta 2003; Li and Gu 2007; Wu et al. 2010, 2011c). To confirm the complete DEP biodegradation (DEP to CO2 and H2O) ability of Pseudomonas putida strain ShA, the orthophthalic acid (PA) consumption test was investigated at a concentration of 2000 mg/L. Results showed that strain ShA could efficiently degrade 2000 mg/L of PA in less than 22 h. Despite the low molecular weight of phthalic acid (166.14 g/mol) and its faster degradation in the environment than DEHP (396.277 g/mol), many studies have shown that some bacteria with the ability to degrade DEHP could not degrade PA. It was reported that during the degradation of DEHP by Gordonia sp. strain JDC-2, PA accumulated in the media, and this strain was not able to consume it (Wu et al. 2010). Furthermore, studies revealed that DEHP and DBP-degrading bacteria Gordonia sp. strain Dop5 (Sarkar et al. 2013) and Camelimonas sp. (Chen et al. 2015) could not degrade PA. Besides, some strains including Agrobacterium sp. (Wu et al. 2011b), Arthrobacter sp. ZH2 (Wang et al. 2012) can degrade PA but not PA isomers, isophthalic acid, and terephthalic acid. In a study, researchers isolated two Pseudomonas aeruginosa sp. strain PP4 and Pseudomonas sp. strain PPD with the capability to utilize phthalic acid isomers from the soil (Krishna et al. 2006). This result confirmed that strain ShA has the high ability to completely degrade DEP, PA, and proceed with the reaction to produce CO2 and H2O. For the catalysis of this reaction, the enzymes esterases/hydrolase and phthalate dioxygenase are probably involved (Liang et al. 2008; Tao et al. 2019; Hu et al. 2021).
Conclusion
For the first time, a novel Pseudomonas Putida strain ShA was isolated from natural sediment (Anzali International Wetland) with the ability to degrade DEP. It also had a high ability to consume DMP, orthophthalic acid, isophthalic acid, and terephthalic acid. Also, this is the first report regarding the isolation of a native strain with the ability to degrade phthalate from the environment of Iran and the Anzali international wetland. Due to the easy working characteristics and versatility of Pseudomonas putida and the non-pathogenic nature of this bacteria that was shown by many types of research, it can be applied as a native bacterial strain in removing PA isomers and low molecular weight phthalate from soil and sediments.
References
Ahuactzin-Pérez M, Tlecuitl-Beristain S, García-Dávila J, Santacruz-Juárez E, González-Pérez M, Gutiérrez-Ruíz MC, Sánchez CA (2018) Novel biodegradation pathway of the endocrine-disruptor di (2-ethyl hexyl) phthalate by Pleurotus ostreatus based on quantum chemical investigation. Ecotoxicol Environ Saf 147:494–499
Ammeri RW, Hidri Y, Hassen W, Mehri I, Khlifi N, Hassen A (2021) Surfactant efficiency on pentachlorophenol-contaminated wastewater enhanced by Pseudomonas putida AJ 785569. Arch Microbiol 203:5141–5152. https://doi.org/10.1007/s00203-021-02486-1
Andersen C, Krais AM, Eriksson AC, Jakobsson J, Löndahl J, Nielsen J, Lindh CH, Pagels J, Gudmundsson A, Wierzbicka A (2018) Inhalation and Dermal Uptake of Particle and Gas-Phase Phthalates-A Human Exposure Study. Environ Sci Technol 52(21):12792–12800
Barrett J, Chase Z, Zhang J, Holl MMB, Willis K, Williams A, Hardesty BD, Wilcox C (2020) Micro plastic pollution in deep-sea sediments from the great Australian bight. Front Mar Sci 7(808):1–10
Boll M, Geiger R, Junghare M, Schink B (2020) Microbial degradation of phthalates: biochemistry and environmental implications. Environ Microbiol Rep 12(1):3–15
Braun JM, Sathyanarayana S, Hauser R (2013) Phthalate exposure and children’s health. Curr Opin Pediatr 25:247–254
Buser MC, Murray HE, Scinicariello F (2014) Age and sex differences in childhood and adulthood obesity association with phthalates: Analyses of NHANES 2007–2010. Int J Hyg Environ Health 217:687–694
Carstens L, Cowan AR, Seiwert B, Schlosser D (2020) Biotransformation of Phthalate Plasticizers and Bisphenol A by Marine-Derived, Freshwater, and Terrestrial Fungi. Front Microbiol 11:317
Chang WH, Wu MH, AnPan H, Guo LG, Lee CC (2017) Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere 173:594–602
Chatterjee S, Dutta TK (2003) Metabolism of butyl benzyl phthalate by Gordonia sp. strain MTCC 4818. Biochem Biophys Res Commun 309:36–43
Chen N, Shuai W, Hao X, Zhang H, Zhou D, Gao J (2017) Contamination of Phthalate Esters in Vegetable Agriculture and Human Cumulative Risk Assessment. Pedosphere 27(3):439–451
Chen X, Zhang X, Yang Y, Yue D, Xiao L, Yang L (2015) Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate by newly isolated Camelimonas sp. and enzymatic properties of its hydrolase. Biodegradation 26:171–182
Costa-Gutierrez SB, Lami MJ, Santo MCCD et al (2020) Plant growth promotion by Pseudomonas putida KT2440 under saline stress: role of eptA. Appl Microbiol Biotechnol 104:4577–4592. https://doi.org/10.1007/s00253-020-10516-z
Demirtaş G, Çavuşoğlu K, Yalçin E (2020) Aneugenic, clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ Sci Pollut R 27:5503–5510
Dutta K, Shityakov S, Das PP, Ghosh C (2017) Enhanced biodegradation of mixed PAHs by mutated naphthalene 1,2-dioxygenase encoded by Pseudomonas putida strain KD6 isolated from petroleum refinery waste. 3 Biotech 7(6):365
Ebenau-Jehle C, Mergelsberg M, Fischer S, Brüls T, Jehmlich N, Bergen M, Boll M (2017) An unusual strategy for the anoxic biodegradation of phthalate. ISME J 11:224–236
Edison TE, Cruz D, Martin J, Torres O (2012) Gelatin hydrolysis test protocol. Am Soc Microbiol
Eltoukhy A, Jia Y, Nahurira R, Abo-Kadoum MA, Khokhar I, Wang J, Yan Y (2020) Biodegradation of endocrine disruptor Bisphenol A by Pseudomonas putida strain YC-AE1 isolated from polluted soil, Guangdong, China. BMC Microbiol 20(11):1–14
Fan JC, Ren R, Jin Q, He HL, Wang ST (2019) Detection of 20 phthalate esters in breast milk by GC-MS/MS using QuEChERS extraction method. Food Addit Contam A 36(10):1551–1558
Fang HHP, Liang D, Zhang T (2007) Aerobic degradation of diethyl phthalate by Sphingomonas sp. Bioresour Technol 98(3):717–720
Feng Z, Cui K, Li X, Fu J, Sheng G (2004) Biodegradation kinetics of phthalateesters by Pseudomonas fluoresences FS1. Process Biochem 39:1125–1129
Gao DW, Wen DZ (2016) Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ 541:986–1001
Guo Y, Kannan K (2013) A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol 47:14442–14449
Hassanzadeh N, Esmaili Sari A, Khodabandeh S, Bahramifar N (2014) Occurrence and distribution of two phthalate esters in the sediments of the Anzali wetlands on the coast of the Caspian Sea (Iran). Mar Poll Bull 89:128–135
Hatch E, Nelson J, Qureshi M (2008) Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A crosssectional study of NHANES data 1999–2002. Environ Health 15:1–15
Hu R, Zhao H, Xu X, Wang Z, Yu K, Shu L, Yan Q, Wu B, Mo C, He Z, Wang C (2021) Bacteria-driven phthalic acid ester biodegradation: Current status and emerging opportunities. Environ Int 154(106560):1–14
Hu X, Gu Y, Huang W, Yin D (2016) Phthalate monoesters as markers of phthalate contamination in wild marine organisms. Environ Pollut 218:410–418
Huang YW, Ren WJ, Liu HR, Wang HM, Xu YF, Han YJ, Teng Y (2021) Contrasting impacts of drying-rewetting cycles on the dissipation of di-(2-ethylhexyl) phthalate in two typical agricultural soils. Sci Total Environ 792:148433
Jamshidi S, Bastami KD (2016) Metal contamination and its ecological risk assessment in the surface sediments of Anzali wetland, Caspian Sea. Mar Pollut Bull 113:559–565
Jin D, Kong X, Li Y, Bai Z, Zhuang G, Zhuang X, Deng Y (2015) Biodegradation of di-n-butyl phthalate by Achromobacter sp. isolated from rural domestic wastewater. Int J Environ Res Public Health 12:13510–13522
Jin DC, Bai ZH, Chang DD, Hoefel D, Jin B, Wang P (2012) Biodegradation of din-butyl phthalate by an isolated Gordonia sp. strain QH-11: genetic identification and degradation kinetics. J Hazard Mater 221:80–85
Kai S, Matsuo Y, Nakagawa S, Kryukov K, Matsukawa S, Tanaka H, Iwai T, Imanishi T, Hirota K (2019) Rapid bacterial identification by direct PCR amplification of 16S rRNA genes using the MinIONTM nanopore sequencer. FEBS Open Bio 9:548–557
Khadka S, Nshimiyimana JB, Zou P, Koirala N, Xion L (2020) Biodegradation Kinetics of Diethyl Phthalate by Three Newly Isolated Strains of Pseudomonas. Scientific African 8(e00380):1–14
Khan Z, Roman D, Kintz T, Alas MD, Yap R, Doty S (2014) Degradation, Phytoprotection and Phytoremediation of Phenanthrene by Endophyte Pseudomonas putida, PD1. Environ Sci Technol 48(20):12221–12228
Kim S, Lee J, Park J, Kim HJ, Cho G, Kim GH, Eun SH, Lee JJ, Choi G, Suh E, Choi S, Kim S, Kim YD, Kim SK, Kim SY, Kim S, Eom S, Moon HB, Kim S, Choi K (2015) Concentrations of phthalate metabolites in breast milk in Korea: Estimating exposure to phthalates and potential risks among breast-fed infants. Sci Total Environ 508:13–19
Kim S, Lee YS, Moon HB (2020) Occurrence, distribution, and sources of phthalates and non-phthalate plasticizers in sediment from semi-enclosed bays of Korea. Mar Pollut Bullet 151(110824):1–8
Kolena B, Petrovicova I, Sidlovska M, Pilka T, Neuschlova M, Valentova I, Rybansky L, Trnovec T (2017) Occupational phthalate exposure and health outcomes among hairdressing apprentices. Hum Exp Toxicol 36:1100–1112
Krieg NR, Holt JG (1984) Bergey’s Manual of Systematic Bacteriology”, vol 1. Williams & Wilkins Co., Baltimore, pp 161–172
Krishna CV, Mohan Y, Phale PS (2006) Biodegradation of phthalate isomers by Pseudomonas aeruginosa PP4, Pseudomonas sp. PPD and Acinetobacter lwoffii ISP4. Appl Microbiol Biotechnol 72:1263–1269
Krishna CV, Phale PS (2008) Bacterial degradation of phthalate isomers and their esters. Indian J Microbiol 48:19–34
Kumar V, Maitra SS (2016) Biodegradation of endocrine disruptor dibutyl phthalate (DBP) by a newly isolated Methylobacillus sp. V29b and the DBP degradation pathway. 3 Biotech 6(200):1–12
Kumar V, Sharma N, Maitra SS (2017) Comparative study on the degradation of dibutyl phthalate by two newly isolated Pseudomonas sp. V21b and Comamonas sp. 51F. Biotech Rep 15:1–12
Lee YM, Lee JE, Choe W, Kim T, Lee JY, Kho Y, Choi K, Zoh KD (2019) Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ Int 126:635–643
Li H, Gu JD (2007) Complete degradation of dimethyl isophthalate requires the biochemical cooperation between Klebsiella oxytoca Sc and Methylobacterium mesophilicum Sr isolated from wetland sediment. Sci Total Environ 380:181–187
Li R, Liang J, Gong Z, Zhang N, Duan H (2017) Occurrence, spatial distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Sci Total Environ 580:388–397
Liang DW, Zhang T, Fang HHP, He JZ (2008) Phthalates biodegradation in the environment. Appl Microbiol Biotechnol 80(2):183–198
Liao CS, Chen LC, Chen BS, Lin SH (2010) Bioremediation of endocrine disruptor di-n-butyl phthalate ester by Deinococcus radiodurans and Pseudomonas stutzeri. Chemosphere 78:342–346
Lu Y, Tang F, Wang Y, Zhao JH, Zeng X, Luo QF et al (2009) Biodegradation of dimethyl phthalate, diethyl phthalate, and di-n-butyl phthalate by Rhodococcus sp L4 isolated from activated sludge. J Hazard Mater 168(2–3):938–943
MacFadden JF (1980) Biochemical Tests for Identification of Medical Bacteria. Williams and Wilkins, Baltimore
Mahto JK, Neetu N, Sharma M, Dubey M, Vellanki BP, Kumar P (2022b) Structural Insights into Dihydroxylation of Terephthalate, a Product of Polyethylene Terephthalate Degradation. J Bacteriol 204(3):e0054321. https://doi.org/10.1128/JB.00543-21
Mahto JK, Neetu N, Waghmode B, Kuatsjah E, Sharma M, Sircar D, Sharma AK, Tomar S, Eltis LD, Kumar P (2021) Molecular insights into substrate recognition and catalysis by phthalate dioxygenase from Comamonas testosteroni. J Biol Chem 297(6):101416. https://doi.org/10.1016/j.jbc.2021.101416
Mahto JK, Sharma M, Neetu N, Kayastha A, Aggarwal S, Kumar P (2022a) Conformational flexibility enables catalysis of phthalate cis-4,5-dihydrodiol dehydrogenase. Arch Biochem Biophys 3(727):109314. https://doi.org/10.1016/j.abb.2022.109314
Maia M, Capão A, Procópio L (2019) Biosurfactant produced by oil-degrading Pseudomonas putida AM-b1 strain with potential for microbial enhanced oil recovery. Bioremediat J 23(4):302–310. https://doi.org/10.1080/10889868.2019.1669527
Molina L, Segura A, Duque E, Ramos JL (2020) Chapter Four - The versatility of Pseudomonas putida in the rhizosphere environment. Adv Appl Microbiol 110:149–180
Navacharoen A, Vangnai AS (2011) Biodegradation of diethyl phthalate by an organic-solvent-tolerant Bacillus subtilis strain 3C3 and effect of phthalate ester coexistence. Int Biodeter Biodeg 65:818–826
Net S, Delmont A, Sempéré R, Paluselli A, Baghdad O (2015) Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ Sci Technol 49(7):4019–4035
Nozawa T, Maruyama Y (1988) Anaerobic metabolism of phthalate and other aromatic compounds by a denitrifying bacterium. J Bacteriol 170:5778–5784
Perpetuo EA, da Silva ECN, Karolski B, Nascimento CAO (2020) Biodegradation of diethyl-phthalate (DEP) by halotolerant bacteria isolated from an estuarine environment. Biodegradation 31:331–340. https://doi.org/10.1007/s10532-020-09913-y
Sarkar J, Chowdhury PP, Dutta TK (2013) complete degradation of di-n-octyl phthalate by Gordonia sp. strain Dop5. Chemosphere 90:2571–2577
Schwedler G, Seiwert M, Fiddicke U, Issleb S, Holzer J, Nendza J, Wilhelm M, Wittsiepe J, Koch HM, Schindler BK et al (2017) Human biomonitoring pilot study DEMOCOPHES in Germany: Contribution to a harmonized European approach. Int J Hyg Environ Health 220:686–696
Selvaraj KK, Sundaramoorthy G, Ravichandran PK, Girijan GK, Sampath S, Ramaswamy BR (2015) Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ Geochem Health 37(1):83–96
Seyoum A, Pradhan A (2019) Effect of phthalates on development, reproduction, fat metabolism and lifespan in Daphnia Magna. Sci Total Environ 654(1):969–977
Shariati S, Ebenau-Jehle C, Pourbabaee AA, Alikhani HA, Rodriguez-Franco M, Agne M, Jacoby M, Geiger R, Shariati F, Boll M (2022a) Degradation of dibutyl phthalate by Paenarthrobacter sp. Shss isolated from Saravan landfill, Hyrcanian Forests, Iran. Biodegradation 33(1):59–70. https://doi.org/10.1007/s10532-021-09966-7
Shariati S, Pourbabaee AA, Alikhani HA, Rezaei KA (2019a) Assessment of phthalic acid esters pollution in Anzali wetland, north of Iran. Int J Environ Sci TE 16:7025–7036
Shariati S, Pourbabaee A, Alikhani H, Rezaei K (2019b) Investigation of Heavy Metal Contamination in the Surface Sediments of Anzali wetland in North of Iran. Pollution 5(1):211–224
Shariati S, Pourbabaee AA, Alikhani HA, Rezaei KA (2021) Anaerobic biodegradation of phthalic acid by an indigenous Ralstonia pickettii strain SHAn2 isolated from Anzali international wetland. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03677-5
Shariati S, Pourbabaee AA, Alikhani HA, Rezaei KA (2022b) Anaerobic biodegradation of phthalic acid by an indigenous Ralstonia pickettii strain SHAn2 isolated from Anzali international wetland. Int J Environ Sci Technol 19:4827–4838. https://doi.org/10.1007/s13762-021-03677-5
Sicińska P, Mokra K, Wozniak K et al (2021) Genotoxic risk assessment and mechanism of DNA damage induced by phthalates and their metabolites in human peripheral blood mononuclear cells. Sci Rep 11:1658
Silva MJ, Reidy JA, Samandar E, Herbert AR, Needham LL, Calafat AM (2005) Detection of phthalate metabolites in human saliva. Arch Toxicol 79:647–652
Singh N, Dalal V, Mahto JK, Kumar P (2017) Biodegradation of phthalic acid esters (PAEs) and in silico structural characterization of mono-2-ethylhexyl phthalate (MEHP) hydrolase on the basis of close structural homolog. 2017. Jour Hazard Mater 338:11–22
Song X, Zhuo Q, Tang S, Xie T, Chen Z, Zeng Z, Zhang Y, Niu X, Yin H, Zeng F, He C (2020) Concentrations of phthalates metabolites in blood and semen and the potential effects on semen concentration and motility among residents of the Pearl River Delta region in China. Emerg Contam 6:39–43
Sun J, Wu X, Gan J (2015) Uptake and Metabolism of Phthalate Esters by Edible Plants. Environ Sci Technol 49(14):8471–8478
Tang S, He C, Tahi P, Vijayasarathy S, Mackie R, Toms LML, Thompson K, Hobsond P, Tscharke B, O’Brien JW, Mueller JF (2020) Concentrations of phthalate metabolites in Australian urine samples and their contribution to the per capita loads in wastewater. Environ Int 137(105534):1–8
Tao Y, Lia H, Gub J, Shi H, Han S, Jiao Y, Zhong G, Zhang Q, Akindolie MS, Lina Y, Chen Z, Zhang Y (2019) Metabolism of diethyl phthalate (DEP) and identification of degradation intermediates by Pseudomonas sp. DNE-S1. Ecotoxicol Environ Saf 173:411–418
Tran TM, Kannan K (2015) Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Arch Environ Contam Toxicol 68:489–499
Tsai CK, Cheng H, Hsu TY, Wang JY, Hung CH, Tsai CC, Lai YJ, Lin YJ, Chin JYH, Tain YL, Chen CC, Yu HR (2021) Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells. Int J Env Res Pub He 18(12):6364
Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL (2013) Phthalates and risk of endometriosis. Environ Res 126:91–97
Wang Y, Kannan ZhuH, K, (2019) A review of biomonitoring of phthalate exposures. Toxics 7(21):1–28
Wang Y, Miao B, Hou D, Wu X, Peng B (2012) Biodegradation of di-n-butyl phthalate and expression of the 3,4-phthalate dioxygenase gene in Arthrobacter sp. ZH2 strain. Process Biochem 47:936–940
Wang Y, Zhan W, Liu Y, Cheng S, Zhang C, Ma J, Chen R (2020) Di-n-octyl phthalate degradation by a halotolerant bacterial consortium LF and its application in soil. Environ Technol 42:2749–2756
Ward PG, de Roo G, O’Connor KE (2005) Accumulation of Polyhydroxyalkanoate from Styrene and Phylacetic Acid by Pseudomonas putida CA-3”. Appl Environ Microbiol 71:2046–2052
Weaver JA, Beverly BEJ, Keshava N, Mudipalli A, Arzuaga X, Cai C, Ak H, Makris SL, Yost EE (2020) Hazards of diethyl phthalate (DEP) exposure: A systematic review of animal toxicology studies. Environ Int 145:105848
Weschler CJ, Bekö G, Koch HM, Salthammer T, Schripp T, Toftum J, Clausen G (2015) Transdermal Uptake of Diethyl Phthalate and Di(n-butyl) Phthalate Directly from Air: Experimental Verification. Environ Health Perspect 123:928–934
Wu RX, Liang QY, Dai DC, Jin YY, Wang WL, Chao W (2010) Complete degradation of di-n-octyl phthalate by biochemical cooperation between Gordonia sp. strain JDC-2 and Arthrobacter sp. strain JDC-32 isolated from activated sludge. J Hazard Mater 176:262–268
Wu X, Monchy S, Taghavi S, Zhu W, Ramos J, van der Lelie D (2011a) Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol Rev 35(2):299–323
Wu X, Wang Y, Liang R, Dai Q, Jin D, Chao W (2011b) Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate by newly isolated Agrobacterium sp. and the biochemical pathway. Process Biochem 46:1090–1094
Wu XL, Wang YY, Dai QY, Liang RX, Jin DC (2011c) Isolation and characterization of four di-n-butyl phthalate (DBP)-degrading Gordonia sp. strains and cloning the 3,4-phthalate dioxygenase gene. World J Microbiol Biotechnol 27:2611–2617
Xu X, Li H, Gu J (2005) Biodegradation of an endocrine-disrupting chemical di-nbutyl phthalate ester by Pseudomonas fluorescens B-1. Int Biodeterior Biodegrad 55:9–15
Yang T, Ren L, Jia Y, Fan S, Wang J, Wang J, Nahurira R, Wang H, Yan Y (2018) Biodegradation of di-(2-ethylhexyl) phthalate by Rhodococcus ruber YC-YT1 in contaminated water and soil. Int J Environ Res Public Health 15(964):1–20
Zeng F, Cui K, Li X, Fu J, Sheng G (2004) Biodegradation kinetics of phthalate esters by Pseudomonas fluoresences FS1. Process Biochem 39(9):1125–1129
Zhang M, Yu X, Wang Y, Hu Y, Liu S (2013) A Highly Sensitive Indirect Competitive Enzyme-Linked Immunosorbent Assay (ic-ELISA) by Antigen Coating for Diethyl Phthalate Analysis in Foods. Food Anal Methods 6:1223–1228
Zhang Y, Jiao Y, Li Z, Yang Y (2021) Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci Total Environ 771(145418):1–23
Zhao HM, Du H, Lin J, Chen XB, Li YW, Li H, Cai QY, Mo CH, Qin HM, Wong MH (2016) Complete degradation of the endocrine disruptor di-(2-ethylhexyl) phthalate by a novel Agromyces sp. MT-O strain and its application to bioremediation of contaminated soil. Sci Total Environ 562:170–178
Acknowledgements
The authors are grateful to Prof. Matthias Boll and Ag Boll group, Albert Ludwig University, Freiburg, Germany, for their support in the GC analysis and 16S rRNA sequencing. Also, financial support for this research was provided by Iran’s National Elites Foundation and the Iran National Science Foundation (INSF) under Grant 4013906.
Funding
Financial support for this research was provided by Iran’s National Elites Foundation and the Iran National Science Foundation (INSF) under Grant 4013906.
Author information
Authors and Affiliations
Contributions
Shayan Shariati: Data curation, analytical method, biodegradation tests, data analysis, funding acquisition, isolation of bacteria, methodology, project administration, writing (original draft), writing (review and editing). Ali Pourbabaee: Data curation, methodology, validation, writing (review and editing), project administration, supervision. Hossein Ali Alikhani: Data curation, methodology, validation, writing (review and editing), project administration, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shariati, S., Pourbabaee, A.A. & Alikhani, H.A. Biodegradation of diethyl phthalate and phthalic acid by a new indigenous Pseudomonas putida. Folia Microbiol 68, 477–488 (2023). https://doi.org/10.1007/s12223-022-01022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-022-01022-y