Abstract
Diethyl phthalate (DEP) is a compound which is used in many industrial fields, especially in cosmetic sector and causes contamination in air, water, and soil due to its widespread usage. In this study, the potential toxic effects of DEP were investigated by using physiological, anatomical, biochemical, and cytogenetic parameters in Allium cepa. The micronucleus (MN) test specifically aimed to elucidate the aneugenic and clastogenic effects of DEP. Physiological effects were determined by germination percentage, root length, weight gain parameters, and cytogenetic effects were investigated by mitotic index (MI) and chromosomal abnormality (CA) test. Malondialdehyde (MDA) level, catalase (CAT), and superoxide dismutase (SOD) activities were investigated as oxidative damage indicators and structural changes were investigated with anatomical cross sections. For this purpose, Allium cepa bulbs were divided into four groups as control and application groups and the application groups were germinated with 1.0, 2.2, and 4.4 μM DEP for 72 h. As a result, it was determined that germination percentage, weight gain and root length decreased, CA frequency, MDA level, SOD, and CAT activities were increased in DEP-treated groups when compared with the control group. DEP has been found to induce CA in root tip cells such as fragment, chromosome bridge, c-mitosis, sticky chromosome, and unequal chromatin distribution. When MN formations induced by DEP application were examined, both large-scale and small-scale MNs were determined. MN formation in both sizes indicates that DEP has both clastogenic and aneugenic effects. And also, it was found that DEP application caused structural changes and especially anatomic damages such as necrosis in 4.4 μM DEP application. As a result, it was found that DEP caused various toxic effects depending on the dose and that A. cepa test material was a useful indicator in determining these effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developing technology in our daily lives and efforts to improve the quality of life has led us to interact directly or indirectly with many chemicals. Increased exposure to chemicals causes many negative effects on the environment and living organisms. Plastics are the most exposed chemicals and their useful properties such as easy forming, electrical and thermal insulation, flexible structure, and resistance to abrasion extend the application area of plastics. It is used frequently in the main material of kitchen equipment, mobile phones, automobile and computer parts, bicycle helmets, electrical industry, and many other sectors (Andrady and Neal 2009; Benjamin et al. 2015). Although the raw materials of plastics are coal, oil, and natural gas, their properties such as strength and flexibility can be increased with some additives during the production stages. Phthalates are also one of these additives and used to improve the properties of plastic materials and to make them more flexible. Over time, phthalates have been used in many different sectors in different fields and are widely used in cosmetics and perfume production to provide softness and as solvent or binder. Phthalates are divided into two groups as high and low molecular weight phthalates according to the number of carbon atoms in the alcohol chain. Their use largely depends on molecular weight, and high molecular weight phthalates such as diethyl phthalate (DEP) are used as a component of 67 cosmetic formulations such as bath products, eye shadows, perfumes, hair sprays, bathing tools, detergents, shaving lotions, and skin care products (Anonymous 1985; Kamrin and Mayor 1991).
DEP (C12H14O4) is a colorless liquid with a slightly aromatic smell. DEP reacts chemically with hydroxyl radicals in the air and has an estimated half-life of 22.2 h. DEP is present in the form of vapor in the atmosphere, causing contamination by adsorbing to particles in air and aquatic environments (Peakall 1975). In the soil, DEP undergoes biodegradation and the reaction takes place in a series of steps, such as in the decomposition of phthalates (Cartwright et al. 2000). The adverse effects caused by the contamination of phthalates to living things reveal the need to investigate the toxic effects of phthalates on organisms in detail. The main source of phthalate exposure in living things is mostly the nutrition with contaminated foods. In particular, contaminated fatty foods (e.g., fish or oils) can cause high levels of phthalate exposure (Meek and Chan 1994; Wormuth et al. 2006). Another source of contamination is the exposure to phthalate in the structure of blood transfusion materials and blood storage bags while performing blood transfusions and dialysis (Mettang et al. 1999; Nässberger et al. 1987). The widespread use of phthalates has raised the concern that it may cause toxic effects to animals and humans as a result of their biological effects. For this purpose, several tests such as chromosomal aberration test, micronucleus test, AMES test, and the other mutation tests performed to evaluate the genotoxic potential of phthalates. As a result of these tests, while some studies reported various toxic effects, some studies have proved that no effect has occurred. In literature, Turner et al. (1974) reported that di-2-ethylhexyl phthalate (2-dEHp) induces single chromatid damage in human lymphocytes. Anderson et al. (1999) showed that 2-dEHp and its major metabolites, mono-2-ethylhexyl phthalate, cause DNA damage in human leukocytes. Astill et al. (1986) stated that the application of 2-dEHp did not cause a change in rat hepatocytes in AMES, micronucleus, and cell transformation tests. Agarwal et al. (1985) reported a mild positive response in both Salmonella typhimurium TA 100 and TA 1535 cultures applied to DEP. As opposed to this, Lee and Lee (2007) observed that phthalic acid and derivatives does not produce any mutagenic response in Salmonella typhimurium strains in the absence or presence of S-9 mixture. These results are an overall summary of the conflicting results and need further work to eliminate this conflict. In this study, the genotoxic effects of DEP were investigated by micronucleus (MN) test, mitotic index (MI), and chromosomal abnormality (CA) tests. In determining the aneugenic and clastogenic effects of DEP, MN size was examined. In addition, changes in physiological parameters induced by DEP, potential oxidative damage, and anatomical changes were investigated. In order to determine all these effects, the Allium test, which is very acceptable and gives the same results as eukaryotic tests especially performed in rodents, was used (Rank and Nielsen 1994; Vicentini et al. 2001).
Materials and methods
Test material and treatment principles
DEP was purchased from Sigma-Aldrich (CAS Number: 84-66-2, ≥ 99%) as chemical agent, and A. cepa (n = 12) bulbs, approximately 3.85 g in weight, were supplied from a commercial market in Giresun Province. Bulbs were divided into four groups as one control and three treatment groups. The bulbs in the control group (group I) were germinated in tap water and the bulbs in group II, group III, and group IV were germinated with 1.0, 2.2, and 4.4 μM doses of DEP, respectively, at 24 °C for 72 h.
Physiological parameter measurements
The root lengths were measured on the basis of the radicle with a millimetric ruler and the weights were measured with precision scales. The weight gain was calculated by the differences between the weights measured before and after DEP exposure (Yalçın et al. 2019). Percentage of germination was determined with the help of Eq. (1).
Germination percentage (%) = number of germinated bulb/total number of bulb × 100 (1)
Cytogenetic parameter analysis
To determine the MI, MN and CA frequency as cytogenetic parameters A. cepa root tip preparations were prepared. For this aim, root tips of the bulbs were cut 1–2 cm in length and fixed in the Carnoy solution for 2 h at 4 °C. After hydrolyzing with 1 N HCl for 17 min at 60 °C, samples were stained with acetocarmine during one night. Root tip preparations were examined for CA, MI, and MN analysis with a research microscope (IRMECO IM-450 TI) and photographed at × 500 magnification (Yalçın et al. 2019). MN and CA frequency was calculated by analyzing 1000 cells from each group. MI was calculated using the formula given in Eq. (2) and a total of 10,000 cells were counted for each group.
MI = number of cells entering to mitosis/total cell count × 100 (2)
Biochemical analysis
Determination of lipid peroxidation
In MDA level, the indicator of lipid peroxidation was measured by the method proposed by Unyayar et al. (2006). A 0.5 g of the root tips was homogenized by adding trichloroacetic acid (TCA, 5%) solution. The homogenates were centrifuged at 12,000g for 24 min at 24 °C. In 20% TCA solution, equal volumes of 0.5% thiobarbituric acid (TBA) and supernatant were incubated at 96 °C for 30 min. After incubation, the tubes were transferred to an ice bath and centrifuged at 10,000g for 5 min. The absorbance of the supernatant was measured at 532 nm and the MDA content was expressed in μM/g FW.
CAT and SOD analysis
For sample extraction, 0.5 g of fresh root material was collected, washed with distilled water, and homogenized in sodium phosphate buffer (5 mL, pH 7.8). The homogenates were then centrifuged at 10,500g for 20 min and stored at 4 °C before analysis.
SOD activity was measured with the method proposed by Beauchamp and Fridovich (1971). A reaction mixture was prepared containing sodium phosphate buffer (1.5 mL, pH 7.8), 0.3 mL methionine, 0.3 mL nitroblue tetrazolium chloride, 0.3 mL EDTA-Na2, 0.3 mL riboflavin, 0.01 mL extract, and 0.28 mL deionized water. The reaction was initiated by placing the tubes under 215 W fluorescent lamps for 10 min. The reaction mixture which was not exposed to light used as control. The absorbance was recorded at 560 nm and SOD activity was expressed as U/mg FW.
>CAT activity was analyzed with the method proposed by Beers and Sizer (1952). A reaction mixture was prepared containing 0.3 mL of 0.1 M H2O2, 1.0 mL of distilled water, and 1.5 mL of 200 mM sodium phosphate buffer (pH 7.8). The reaction was started by adding 0.2 mL of extract. CAT activity was measured by measuring the absorbance at 240 nm and expressed as OD240 nm min/g.
Anatomical damage observations
Root tips were rinsed with dH2O distilled water for removing the residues on the surface. Then, cross sections were taken from the root tips and stained with methylene blue. Anatomical structures of each group were photographed at the × 500 magnification with the research microscope (Acar et al. 2015).
Statistical analysis
Statistical analyses were performed using the “IBM SPSS Statistics 22 SP” package program. Data were shown as mean ± SD. The statistical significance between the means was determined by one-way ANOVA and Duncan’s test, and p value < 0.05 was considered statistically significant.
Results and discussion
In this study, the toxic effects and anatomical changes induced by 1.0, 2.2, and 4.4 μM of DEP treatment in A. cepa were investigated. The effect of DEP treatment on root length, which is one of the physiological parameter tested in this study, is shown in Table 1. At the end of the application period, maximum root length was measured in the control group and the minimum root length was determined in group IV, which received 4.4 μM dose of DEP. There was an average reduction of 27.0% in root length of the group treated with 1.0 μM DEP compared with the control group. This reduction was 81.4% in the 4.4 μM DEP-treated group and severe inhibition was observed. These decreases in root length were found to be statistically significant (p < 0.05) in DEP treatment groups compared to control group.
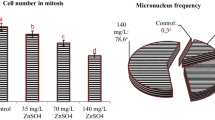
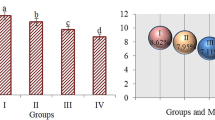
The effects of DEP exposure on germination rate, weight gain, and MI ratio are given in Table 2. While 100% germination was observed in the control group as expected, it was determined that this rate decreased with DEP application. The most significant decrease in germination percentage was observed in the group treated with 4.4 μM DEP and a 50.0% decrease was observed compared with the control group. Abnormalities observed in rooting and germination rates were reflected in weight gain and it was determined that DEP application decreased weight gain of bulbs. In group IV, the average weight gain decreased by 85.2% compared with the control group. In group II and group III, there were important decrease in weight gain as 40.3% and 62.9%, respectively. It was also found that these decreases were statistically significant (p < 0.05). Germination starting with root formation causes weight gain in the plants and these three parameters directly affect each other. This was confirmed by DEP application results and the reduction in root length reduced germination rate and led to a decrease in weight gain. MI provides information on the ratio of dividing cells in a tissue and is a good indicator of proliferation. While the number of dividing cells in the root tissue of the control group was 912.60 ± 23.86, it was determined that the division rate of the cells decreased with DEP application. After 4.4 μM DEP treatment, the number of dividing cells decreased to 548.50 ± 24.54. This decrease was also observed in the MI ratio and the MI ratio in group IV decreased from 9.12 to 5.48%. Applications of DEP have been reported to cause mitodepressive effect and the effect is associated with blocking of the mitosis in the interphase by inhibiting the normal development of the cells (Rijstenbil and Poortvliet 1992; Duan and Wang 1995). In the literature, it has been reported that di (2-ethylhexyl) phthalate exposure causes a decrease in mitotic rate and lymphotic mitotic inhibition (Turner et al. 1974; Stenchever et al. 1976). In plant physiology, root growth and germination are essential for the growth period of the plant. Root growth and elongation are dependent on mitotic velocity and changes in MI ratio directly affect growth. In this study, abnormalities observed in physiological parameters after DEP application can be explained by suppression of mitotic activity. There is no data in the literature regarding the effect of DEP on physiological parameters in plants, but parallel effects of similar chemicals are reported. Rank and Nielsen (2002) reported that 100 mg/L di(2-ethylhexyl) phthalate treatment reduced the MI rate from 40.3 ± 6.3 to 9.01 ± 1.2. In another study, Liu et al. (2014) reported that 50 and 100 mg/kg of phthalate ester treatment decreased the germination percentage in Phaseolus radiatus by 37.5% and 62.5%, respectively compared with the control group.
The frequencies of MN and CA induced by DEP are given in Table 3. In the control group, low levels of MN were observed at a rate of 0.20 ± 0.42, whereas in DEP-treated groups II, III, and IV, a total of MN frequencies as 7.20 ± 2.5, 22.20 ± 5.39, and 41.90 ± 3.78 were observed, respectively. It was determined that the MN frequency increased due to the increase in DEP concentration and the increase was statistically significant (p < 0.05). In addition to the frequency of MN detected in the study, MN size was evaluated as a separate parameter. MN size allows the toxic effect to be defined as either clastogenic or aneugenic. For this purpose, MN formation in DEP-treated cells was also examined according to their size and the frequency of MN caused by clastogenic effect was higher than that of aneugenic effect. Although small-sized MNs containing acentric chromosomal fragments or breaks in cells stimulated by clastogen, large-sized MNs containing full chromosomes appear in cells stimulated by aneugens. Compared with clastogens, aneugens cause more damage to mitotic spindles, resulting in a complete loss of chromosomes and large-scale formation of MN (Von Ledebur and Schmid 1973; Högstedt and Karlsson 1985). In this study, it was determined that DEP application caused both small-scale MN and large-scale MN formation and therefore showed both clastogenic and aneugenic effects (Fig. 1). While the fragment is the most common CA formation, the other observed damages are chromosome bridge, sticky chromosome, c-mitosis, unequal distribution of chromatin, reverse polarization, and spindle thread abnormality. Chromosomal abnormalities such as reverse polarization, unequal distribution of chromatin, and spindle abnormality observed in this study result from aneugenic effect. However, CA types such as fragments and bridges are mostly formed by clastogenic effects. The presence of fragments with a high frequency confirms the small-scale MN should be high and also in this study, MN frequency with small scale was found 2.4 times higher than large-sized MN in group IV. Similarly, Rank & Nielsen (2002) have found that 2dEHp, which has similar structure with DEP, causes total CAs formation in Allium cepa at a rate of 19.19% and fragment formation is higher than other abnormalities. They also reported that the observed chromosomal abnormalities were associated with both clastogenic and spindle yarn disorders.
The effects of DEP treatment on MDA, SOD, and CAT activities, the important indicators of oxidative stress, are shown in Table 4. It was determined that DEP treatment significantly increased MDA levels compared with the control group (p < 0.05) and the increase was dose dependent. While the mean MDA level in the control group was measured as 9.20 μmol/g, after 1.0 μM, 2.2 μM, and 4.4 μM DEP treatment, MDA levels were determined to be 17.30 μmol/g, 22.20 μmol/g, and 28.80 μmol/g, respectively. In group IV treated with 4.4 μM DEP, MDA increased by 213% compared with the control group. When SOD levels were examined, mean SOD activity in the control group was measured as 101.00 U/mg and it was found that the activity increased continuously with DEP application. The most significant increase was detected in the 4.4 μM DEP-treated group and the SOD level increased 115% compared with the control group. Similar to SOD activity, there was an increase in CAT activity against DEP application. In groups II, III, and IV treated with DEP, CAT activity increased 29.2%, 93.8%, and 173.9%, respectively, compared with the control group. Increased MDA activity against DEP exposure indicates oxidative damage formation in tissues. DEP administration may cause lipid peroxidation by causing stress in cells and increase MDA level. Against oxidative damage in a cell, cell defense systems are activated and in particular various antioxidant enzymes are induced. Increased SOD and CAT activities after DEP administration may be associated with the formation of an adaptive response and increased detoxification capacity (Liu et al. 2014). Stress conditions such as salinity, excessive temperature, or chemical exposure in plants can cause oxidative stress and ROS production. ROS production in plants under normal conditions is as low as 240 μM/s, while in the presence of stress ROS production level up to 720 μM/s. Throughout the evolution of plants, an enzymatic antioxidant defense system containing SOD, CAT, and APX was developed. In plants, the superoxide radical can be inactivated by SOD activity, then hydrogen peroxide and oxygen are released as a result of this reaction. Hydrogen peroxide damages proteins and DNA, causes lipid peroxidation, but these negative effects are eliminated by CAT activity (Bahmani et al. 2015). The increased activities of CAT and SOD observed in this study were evaluated as the tolerance mechanism developed against potential oxidative stress induced by DEP application. Similarly, Liu et al. (2014) reported that phthalate ester exposure caused changes in SOD and CAT activity in the model plant and these changes are associated with the adaptive response. In another study, Yu et al. (1999) reported significant alterations in SOD activity in Pagrosomus major exposed to hydrocarbon (Fig. 2).
As a result of the examination of the anatomical cross sections, it was observed that DEP application caused some structural changes and anatomical damages. After DEP application, structural changes such as accumulation of substance in epidermis cells and flattened cell nucleus were observed in stem tip cells. However, anatomical damages such as unclear vascular bundle, necrosis, deformation of cortex, and epidermis cell were observed especially at high doses of DEP at 4.4 mM. Plants develop specific structural and physiological modifications to cope with stress conditions. Epidermis is the first cell layer exposed to chemical exposure. The accumulation of the exposed chemical in the epidermal wall prevents the transport to other tissues and provides the development of tolerance and resistance (Bahmani et al. 2015). Therefore, accumulation of substance in epidermis cells observed after DEP application (Fig. 3c) is an anatomical adaptation to chemical exposure rather than damage. Necrosis which is observed more predominant in high DEP administration (Fig. 3f) is irreversible. Plants or plant cell cultures, when exposed to stress, initiate rapid cell death by necrotic morphology. Necrotic areas appear as brown spots. From these data, it was concluded that more anatomical changes were observed in low DEP applications and serious anatomic damages occurred in high DEP treatment. In the literature, Liu et al. (2004) examined the effects of toxic substance exposure on plant tissue and reported that anatomical changes such as cell wall thickening occur and this change is related to adaptation mechanism.
Conclusion
In this study, it was determined that DEP has a dose-dependent toxic effect on A. cepa test material. The highest toxic effect was obtained at a concentration of 4.4 mm, but it was observed that the toxicity increased with the dose; however, there was no direct proportional increase. In particular, anatomical changes such as deposition of substances in the epidermis and the induction of antioxidant enzymes prevent a proportional increase in toxicity. The plant continued to survive in the presence of DEP through these defense and tolerance mechanisms. As a result, diethyl phthalate, which is present in the structure of many products we use continuously in our daily lives, has been found to cause toxic effects in a eukaryotic model system at all tested doses. In such studies, it is especially important to clarify the mechanism of toxic effect. In this study, the toxic effect mechanism of DEP was investigated especially using MN size and both clastogenic and aneugenic effects were determined. This result shows that DEP damages mitotic spindle in cells and causes fragments. Considering the role of mitotic spindle damages in eukaryotic systems involving animals and humans, it can be said that DEP can cause mitotic abnormalities in eukaryotes. Therefore, the use of DEP should be limited, and when it is essential, dose levels that do not cause toxic effects in living things should be preferred.
References
Acar A, Çavuşoğlu K, Türkmen Z, Çavuşoğlu K, Yalçın E (2015) The investigation of genotoxic, physiological and anatomical effects of paraquat herbicide on Allium cepa L. Cytologia 80(3):343–351
Agarwal DK, Lawrence WH, Nunez LJ, Autian J (1985) Mutagenicity evaluation of phthalic acid esters and metabolites in Salmonella typhimurium cultures. J Toxicol Environ Health 16(1):61–69
Anderson D, Yu TW, Hinçal F (1999) Effect of some phthalate esters in human cells in the Comet assay. Teratog Carcinog Mutagen 19:275–280
Andrady A, Neal M (2009) Applications and societal benefits of plastics. Philos Trans R Soc Lond 364:1526
Anonymous (1985) Final report on the safety assessment of dibutylphthalate, dimethylphthalate, and diethylphthalate. J Am Coll Toxicol 4(3):267–303
Astill B, Barber E, Lington A, Moran E, Mulholland A, Robinson E, Scheider B (1986) Chemical industry voluntary test program for phthalate esters: health effects studies. Environ Health Perspect 65:329–336
Bahmani K, Noori SAS, Darbandi AI, Akbari A (2015) Molecular mechanisms of plant salinity tolerance: a review. Aust J Crop Sci 9(4):321
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Beers RF, Sizer IW (1952) Colorimetric method for estimation of catalase. J Biol Chem 195:133–139
Benjamin S, Pradeep S, Josh MS, Kumar S, Masai E (2015) A monograph on the remediation of hazardous phthalates. J Hazard Mater 298:58–72
Cartwright CD, Owen SA, Thompson IP, Burns RG (2000) Biodegradation of diethyl phthalate in soil by a novel pathway. FEMS Microbiol Lett 186(1):27–34
Duan CQ, Wang HX (1995) Cytogenetical toxical effects of heavy metals on Vicia faba, and inquires into the Vicia micronucleus. Acta Bot Sin 37:14–24
Högstedt B, Karlsson A (1985) The size of micronuclei in human lymphocytes varies according to inducing agent used. Mutat Res 156:229–232
Kamrin MA, Mayor GH (1991) Diethyl phthalate—a perspective. J Clin Pharmacol 31(5):484–489
Lee KH, Lee BM (2007) Study of mutagenicities of phthalic acid and terephthalic acid using in vitro and in vivo genotoxicity tests. J Toxicol Environ Health A 70:1329–1335
Liu W, Zhang C, Liu S (2014) Effects of phthalate ester treatment on seed germination and antioxidant enzyme activities of Phaseolus radiatus L. B Environ Contam Tox 92(5):621–624
Liu H, Liao B, Lu S (2004) Toxicity of surfactant, acid rain and Cd2+ combined pollution to the nucleus of Vicia faba root tip cells. Chin J Appl Ecol 15:493–496
Meek ME, Chan PKL (1994) Bis(2-ethylhexyl)phthalate: evaluation of risks to health from environmental exposure in Canada. Environ Carcinog Rev 12:179–194
Mettang T, Alscher DM, Pauli-Magnus C, Dunst R, Kuhlmann U, Rettenmeier AW (1999) Phthalic acid is the main metabolite of the plasticizer di(2-ethylhexyl) phthalate in peritoneal dialysis patients. Adv Perit Dial 15:229–233
Nässberger L, Arbin A, Ostelius J (1987) Exposure of patients to phthalates from polyvinyl chloride tubes and bags during dialysis. Nephron 45:286–290
Peakall DB (1975) Phthalate esters: occurrence and biological effects. Residue Rev 54:1–41
Rank J, Nielsen MH (1994) Evaluation of the Allium anaphase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat Res 312:17–24
Rank JL, Nielsen MH (2002) Genotoxicity of maleic hydrazide, acridine and DEHP in Allium cepa root cells performed by two different laboratories. Hereditas 135:13
Rijstenbil JW, Poortvliet TCW (1992) Copper and zinc in estuarine water: chemical speciation in relation to bioavailability to the marine planktonic diatom Ditylum brightwellii. Environ Toxicol Chem 11:1615–1625
Stenchever MA, Allen MA, Jerominski L, Petersen RV (1976) Effects of bis(2-ethylhexyl) phthalate on chromosomes of human leukocytes and human fetal lung cells. J Pharm Sci 65:1648–1651
Turner JH, Petricciani JC, Crouch ML, Wenger S (1974) An evaluation of the effects of diethylhexyl phthalate (DEHP) on mitotically capable cells in blood packs. Transfusion 14:560–566
Unyayar S, Celik A, Cekic FO, Gozel A (2006) Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 21:77–81
Vicentini VEP, Camparoto ML, Teixeira RO, Mantovani MS (2001) Averrhoa carambola L., Syzygium cumini (L.) Skeels and Cissus sicyoides L.: medicinal herbal tea effects on vegetal and test systems. Acta Sci 23:593–598
Von Ledebur MM, Schmid W (1973) The micronucleus test: methodological aspects. Mutat Res 19:109–117
Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K (2006) What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 26:803–824
Yalçın E, Uzun A, Çavusoglu K (2019) In vivo epiclorohidrine toxicity: cytogenetic, biochemical, physiological and anatomical evidences. Environ Sci Pollut R 26:22400–22406
Yu Q, Zheng WY, Weng Y (1999) Effect of petroleum pollutant on SOD and CAT enzyme in viscera tissue of Pagrosomus major. J Xiamen Univ 38:429–434
Acknowledgments
The authors would like to thank the Giresun University Scientific Research Department (FEN-BAP-C-220413-19, FEN-BAP-C-200515-11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demirtaş, G., Çavuşoğlu, K. & Yalçin, E. Aneugenic, clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ Sci Pollut Res 27, 5503–5510 (2020). https://doi.org/10.1007/s11356-019-07339-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07339-5