Abstract
In yeast, the STB5 gene encodes a transcriptional factor belonging to binuclear cluster class (Zn2Cys6) of transcriptional regulators specific to ascomycetes. In this study, we prepared the Kluyveromyces lactis stb5Δ strain and assessed its responses to different stresses. We showed that KlSTB5 gene is able to complement the deficiencies of Saccharomyces cerevisiae stb5Δ mutant. The results of phenotypic analysis suggested that KlSTB5 gene deletion did not sensitize K. lactis cells to oxidative stress inducing compounds but led to Klstb5Δ resistance to 4-nitroquinoline-N-oxide and hygromycin B. Expression analysis indicated that the loss of KlSTB5 gene function induced the transcription of drug efflux pump encoding genes that might contribute to increased 4-nitroquinoline-N-oxide and hygromycin B tolerance. Our results show that KlStb5p functions as negative regulator of some ABC transporter genes in K. lactis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kluyveromyces lactis, the yeast species with predominantly respiratory metabolism, represents an alternative model to the commonly used Saccharomyces cerevisiae for studies related to carbon and oxygen metabolism. While these two yeast species are phylogenetically close to each other, they display some important differences. K. lactis is a Crabtree-negative yeast with a respiratory-fermentative lifestyle, respiration being regulated by oxygen availability. The Crabtree-positive S. cerevisiae is adapted to high-sugar supply in its natural environment, performs aerobic fermentation, and compensates the low-energy yield by a high-glycolytic flux. The glucose is metabolized through glycolysis in S. cerevisiae; in K. lactis, it is mainly channeled into the pentose phosphate pathway (PPP). K. lactis is able to utilize xylose, xylitol, cellobiose, arabinose, and lactose. The important advantage over other types of yeast is the ability of K. lactis to grow on whey, which has economic and ecological benefits. With its potential to secrete large proteins and rapidly achieve high-culture densities, K. lactis has been used to produce proteins on an industrial scale in the food and feed industry. K. lactis unlike S. cerevisiae has not undergone whole genome duplication (WGD), the redundancy of genes is, therefore, lower (Bussereau et al. 2006; González-Siso et al. 2009; Rodicio and Heinisch 2013). Previous studies have shown that most genes are conserved in these yeast species. The different physiological responses result from modified regulatory interactions (Breunig et al. 2000; Mehlgarten et al. 2015).
The most important class of yeast transcriptional regulators is composed of proteins bearing the Zn(II)2Cys6 zinc cluster (MacPherson et al. 2006; Clarke et al. 2013). These proteins display variable secondary structures and enormous functional diversity. They are involved in the regulation of numerous processes such as the control of the metabolism of sugars, amino acids, fatty acids, as well as drug resistance. Different combinations of transcription factors (forming homodimers or heterodimers) may differentially regulate the expression of their target genes. The genome of S. cerevisiae encodes 56 proteins in this category, and 58 putative Zn(II)2Cys6 proteins were detected in the genome of K. lactis (Bussereau et al. 2006). However, in K. lactis, only a handful of them have been characterized.
In our previous work, we have characterized the master regulator of drug resistance in K. lactis, the KlPdr1p (Balkova et al. 2009). In S. cerevisiae, Pdr1p has been shown to form a heterodimer with another transcription factor, Sin3 binding protein 5 (ScStb5p) (Akache et al. 2004). Studies in S. cerevisiae found that ScStb5p is a Zn(II)2Cys6 transcription factor (Kasten and Stillman 1997; Akache et al. 2001) which regulates genes involved in the oxidative stress response by increasing the supply of NADPH through the pentose phosphate pathway (Larochelle et al. 2006). The absence of ScStb5p results in a growth defect, sensitivity to cold (below 20 °C), and susceptibility to various drugs (Akache et al. 2001; Larochelle et al. 2006; Matsufuji et al. 2010).
Here, we investigated the role of the protein encoded by the open reading frame (ORF) KLLA0A09251g and show that it is a K. lactis orthologue of ScStb5p. Deletion or overexpression of KlSTB5 had substantial effect on K. lactis drug susceptibility. We also showed that the KlSTB5 gene can complement the defects in the S. cerevisiae stb5Δ mutant strain and acts as a transcriptional regulator of several genes implicated in K. lactis drug resistance.
Materials and methods
Strains and culture conditions
The following yeast haploid strains were used: K. lactis CBS2359ku80 (MATα ku80::loxP; Kooistra et al. 2004) and S. cerevisiae BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) as parental strains and their isogenic stb5Δ deletion mutants. Cells were grown in liquid glucose-rich (YPD) medium (2% glucose, 1% yeast extract, 2% Bacto peptone), or minimal (YNB) medium containing 0.67% yeast nitrogen base without amino acids and 2% glucose, supplemented with the required amino acids and/or bases at 28 °C to a density of 1.0 × 108 cells/mL. Phenotypic analyses were performed on YP or YNB medium containing 2% glucose (w/v), 2% glycerol, or 2% lactate as carbon source in serial tenfold dilutions. The media were solidified with 2% Bacto agar. YP and YNB plates were incubated at 28 °C for 2 and 4 days respectively. For drug susceptibility testing, yeast cell suspensions were prepared from mid-exponential cell cultures grown in YNB, diluted to 1.0 × 107 cells/mL. Five-microliter aliquots from tenfold dilutions were spotted onto YPD media containing respective drugs. Drugs were applied at the concentrations indicated in the figures. The Escherichia coli DH5α strain was used as a host for transformation, plasmid amplification, and preparation. Bacteria were grown at 37 °C in Luria-Bertani (LB) medium (1% tryptone, 1% NaCl, 0.5% yeast extract, and pH 7.6) supplemented with 100 μg/mL ampicillin for the selection of transformants.
Cloning of the KlSTB5 gene
Genomic DNA from K. lactis CBS2359ku80 was extracted and used as a template for the amplification of KlSTB5 gene. PCR was carried out with a high-fidelity DNA polymerase (ThermoFisher Scientific, Frankfurt am Main, Germany) using forward and reverse primers (Table 1) and generated 4 kb fragment. It was then ligated into the BamHI-XhoI digested pRS306K (URA3 ARS1 KARS2 ori AmpR) vector, obtained from J.J. Heus (Leiden University, Leiden, The Netherlands). The pRS306K-KlSTB5 plasmid amplified in E. coli DH5α was verified by restriction analysis and sequencing with automated DNA sequences (ABI Prism 3100, Applied Biosystems, Foster City, USA).
Construction of the K. lactis deletion strain
The chromosomal KlSTB5 gene was deleted by one-step gene replacement procedure (Rothstein 1983). Plasmid pUG6 (Güldener et al. 1996) was used as template for amplification of the kanMX cassette using primers listed in Table 1. The 2 kb fragment obtained containing 49/43 bp target homologous flanking sequences of the KlSTB5 gene was used to transform K. lactis CBS2359ku80 cells. The KlSTB5 gene deletion was verified by PCR.
Genetic manipulations, transformations, and DNA preparations
Standard protocols for generating recombinant DNAs, the restriction enzyme analyses, gel electrophoresis, and hybridization were used (Sambrook et al. 1989). Plasmid DNA from E. coli was prepared by the alkaline lysis method. K. lactis strains were transformed by electroporation (Sánchez et al. 1993) using Bio-Rad gene pulser at 1.0 kV, 25 μF, and 400 Ω in 0.2-cm cuvettes. S. cerevisiae transformation was carried out using the modified lithium acetate protocol (Thompson et al. 1998).
Quantitative real-time PCR
Yeast cells were grown in minimal medium containing 2% glucose to the mid-logarithmic phase. Total RNA was isolated by the hot acidic phenol extraction method (Ausubel et al. 1989). First-strand cDNA was synthetized from 1 μg of total RNA using 200 U of the Revert AID™H Minus M-MuLV Reverse transcriptase (ThermoFisher Scientific, Frankfurt am Main, Germany). Quantitative real-time PCRs were performed in triplicate using the Applied Biosystems 7900 HT fast real-time PCR system. Independent PCRs were performed using the same cDNA for both the gene of interest and the KlACT1 gene, using the HOT FIREPol® EvaGreen® qPCR Mix Plus (ROX) (Solis BioDyne, Tartu, Estonia). The KlACT1 mRNA level was used as the internal control. The relative value obtained for each target gene in the wild type was set as 1 and the remaining values are relative to that value.
β-glucuronidase reporter assay
K. lactis cells transformed with the PKlPDR5-gusA chimeric construct were grown in minimal liquid medium to the mid-logarithmic phase and harvested by centrifugation for 5 min at 3000g at 4 °C. Pellet was washed with β-glucuronidase extraction buffer (10 mM sodium phosphate pH 7.0, 10 mM Na2EDTA, 10 mmol/L dithiothreitol, 0.1% sodium lauryl sarcosine, 0.1% Triton X-100) and resuspended in 500 μL of the same buffer. An equal volume of ice-chilled, acid-washed glass beads (0.45 mm diameter) was added and the cells were homogenized ten times for 1 min at 4 °C. After 10-min centrifugation at 10,000g at 4 °C obtained crude extracts were used for the measurement of β-glucuronidase activity. It was determined at 30 °C in the extraction buffer containing 1 mmol/L p-nitrophenyl-β-D-glucopyranoside (PNPG) and the reaction was followed spectrophotometrically. The specific activity was normalized to the amounts of protein determined according to Bradford (1976).
Results
Phenotypic analysis of S. cerevisiae and K. lactis lacking the KlSTB5 gene
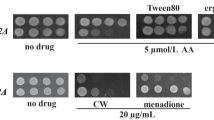
To analyze whether the KlStb5p is required for growth on non-fermentable carbon sources, the K. lactis STB5 gene was disrupted in the wild type and phenotypically analyzed by spotting serial dilutions on different carbon sources and compared to those of the respective S. cerevisiae null mutant. The phenotypes of S. cerevisiae and K. lactis wild type and the stb5Δ deletion mutants were compared on minimal and rich media supplemented either with fermentative or respiratory carbon sources. The S. cerevisiae stb5Δ strain failed to grow on the minimal medium with 2% glycerol or 2% lactate. However, the growth of K. lactis strain lacking KlSTB5 was not impaired on any of these media. Introducing the heterologous KlSTB5 gene into Scstb5Δ mutant cells recovered Scstb5Δ mutant growth to wild-type levels in both glycerol and lactate containing media (Fig. 1a). Previous studies showed that the deletion of ScSTB5 results in sensitivity to cold (20 °C) (Akache et al. 2001; Noble et al. 2013). Our results (Fig. 1b) showed that the overexpression of KlSTB5 gene in Scstb5Δ mutant strain restored its growth at low temperature. On the other hand, the phenotype of Klstb5Δ mutant contrasts with that of the Scstb5Δ mutant as no discernible effect regarding carbon source and decreased temperature was detected.
Complementation assays. KlSTB5 from K. lactis was expressed in S. cerevisiae Scstb5Δ mutant strain. a Growth of S. cerevisiae and K. lactis cells on YNB medium with 2% glucose, 2% glycerol, and 2% lactate; b growth of S. cerevisiae and K. lactis cells on YPD agar plates at 20 and 28 °C respectively. Each spot represents 5 μL of the tenfold cell suspension dilution series. The initial cell concentration was 1 × 107cells/mL
Studies in S. cerevisiae characterized Stb5p as a regulator of oxidative stress response and drug resistance (Larochelle et al. 2006). To analyze whether the KlSTB5 deletion also results in sensitivity to drugs, we compared the growth of Scstb5Δ and Klstb5Δ deletion mutants in the presence of various drugs. Figure 2 demonstrates that Scstb5Δ null mutant is susceptible to 4-nitroquinoline-N-oxide, hygromycin B, caffeine, hydrogen peroxide, and antifungal azoles (itraconazole and miconazole) in comparison to wild-type strain. Overexpression of the KlSTB5 gene in the Scstb5Δ mutant restored the wild-type phenotype. Contrary to S. cerevisiae, the Klstb5Δ strain was able to grow comparably to the wild-type in the presence of 4-nitroquinoline-N-oxide, hygromycin B, caffeine, hydrogen peroxide, itraconazole, and miconazole. The presence of KlSTB5 gene in multiple copies impaired the growth of Klstb5Δ transformant in the presence of 4-nitroquinoline-N-oxide and hygromycin B (Fig. 2).
KlPDR5, KlSNQ2, and KlERG6 genes transcription is upregulated in response to drugs in Klstb5Δ mutant
To identify genes that require the presence of KlStb5p for their expression, mRNA levels of selected genes were compared in K. lactis wild-type and Klstb5Δ mutant. The selected genes listed in Table 2 encode transcription factors (KlPDR1, KlYAP1), genes involved in lipid metabolism (KlPDR16, KlERG6), major facilitator superfamily transporters (KNQ1, KlTPO1), ABC transporters (KlPDR5, KlSNQ2, KlPDR12), and plasma membrane H+-ATPase (KlPMA1). As Fig. 3a shows, there was no significant difference in transcript levels in the Klstb5Δ in comparison with the wild type. However, after exposition of log-phase wild-type and Klstb5Δ cell cultures to 1 mM hydrogen peroxide for 60 min, induced transcription of KlERG6 and KlSNQ2 genes was observed in Klstb5Δ mutant (Fig. 3b). KlERG6 gene encoding S-adenosine dependent sterol C-24 methyltransferase involved in the ergosterol biosynthetic pathway was induced about 2.5-fold and KlSNQ2 encoding ABC transporter involved in multidrug resistance about twofold in the Klstb5Δ mutant cells. Transcription of KNQ1, KlTPO1, and KlPDR5 genes was stimulated to a lesser extent in response to hydrogen peroxide in the Klstb5Δ mutant (Fig. 3b).
mRNA levels from the selected K. lactis genes by qRT-PCR. a Gene transcription levels in Klstb5Δ deletion mutant compared with those in the wild-type strain. b Relative gene transcription levels in Klstb5Δ mutant cells grown in the presence of 1 mM hydrogen peroxide for 60 min. c Relative gene transcription levels of Klstb5Δ mutant cells grown in the presence of itraconazole (0.001 μg/mL) for 60 min. d Relative gene transcription levels in Klstb5Δ mutant cells grown in the presence of 4-nitroquinoline-N-oxide (0.25 μg/mL) for 60 min. The gene transcript levels in parental strain were set as 1. Data represent mean values of three measurements performed on two independent biological samples. Error bars indicate the standard deviation (SD). Note: systematic names of the K. lactis genes of S. cerevisiae othologues: KLLA0A09119g/PDR1; KLLA0A01760g/YAP1; KLLA0D19679g/PDR16; KLLA0A01738g/ ERG6; KLLA0C18931g/ KNQ1; KLLA0F18106g/ TPO1; KLLA0F21692g/ PDR5; KLLA0D03432g/ SNQ2; KLLA0B09702g/ PDR12; KLLA0A09031g/ PMA1
To identify genes that are regulated by KlStb5p under azole stress, we compared the expression of selected genes after exposition of log-phase wild-type and Klstb5Δ cell cultures to 0.001 μg/mL itraconazole for 60 min. Upon itraconazole exposure, the genes encoding transcription factors KlPDR1 and KlYAP1 were significantly upregulated in the Klstb5Δ mutant (Fig. 3c). Transcription of genes encoding MDR transporters KlPDR5, KlSNQ2, KlTPO1, and also the KlPMA1 was stimulated as a result of KlSTB5 gene deletion. As the KlPDR5 gene promoter contains the PDRE elements to which KlStb5p apparently binds, we analyzed the influence of the Klstb5Δ mutation on KlPDR5 promoter activity. KlPDR5 promoter region was fused to the β-glucuronidase gusA reporter gene (PKlPDR5—gusA) and the construct was introduced into the wild-type and Klstb5Δ mutant cells. GUS expression in the wild-type strain and Klstb5Δ transformants was determined. Itraconazole induced the activity of β-glucuronidase both in wild-type and in Klstb5Δ mutant (Table 3). Increased activity of the reporter construct in the Klstb5Δ mutant thus corroborates the results of the qRT-PCR analysis showing increased transcript level of KlPDR5 gene. Itraconazole strongly induced the transcription of KlSNQ2 gene (about sixfold) in the Klstb5Δ mutant cells that correlates with the observed resistance of Klstb5Δ mutant to 4-nitroquinoline-N-oxide in phenotypic assays. The transcription of KlERG6 gene was induced about threefold in the Klstb5Δ mutant compared to the wild-type (Fig. 3c). Levels of mRNA from selected genes (Table 2) were compared also after exposition of log-phase wild-type and Klstb5Δ cell cultures to 0.25 μg/mL 4-nitroquinoline-N-oxide for 60 min. The transcription of genes encoding MDR transporters KlPDR5 and KlSNQ2 genes was markedly stimulated as a result of KlSTB5 gene deletion upon 4-nitroquinoline-N-oxide exposure. Enhanced transcription of the KlERG6 gene was also observed in the Klstb5Δ mutant (Fig. 3d). Collectively, our results suggest a negative role of KlStb5p in the transcription of KlPDR5, KlSNQ2, and KlERG6 genes in the presence of various drugs.
Discussion
KlSTB5 gene (KLLA0A09251g) encodes a binuclear zinc cluster protein conserved among hemiascomycete yeast (Bussereau et al. 2006). The K. lactis protein shares 50% identity and 66% similarity with its S. cerevisiae counterpart. Previous studies in S. cerevisiae showed that ScStb5p activates the expression of most genes of the pentose phosphate pathway and other genes involved in the production of NADPH, a cofactor in conferring resistance to oxidative stress (Akache et al. 2004; Larochelle et al. 2006; Cadiere et al. 2010). The ScStb5p forms heterodimers with ScPdr1p and regulates the expression of different genes involved in S. cerevisiae drug resistance (Akache and Turcotte 2002).
In this work, we have initiated a comparative analysis of Stb5p function in the Crabtree-positive S. cerevisiae and the distantly related Crabtree-negative K. lactis. We demonstrate that the K. lactis ortholog of ScSTB5 gene is able to compensate the loss of ScSTB5 and enables the Scstb5Δ mutant strain to grow on non-fermentable carbon sources, at low temperature, and in the presence of various drugs. In contrast to S. cerevisiae, K. lactis does not require Stb5p for growth on non-fermentable carbon sources. As the non-fermentable substrates provide yeast with energy produced via aerobic respiration, the observed differences could result from the differences in primary carbon metabolism in S. cerevisiae and K. lactis.
In contrast to S. cerevisiae, K. lactis is characterized by a higher glucose flow through the pentose phosphate pathway than through glycolysis and as a consequence by a higher production of NADPH in the cytosol (González-Siso et al. 2009; Rodicio and Heinisch 2013). The production of NADPH by the glucose-6-phosphate dehydrogenase reaction is a major factor contributing to the detoxification of ROS in K. lactis and its tolerance to oxidative stress. KlStb5p does not appear, however, to regulate genes of the pentose phosphate pathway which is in agreement with the data obtained for CgSTB5 in C. glabrata (Klimova et al. 2014). To explore the role of KlStb5p in K. lactis we prepared the Klstb5Δ deletion mutant and compared its ability to grow in the presence of various oxidants and drugs. Although we could not detect any marked difference in growth between wild-type and that of the Klstb5Δ deletion mutant in the presence of 4-nitroquinoline-N-oxide, hygromycin B, hydrogen peroxide, and antifungal azoles, the overexpression of KlSTB5 conferred susceptibility to 4-nitroquinoline-N-oxide in K. lactis. This observation correlates with the upregulated KlSNQ2 gene transcription in the Klstb5Δ mutant, pointing to the fact that KlStb5p could be a negative regulator of KlSNQ2 expression. Besides this, the KlSTB5 gene deletion also positively affects the transcription of genes encoding transcription factors KlPDR1 (master regulator of multidrug resistance), KlYAP1 (involved in oxidative stress response), KlPDR5 (main drug efflux pump), and KlERG6 (involved in ergosterol biosynthesis). K. lactis belongs to the species placed before the whole genome duplication proposed by Wolfe and Shields (1997). Most of the genes duplicated in S. cerevisiae are single in K. lactis. Inspection of the phylogenetic relationship of multidrug resistance-related ATP-binding cassette transporters in five yeast species (Gbelska et al. 2006) revealed that the K. lactis ORF KLLA0D03432g is a homolog of two closely related proteins from S. cerevisiae called ScSnq2p and ScPdr18p. Coordinated activation of transcription of ScPDR18 and several ergosterol biosynthetic genes (ERG2–4, ERG6, ERG24) was observed in stressed S. cerevisiae cells (Godinho et al. 2018). Thus, we suppose that the K. lactis ORF KLLA0D03432g represents a blend of the properties of both of its S. cerevisiae orthologs, ScSNQ2 and ScPDR18.
Based on our results, we propose that there is a shared regulon between KlPDR1 and KlSTB5 in K. lactis. The genes upregulated by overexpression of KlPDR1 are also upregulated in the absence of KlSTB5. KlStb5p, thus, functions as a negative regulator of genes involved in K. lactis multidrug resistance (KlSNQ2, KlPDR5). Similar conclusion was found in the work of Noble et al. (2013), who showed that CgSTB5 gene encodes a transcriptional repressor of several genes implicated in azole resistance in C. glabrata.
Financial information
We gratefully acknowledge financial support from the Slovak Grant Agency of Science (Grant No. VEGA 2/0111/15 and VEGA 1/0697/18), and Comenius University (Grant No. UK/50/2018). This contribution is the result of the project implementation (ITMS 26240120027) supported by the OPRaD funded by the ERDF.
References
Akache B, Turcotte B (2002) New regulators of drug sensitivity in the family of yeast zinc cluster proteins. J Biol Chem 277:21254–21260
Akache B, Wu K, Turcotte B (2001) Phenotypic analysis of genes encoding yeast zinc cluster proteins. Nucleic Acids Res 29:2181–2190
Akache B, MacPherson S, Sylvain MA, Turcotte B (2004) Complex interplay among regulators of drug resistance genes in Saccharomyces cerevisiae. J Biol Chem 279:27855–27860
Ausubel FM, Brent R, Kingston RE et al (1989) Current protocols in molecular biology. Willey, New York
Balkova K, Sarinova M, Hodurova Z, Goffrini P, Gbelska Y (2009) Functional analysis of the Kluyveromyces lactis PDR1 gene. FEMS Yeast Res 9:321–327
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breunig KD, Bolotin-Fukuhara M, Bianchi MM et al (2000) Regulation of primary carbon metabolism in Kluyveromyces lactis. Enzym Microb Technol 26:771–780
Bussereau F, Casaregola S, Lafay JF, Bolotin-Fukuhara M (2006) The Kluyveromyces lactis repertoire of transcriptional regulators. FEMS Yeast Res 6:325–335
Cadiere A, Galeote V, Dequin S (2010) The Saccharomyce cerevisiae zinc factor protein Stb5p is required as a basal regulator of the pentose phosphate pathway. FEMS Yeast Res 10:819–827
Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Bürglin TR, Frech C, Turcotte B, Kopec KO, Synnott JM, Choo C, Paponov I, Finkler A, Heng Tan C, Hutchins AP, Weinmeier T, Rattei T, Chu JSC, Gimenez G, Irimia M, Rigden DJ, Fitzpatrick DA, Lorenzo-Morales J, Bateman A, Chiu CH, Tang P, Hegemann P, Fromm H, Raoult D, Greub G, Miranda-Saavedra D, Chen N, Nash P, Ginger ML, Horn M, Schaap P, Caler L, Loftus BJ (2013) Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol 14:R11
Gbelska Y, Krijger JJ, Breunig KD (2006) Evolution of gene families: the multidrug resistance transporter genes in five related yeast species. FEMS Yeast Res 6:345–355
Godinho CP, Prata CS, Pinto SN, Cardoso C, Bandarra NM, Fernandes F, Sá-Correia I (2018) Pdr18 is involved in yeast response to acetic acid stress counteracting the decrease of plasma membrane ergosterol content and order. Sci Rep 8:7860
González-Siso MI, Garcia-LeiroA TN, Cerdán ME (2009) Sugar metabolism, redox balance and oxidative stress response in the respiratory yeast Kluyveromyces lactis. Microb Cell Factories 8:46
Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 4:2519–2524
Kasten MM, Stillman DJ (1997) Identification of the Saccharomyces cerevisiae genes STB1-STB5 encoding Sin3p binding proteins. Mol Gen Genet 256:376–386
Klimova N, Yeung R, Kachurina N, Turcotte B (2014) Phenotypic analysis of a family of Trancriptional regulators, the zinc cluster proteins, in the human fungal pathogen Candida glabrata. G3 4:931–940
Kooistra R, Hooykaas PJ, Steensma HY (2004) Efficient gene targeting in Kluyveromyces lactis. Yeast 21:781–792
Larochelle M, Drouin S, Robert F, Turcotte B (2006) Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol Cell Biol 26:6690–6701
MacPherson S, Larochelle M, Turcotte B (2006) A fungal family of trancription regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70:583–604
Matsufuji Y, Nakagawa T, Fujimura S, Tani A, Nakagawa J (2010) Transcription factor Stb5p is essential for acetaldehyde tolerance in Saccharomyces cerevisiae. J Basic Microbiol 50:494–498
Mehlgarten C, Krijger JJ, Lemnian I, Gohr A, Kasper L, Diesing AK, Grosse I, Breunig KD (2015) Divergent evolution of the transcriptional network controlled by Snf1-interacting protein Sip4 in budding yeasts. PLoS One 10:e0139464
Noble JA, Tsai HF, Suffis SD, Su Q, Myers TG, Bennett JE (2013) STB5 is a negative regulator of azole resistance in Candida glabrata. Antimicrob Agents Chemother 57:959–967
Rodicio R, Heinisch JJ (2013) Yeast on the milky way: genetics, physiology and biotechnology of Kluyveromyces lactis. Yeast 30:165–177
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101:202–211
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sánchez M, Iglesias FJ, Santamarıa C et al (1993) Transformation of Kluyveromyces lactis by electroporation. Appl Environ Microbiol 59:2087–2092
Thompson JR, Register E, Curotto J, Kurtz M, Kelly R (1998) An improved protocol for the preparation of yeast cells for transformation by electroporation. Yeast 14:565–571
Wolfe KH, Shields DC (1997) Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387:708–713
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Bencova, A., Konecna, A., Toth Hervay, N. et al. Stb5p is involved in Kluyveromyces lactis response to 4-nitroquinoline-N-oxide stress. Folia Microbiol 64, 579–586 (2019). https://doi.org/10.1007/s12223-019-00682-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00682-7