Abstract
During fermentation, yeast cells undergo various stresses that inhibit cell growth and ethanol production. Therefore, the ability to tolerate multiple stresses during fermentation is one of the important characteristics for yeast cells that can be used for commercial ethanol production. In the present study, we evaluated the multi-stress tolerance of parent and ethanol adapted Kluyveromyces marxianus MTCC1389 and their relative gene expression analysis. Multi-stress tolerance was confirmed by determining its cell viability, growth, and spot assay under oxidative, osmotic, thermal, and ethanol stress. During oxidative (0.8% H2O2) and osmotic stress (2 M NaCl), there was significant cell viability of 90% and 50%, respectively, by adapted strain. On the other hand, under 45 °C of thermal stress, the adapted strain was 80% viable while the parent strain was 60%. In gene expression analysis, the ethanol stress responsive gene ETP1 was significantly upregulated by 3.5 folds, the osmotic stress gene SLN1 was expressed by 3 folds, and the thermal stress responsive gene MSN2 was expressed by 7 folds. This study shows adaptive evolution for ethanol stress can develop other stress tolerances by changing relative gene expression of osmotic, oxidative, and thermal stress responsive genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During fermentation, yeast cells encounter various stresses such as osmotic, oxidative, thermal, and ethanol (end-product), which inhibit cell growth and lead to low production. For economical ethanol production, yeast cells should be able to resist fermentation stresses for the utmost ethanol titer and cell viability. Therefore, a strain with improved multi-stress tolerance is a prerequisite for commercial application.
K. marxianus is a cell factory with several advantages, including thermotolerance up to 52 °C, rapid doubling time, and the ability to utilise several agricultural wastes containing sugars not used by traditional yeast, Saccharomyces cerevisiae [1]. Using non-conventional yeast with numerous benefits for utilising agricultural waste is a lucrative option which makes it a more suitable candidate for commercial application. However, K. marxianus is less explored yeast compared to S. cerevisiae due to low ethanol production, the major bottleneck for using non-conventional yeast. Therefore, a previous study was conducted using K. marxianus MTCC1389 by Pal et al. [2], in which it was adapted to improve its ethanol tolerance from 8 to 12% (v/v) ethanol stress in 110 days that significantly increased its tolerance to ethanol and its production. The ethanol titer was 7.9% (v/v) by the parent strain, while the adapted strain was able to produce 11.5%. Though, each transfer for adaptation process improves strain tolerance not only for desirable trait, also for other fermentation stress that improves yeast cell’s overall stress resistance [3]. Also, fermentation stress, such as osmotic, oxidative, thermal, and ethanol stress in yeast, may share certain common anti-stress pathways according to the stress cross-tolerance phenomenon. Therefore, genes responsible for providing tolerance for a single stress also change in expression during other stresses [4, 5].
Few studies have been conducted to examine the multi-stress tolerance of a strain after adaptation to a single stress. Caspeta and Nielsen [6] reported that adaptive evolution of S. cerevisiae for thermotolerance improved its other stress tolerance that affects the cell during fermentation. Kitichantaropas et al. [7] reported that isolated thermotolerant S. cerevisiae C3253, C3752, and C4377 strains were resistant to multiple stresses (ethanol, heat, oxidative, and osmotic stress) with significant higher growth rate compared to control. Mo et al. [4] studied the multi-stress tolerance of K. marxianus FIM1 after adaptation for high ethanol tolerance (6% to 10% v/v), and found that the strain became tolerant to oxidative, osmotic, thermal, and ethanol stress, confirmed by cell viability assay. Zhang et al. [8] conducted a study using evolutionary adaptation of S. cerevisiae strain YF10-5 in which the resistance to osmotic stress and end-product inhibition (ethanol) was found to improve after 10 rounds of freeze thaw treatment by liquid nitrogen, and significantly improved ethanol titer by 16%.
Li et al. [9] reported that P. kudriavzevii was exposed to salt stress that improved its stress tolerance for osmotic, thermal, and high sugar and its 3.9-times bioethanol production by cross protection. Pattanakittivorakul et al. [1] reported the adaptation of K. marxianus DMKU 3-1042 for improving thermotolerance after adaptation of a strain that was able to produce ethanol at a high temperature and enhanced its resistance to acetic acid and formic acid. To date, there are few studies investigating the multi-stress tolerance of K. marxianus after adaptation for a desirable trait. Therefore, in the present study, we aimed to analyse pre-adaptation to multi-stress (such as osmotic, oxidative, thermal, and ethanol) with adapted K. marxianus MTCC1389 and its parent strain by determining their viability of cell, growth curve, and spot assay under multi-stress conditions and differential gene expression analysis of stress related genes using RTPCR.
Material and Methods
Microorganism and Media
K. marxianus MTCC 1389 yeast strain primarily precured from Microbial Type Culture Collection (MTCC) Chandigarh, India. This strain thrives at 37 °C and was kept in a yeast extract-peptone-lactose medium (YPL) medium: peptone, yeast extract (Hi-media Pvt. limited Mumbai, India), lactose (Sigma). The cultures were preserved in 50% glycerol at − 20 °C.
Analysis of Multi-stress
To estimate tolerance to oxidative stress (H2O2), an overnight culture at 37 °C with an OD600 of 1 was transferred to different concentrations of H2O2 broth medium (0.7%, 0.8%, 0.9%, and 1%), and incubated at 37 °C. For osmotic stress, overnight grown cultures were transferred to NaCl concentrations in broth medium were varied (1 M, 1.5 M, 2 M, and 2.5 M) and incubated at 37 °C. To check the ethanol tolerance improvement, an overnight grown culture was transferred to an ethanol containing medium (8–12% v/v). Thermal stress tolerance was analysed by transferring overnight culture to broth tubes and incubating at 30°, 37°, 40°, and 45 °C temperatures.
Cell Viability Under Stress
Cell viability was determined by the growing parent and adapted strain under multi-stress conditions (mentioned in “Analysis of Multi-stress” section) and serial dilution with plating was performed at 0 h, 24 h, and 48 h.
Cell Growth Under Multi-stress
Cell growth of parent and adapted strain was determined by taking OD600 at 6 h intervals for 48 h under the multi-stress conditions mentioned in “Analysis of Multi-stress” section.
Spot Assay
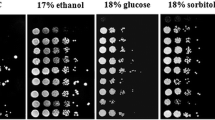
The spot assay was performed as proposed by Ogawa et al. [10] and Kwolek-Mirek and Zadrag-Tecza [11] with some modification. Spot assays were carried out at different time intervals under multi-stress conditions, 5 µL of cell suspension was taken at 6 h of time interval and spotted on YPD (yeast extract-peptone-dextrose) agar plates and incubated at 37 °C for 24 h.
Gene Expression Analysis
The differential gene expression for multiple stresses, i.e., ethanol, osmotic, oxidative, and thermal, was analysed by Real Time Polymerase Chain Reaction (CFX96 Touch ™ thermal cycler Bio-Rad). Primers used for genes were enlisted in Table 1. The yeast cell RNA was extracted from both the strains of K. marxianus MTCC1389, parent and adapted in stress conditions, for control in YPD medium, for sugar stress YPL (Lactose 20%) and under stress of ethanol of 6% and 8% (v/v). A RNA extraction kit (Zymo research—mini yeast) was used, and total RNA was quantified by nanodrop (BIOTEK TEK3). cDNA was synthesised by the solis-biodyne cDNA preparation kit, Estonia. Reverse transcriptase was done at 55 °C for 30 min, then 85 °C for 5 min. RTPCR was performed using qPCR master mix solis-biodyne, Estonia. Two housekeeping genes taken for normalisation were TEF1 (translation elongation factor 1) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
Statistical Analysis
The experiments were performed three times of 5 biological/technical replicates. Data shown in graphs are average from triplicates and error bar represents standard deviation.
The percentage of cell viability was calculated by
Growth curves were performed in 96—well plate. 2-way ANOVA was used for RTPCR data analysis. All graphs and calculations were performed with GraphPad Prism 5.
Results and Discussion
Oxidative Stress Response
In order to investigate the osmotic stress tolerance of parent and adapted strain, the broth was supplemented with by H2O2 concentration ranging from 0.7%, 0.8%, 0.9%, and 1% (v/v). The viability was checked by plating at 0 h, 24 h, and 48 h as shown in Fig. 1a. It represents that viability was 100% in 0.7% of H2O2 after 48 h in both the strains, whereas in 0.8% of H2O2, the viability of the cell was 20% in parent strain and 30% in adapted strain after 24 h of incubation. However, in 48 h, the viability was significantly increased, 80% viability was observed in adapted strain and 50% in parent strain. Whereas in a higher concentration (0.9%), adapted strain was viable at more than 30% while parent was less than 20%. Similarly, in 1% of H2O2, the viability was less than 20%. This clearly indicated adapted strain was significantly more viable in oxidative stress conditions compared to parent strain. Furthermore, in the growth analysis of both the strains under the same stress conditions (Fig. 2a), there was a significant increase in growth after 24 h of incubation in 0.7% and 0.8% of H2O2 by both the strains, while in 0.9% of stress the growth was found sluggish. However, in 1% of H2O2 stress, there was a decline in growth by both the strains which shows 0.7%, 0.8% of stress was less affecting to the strains, as yeast cells were able to grow while higher concentrations were disrupting cells. A spot assay was performed simultaneously and it shows comparable results between parent and adapted strain (Fig. 3a). Alike, to cell viability and growth analysis, adapted strain found to have more colonies than parent strain under all the oxidative stress conditions. Therefore, it may be concluded that in a high concentration of oxidative stress (> 0.8%), there was a decreasing of cell growth and viability. Bhurphen et al. [12] reported that during fermentation, yeast cells undergo several stresses such as hyperosmolarity, high ethanol, and high temperature, which induces oxidative stress that leads to increased production of ROS and O2·—by mitochondrial independent pathways. Arellano‐Plaza et al. [13] studied that by inducing the oxidative stress (H2O2) in S. cerevisiae and K. marxianus enhanced its ethanol productivity and yield with other aromatic compounds such as ethyl lactate and esters which were not detected in control strains.
Osmotic Stress Response
In order to determine the osmotic stress tolerance of parent and adapted strain, the liquid media was supplemented with NaCl concentrations ranging from 1 M, 1.5 M, 2 M, and 2.5 M. The viability under osmotic stress shown in Fig. 1b. represents that parent strain under stress of 1 M was 100% viable in 24 h and 48 h. However, in further concentrations, the viability of the cell sharply decreased. On the contrary, in adapted strain, the viability of the cell was more than 90% in 1 M, 1.5 M, and in 2 M, 2.5 M of osmotic stress, was more than 50% at 0 h while in 24, 48 h of time interval, it was less than 50%. Growth under osmotic stress was also determined as shown in Fig. 2b, which represents growth under 1 M NaCl concentration was increasing with respect to time in both the strains, whereas, in higher concentrations, it was not significantly increased. Therefore, it can be stated that the yeast cell was viable under osmotic stress but not growing at a higher concentration (1.5 M). The Spot assay was performed using the same sample and the results shown in Fig. 3b represent viability at different time intervals. As a result, the viability of both strains was highest at 1 M concentration. While under increasing stress, the cell colonies were decreased subsequently, which clearly indicates that with increasing time of incubation, cell viability decreases. However, the adapted strain was found with more colonies under all the concentration than the parent strain. Ahmad et al. [14] reported that NaCl was used as a stress inducer in S. cerevisiae as it was less toxic to cells than KCl and induced more cytoprotective pathways in growth medium and adapted the strain for higher salt stress, which improved its production rate by 50%. Illarionov et al. [15] studied the common salt stress response in S. cerevisiae and K. marxianus and found that in S. cerevisiae, the high concentration of Na+, K+ impaired ethanol and acetate production, but their specific growth rate was increased by fourfold. In K. marxianus the vacuole to cell size ratio was increased under osmotic stress.
Thermal Stress Response
In order to measure the thermal stress tolerance of parent and adapted strain, the inoculated broth was incubated at 30°, 37°, 40°, and 45 °C. The viability was checked by plating at 0 h, 24 h, and 48 h. Under low temperature stress, the viability was increased to 70% in parent strain and 80% in adapted strain in 48 h of incubation compared to control, i.e., 37 °C (Fig. 1c). As a result, 37 °C was determined to be the optimum temperature for both strains, as they demonstrated 100% viability at that temperature, whereas, at 40 °C, viability was higher in adapted strain than in parent strain by 20%. Similarly, at 45°C, adapted strain was more surviving with 80% viability and parent strain was 60% viable. The adapted strain shows notable thermotolerance in the cell viability assay, with maximum viability during stress conditions. Corresponding to the viability test, the growth assay represents similar results as adapted strain shows maximum growth at 37 °C and at other temperatures such as 40 °C and 45 °C, adapted strain has rampant growth. However, compared to parent strain, which also shows slow growth at the same temperatures (Fig. 2c). The spot assay was also performed along with the cell viability and growth assay shown in Fig. 3c, and that shows colonies by both the strains due to the presence of notable growth at all the temperatures. K. marxianus MTCC1389 was a thermotolerant yeast that could grow at temperatures as high as 52 °C, but efficient yeast growth was optimised at 37 °C [16]. Matsumoto et al. [17] studied the comparison in S. cerevisiae and K. marxianus respectively, and found that K. marxianus grew at 45 °C and survived heat shock at 50 °C, whereas S. cerevisiae was not able to grow at 45 °C while at 50 °C heat shock damage of cell membrane was not appeared however, at 60 °C K. marxianus recovered from shock and its metabolic activity was preserved compare to S. cerevisiae which was disrupted.
Ethanol Stress Response
To assess the enhancement in ethanol tolerance of parent and adapted strain, it was carried out by growing yeast cells in YPL broth containing ethanol (8–12% v/v) as shown in Fig. 1d. It was found that parent strain was 50% viable in 8% of ethanol stress after 48 h. In comparison to adapted strain, which shows 100% survivability in 24 h and 48 h under 8% ethanol stress, in a higher concentration of ethanol, parent strain survivability was negligible compared to adapted strain. However, adapted strain was 100% viable in up to 10% (v/v) ethanol stress after 48 h. Further increasing ethanol concentration, the cell viability in 11% ethanol was 60% after 24 h and 80% after 48 h. Under 12% stress, the viability of the cell was 60%. A growth assay was performed in similar stress conditions, and it was found that adapted strain started growing after 6 h of incubation and was in progress up to 48 h under all the concentrations of ethanol stress. However, parent strain was found to grow only in 8% of stress after 24 h of incubation, and in further higher concentrations the growth was sluggish (Fig. 2d). Similarly in spot assay analysis, it was found that adapted strain was having a higher number of colonies under stress of ethanol in all the concentrations after 24 h, while parent strain was having colonies up to 12 h, and later there was a lower number of colonies may be due to stress; cell growth was sluggish (Fig. 3d). Sostric et al. [18] reported that an ethanol adapted strain produces energy mainly from glycolysis and ethanol fermentation and found that increased gluconeogenesis flow and large levels of high-affinity hexose transporters are likely to counteract ethanol stress-induced pseudo-starvation.
Multi-stress Tolerance Gene Expression Analysis
We examined relative gene expression of multi-stress tolerance genes, i.e., ethanol (ETP1, ADH6), osmotic (SLN1, SSK1), oxidative (SKN7, HYR1), and thermal (HSF1, MSN2) by real-time polymerase chain reaction under sugar stress (20%) and ethanol stress of 6% and 8% (v/v) as shown in Table 2.
Relative Gene Expression Analysis of Oxidative Stress Tolerance Gene
SKN7 and HYR1 genes mainly expressed under oxidative stress caused by ethanol. SKN7 (Suppressor of Kre Null) function as transcription factor and HYR1 (Hydroperoxide Resistance) function as developing resistance under oxidative stress. Figure 4a represents the relative gene expression of the SKN7 gene under high sugar and ethanol stress. Under high sugar stress, parent strain was significantly (***P < 0.001) upregulated by 1.75 folds while adapted strain was 1.5-fold upregulated, whilst under 6% ethanol stress, parent strain was upregulated by 1.75 folds and adapted strain was upregulated by twofold. However, under 8% ethanol stress, the parent and adapted strain was upregulated by 1.3 and 1.6 folds respectively. The data shows that under fermentation stress (osmotic, end-product), both the strains upregulated SKN7 expression, which shows SKN7 was an important gene in managing cellular stress. Fassler et al. [19] studied S. cerevisiae gene SKN7, and observed that it plays a major role in oxidative stress response, function as conserved transcription factor gene and its activity depends on SLN1 gene phosphorylation and under stressful condition, SKN7 maintains cell wall integrity and plays an important role in thermal stress. Figure 4b shows expression of HYR1 gene under sugar and ethanol stress conditions. Under sugar stress both the strains expressed by 0.5 folds. Whereas under 6% of ethanol stress both the strains were expressed by onefold which was also non-significant in compare to control. However, under 8% of ethanol stress, the parent strain was upregulated by 1.75 folds (***P < 0.001) and the adapted strain showed non-significant (onefold) expression. Therefore, under stress conditions, a similar trend of gene expression was found in both the strains without much alteration. Fu et al. [20] reported the stress response of K. marxianus under high temperature and found that expression of HYR1 was downregulated, which led to a reduction in ROS resistance, resulted in fermentation arrest.
Relative gene expression (folds) under sugar stress (YPL 20%) and ethanol stress (6%, 8% v/v) of parent and adapted strain. Expression of gene associated with oxidative stress a SKN7, b HYR1. Gene associated with osmotic stress c SLN1, d SSK1. Gene associated with thermal stress e HSF1, f MSN2. Gene associated with ethanol stress g ETP1, h ADH6. Experiment was conducted in triplicate manner, and two repetition. Differential fold expression was determined by 2− (Δ ΔCt) method using GAPDH as reference gene. Value represent means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001
Relative Gene Expression Analysis of Osmotic Stress Tolerance Gene
SLN1 (Synthetic Lethal of N-end rule) osmotic histidine kinase gene is a plasma membrane osmotic sensor, SSK1 (Suppressor of Sensor Kinase) involved in downstream. Medium containing high sugar and ethanol imposes osmotic stress on yeast cells. Under the 20% sugar stress, there was significant (***P < 0.001) upregulation of adapted strain by 3 folds while parent strain was expressed by 1.5 folds (Fig. 4c). Moreover, under ethanol stress of 6%, both the strain was upregulated by 1.8–2 folds, similar trend was found, in 8% of ethanol stress, where expression was 1.2, 1.8 folds by parent and adapted strain respectively. Hohman [21] reported that mutation of the SLN1 gene affects activity of the HOG (high osmolarity glycerol) pathway, by affecting the plasma membrane of yeast cells. Figure 4d represents the relative gene expression of SSK1, and it showed that under high sugar stress, the expression was non-significant in both the strains compared to control. Under the 6% ethanol stress, expression of SSK1 was 0.5 folds in the parent strain and 0.8 folds in the adapted strain while under 8% ethanol stress, the parent strain showed insignificant expression however, the adapted strain was expressed by 1.4 folds, which clearly indicated that under high ethanol stress, SSK1 was more expressed, whereas in sugar stress and low ethanol stress there was insignificant expression of it compared to control. Parmar et al. [22] studied the roles of signalling pathways under osmotic stress, which are SHO1, SLN1, and it was found that SLN1 plays a major role in the stress response cascade, while SHO1 was downregulated because of inhibition by HOG1P2, and concluded that both stress-response pathways sense osmotic shock in different way.
Relative Gene Expression Analysis of Thermal Stress Tolerance Gene
HSF1 (Heat Shock transcription Factor) and MSN2 (Multicopy suppressor of SNF1 mutation) are transcription factors that regulate cell gene expression and are expressed under thermal stress conditions. Figure 4e represents the relative gene expression of HSF1, under sugar stress of 20%, both the strains were expressed by 0.8 folds, which was insignificant in expression compared to control. Moreover, under 6% ethanol stress, parent strain was upregulated by 1.25 folds while adapted strain was 1.75 folds and in 8% ethanol stress, parent strain was expressed by 0.5 folds and adapted strain was upregulated by 1.5 folds (***P < 0.001). This clearly indicated that under sugar stress, there was a trivial difference in the expression of HSF1 by both the strains while under ethanol stress there was significantly higher expression in adapted strain than in parent strain, which gives better survivability to strain. Nurcholis et al. [23] reported the differential gene expression by string database analysis, and found that HSF1 gene regulated genes responsible for transporting glucose in glycolysis and gluconeogenesis pathways, while MSN2 controlled the genes in lipid metabolism and in glycolysis. Figure 4f shows the relative gene expression of MSN2 and it was found that under sugar stress, both strains were found upregulated by 2 folds, while under ethanol stress of 6%, parent strain was upregulated by 3 folds, whereas adapted strain was significantly (***P < 0.001) expressed by 7 folds. However, under 8% ethanol stress, the gene was expressed by 4.8 folds in parent strain and 7 folds in adapted strain. The results presented here show that MSN2 was an important gene for increasing ethanol stress resistance. Watanabe et al. [24] reported that over-expression of the MSN2 strain was more tolerant than the control strain of sake yeast and the expression of MSN2 was sevenfolds higher in the tolerant strain in comparison to control and without ethanol stress. Li et al. [25] studied the role of MSN2 genes of K. marxianus and reported KmMsn2 improved ethanol fermentation by regulating genes related to sugar metabolism and glycolysis/gluconeogenesis.
Relative Gene Expression Analysis of Ethanol Stress Tolerance Gene
Genes associated with ethanol stress includes ETP1 (ethanol tolerance protein) and ADH6 (alcohol dehydrogenase 6), which primarily aid in ethanol tolerance by regulating genes in various pathways. The expression of ETP1 was significantly (**P < 0.01) increased by 2 folds in adapted strain in sugar stress as shown in Fig. 4g. It also shows that under ethanol stress of 6%, the adapted strain was significantly increased by 2.6 folds, and found insignificant in parent strain compared to control whereas, in 8% of ethanol stress, adapted strain expression was 3.5 folds, while in parent strain was 1.2-folds. Hiller [26] investigated the role of ETP1 in wine fermentation and found that etp1/etp1 mutants ferment sugar poorly, which results in incomplete fermentation due to genes involved in sugar transport. Figure 4h represents the expression of ADH6, under high sugar stress was expressed by 1.5 folds in parent strain, whereas it was expressed only by 0.75 folds in the adapted strain. However, under ethanol stress of 6% and 8%, in the adapted strain, there was significant expression (***P < 0.001) by 0.5 folds, while in the parent strain, it was expressed by 1.2–1.5 folds, it indicated that under stress condition, ADH6 was more expressed in parent strain than in adapted strain as the yeast cell reduces expression for reducing ethanol toxicity. Feng et al. [27] studied the micro-aeration effect on growth of K. marxianus using lignocellulosic hydrolysate and found the upregulation of genes related to the acetic acid pathway, i.e., ADH2, ADH4, and ADH6, increased significantly by 2.54, 2, and 1.75 folds after transcriptomic analysis. Zhang et al. [28] reported the transcriptomic analysis of K. marxianus in apple cider fermentation and found that alcohol dehydrogenase genes such as ADH1, ADH2, ADH6 were significantly overexpressed in K. marxianus.
Conclusion
Based on the results and discussion, it can be concluded that the multi-stress tolerance of the adapted K. marxianus MTCC1389 strain was significantly increased after evolutionary adaptation for single stress (ethanol). The viability of the cell was comparatively higher than its control strain under stress conditions, which shows greater survivability. Moreover, the relative gene expression was also changed under the stress conditions of sugar and (end-product) ethanol. Genes such as ETP1, SLN1, and MSN2 were found with significant upregulation under stress condition hence can be used in further studies of stress tolerance. As a result, the adapted strain was also multi-stress tolerant and can be used commercially for whey-based ethanol production.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pattanakittivorakul S, Tsuzuno T, Kosaka T, Murata M, Kanesaki Y, Yoshikawa H, Yamada M (2022) Evolutionary adaptation by repetitive long-term cultivation with gradual increase in temperature for acquiring multi-stress tolerance and high ethanol productivity in Kluyveromyces marxianus DMKU 3–1042. Microorganisms 10:798. https://doi.org/10.3390/microorganisms10040798
Pal U, Vij S (2022) Adaptive evolution of Kluyveromyces marxianus MTCC1389 for high ethanol tolerance. Biocatal Agric Biotechnol 45:1025. https://doi.org/10.1016/j.bcab.2022.102533
Zheng DQ, Wu XC, Tao XL, Wang PM, Li P, Chi XQ, Zhao YH (2011) Screening and construction of Saccharomyces cerevisiae strains with improved multi-tolerance and bioethanol fermentation performance. Bioresour Technol 102:3020–3027. https://doi.org/10.1016/j.biortech.2010.09.122
Mo W, Wang M, Zhan R, Yu Y, He Y, Lu H (2019) Kluyveromyces marxianus developing ethanol tolerance during adaptive evolution with significant improvements of multiple pathways. Biotechnol Biofuels 12:1–15. https://doi.org/10.1186/s13068-019-1393-z
Auesukaree C (2017) Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J Biosci Bioeng 124:133–142. https://doi.org/10.1016/j.jbiosc.2017.03.009
Caspeta L, Nielsen J (2015) Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses. MBio 6:e00431-e515. https://doi.org/10.1128/mBio.00431-15
Kitichantaropas Y, Boonchird C, Sugiyama M, Kaneko Y, Harashima S, Auesukaree C (2016) Cellular mechanisms contributing to multiple stress tolerance in Saccharomyces cerevisiae strains with potential use in high-temperature ethanol fermentation. AMB Express 6:1–14. https://doi.org/10.1186/s13568-016-0285-x
Zhang Q, Jin YL, Fang Y, Zhao H (2019) Adaptive evolution and selection of stress-resistant Saccharomyces cerevisiae for very high-gravity bioethanol fermentation. Electron J Biotechnol 41:88–94. https://doi.org/10.1016/j.ejbt.2019.06.003
Li C, Liu Q, Wang Y, Yang X, Chen S, Zhao Y, Li L (2021) Salt stress improves thermotolerance and high-temperature bioethanol production of multi-stress-tolerant Pichia kudriavzevii by stimulating intracellular metabolism and inhibiting oxidative damage. Biotechnol Biofuels 14:1–17. https://doi.org/10.1186/s13068-021-02071-0
Ogawa Y, Nitta A, Uchiyama H, Imamura T, Shimoi H, Ito K (2000) Tolerance mechanism of the ethanol-tolerant mutant of sake yeast. J Biosci Bioeng 90:313–320. https://doi.org/10.1016/S1389-1723(00)80087-0
Kwolek-Mirek M, Zadrag-Tecza R (2014) Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res 14:1068–1079. https://doi.org/10.1111/1567-1364.12202
Burphan T, Tatip S, Limcharoensuk T, Kangboonruang K, Boonchird C, Auesukaree C (2018) Enhancement of ethanol production in very high gravity fermentation by reducing fermentation-induced oxidative stress in Saccharomyces cerevisiae. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-31558-4
Arellano-Plaza M, Noriega-Cisneros R, Clemente-Guerrero M, González-Hernández JC, Robles-Herrera PD, Manzo-Ávalos S, Gschaedler-Mathis A (2017) Fermentative capacity of Kluyveromyces marxianus and Saccharomyces cerevisiae after oxidative stress. J Inst Brew 123:519–526. https://doi.org/10.1002/jib.451
Ahmad M, Pathania R, Chowdhury A, Gupta JK, Dev C, Srivastava S (2021) Salt-stress adaptation of yeast as a simple method to improve high-gravity fermentation in an industrial medium. Appl Microbiol Biotechnol 105:8009–8018. https://doi.org/10.1007/s00253-021-11566-7
Illarionov A, Lahtvee PJ, Kumar R (2021) Potassium and sodium salt stress characterization in the yeasts Saccharomyces cerevisiae, Kluyveromyces marxianus, and Rhodotorula toruloides. Appl Environ Microbiol 87:e03100-e3120. https://doi.org/10.1128/AEM.03100-20
Saini P, Beniwal A, Kokkiligadda A, Vij S (2017) Evolutionary adaptation of Kluyveromyces marxianus strain for efficient conversion of whey lactose to bioethanol. Process Biochem 62:69–79. https://doi.org/10.1016/j.procbio.2017.07.013
Matsumoto I, Arai T, Nishimoto YUI, Leelavatcharamas V, Furuta M, Kishida M (2018) Thermotolerant yeast Kluyveromyces marxianus reveals more tolerance to heat shock than the brewery yeast Saccharomyces cerevisiae. Biocontrol Sci 23:133–138. https://doi.org/10.4265/bio.23.133
Šoštarić N, Arslan A, Carvalho B, Plech M, Voordeckers K, Verstrepen KJ, van Noort V (2021) Integrated multi-omics analysis of mechanisms underlying yeast ethanol tolerance. J Proteome Res 20:3840–3852. https://doi.org/10.1021/acs.jproteome.1c00139
Fassler JS, West AH (2011) Fungal Skn7 stress responses and their relationship to virulence. Eukaryot Cell 10:156–167. https://doi.org/10.1128/EC.00245-10
Fu X, Li P, Zhang L, Li S (2019) Understanding the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation based on integration of RNA-Seq and metabolite data. App Microbiol Biotechnol 103:2715–2729. https://doi.org/10.1007/s00253-019-09637-x
Hohmann S (2002) Osmotic stress signalling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372. https://doi.org/10.1128/MMBR.66.2.300-372.2002
Parmar JH, Bhartiya S, Venkatesh KV (2009) A model-based study delineating the roles of the two signaling branches of Saccharomyces cerevisiae, Sho1 and Sln1, during adaptation to osmotic stress. Phys Biol 6:036019. https://doi.org/10.1088/1478-3975/6/3/036019
Nurcholis M, Lertwattanasakul N, Rodrussamee N, Kosaka T, Murata M, Yamada M (2020) Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl Microbiol Biotechnol 104:475–488. https://doi.org/10.1007/s00253-019-10224-3
Watanabe M, Watanabe D, Akao T, Shimoi H (2009) Overexpression of MSN2 in a sake yeast strain promotes ethanol tolerance and increases ethanol production in sake brewing. J Biosci Bioeng 107:516–518. https://doi.org/10.1016/j.jbiosc.2009.01.006
Li P, Fu X, Zhang L, Zhang Z, Li J, Li S (2017) The transcription factors Hsf1 and Msn2 of thermotolerant Kluyveromyces marxianus promote cell growth and ethanol fermentation of Saccharomyces cerevisiae at high temperatures. Biotechnol Biofuels 10:1–13. https://doi.org/10.1186/s13068-017-0984-9
Hillier AE (2013) Investigating the impact of ETP1 in Saccharomyces cerevisiae during Chardonnay fermentation. Doctoral dissertation, University of Guelph. http://hdl.handle.net/10214/5627
Feng H, Li Y, Du C, Yuan W (2021) Effect of microaeration on cell growth and glucose/xylose fermentation of Kluyveromyces marxianus from the imitate lignocellulosic-derived hydrolysate. Process Biochem 101:247–255. https://doi.org/10.1016/j.procbio.2020.11.025
Zhang Z, Lan Q, Yu Y, Zhou J, Lu H (2022) Comparative metabolome and transcriptome analyses of the properties of Kluyveromyces marxianus and Saccharomyces yeasts in apple cider fermentation. Food Chem Mol Sci 4:100095. https://doi.org/10.1016/j.fochms.2022.100095
Acknowledgements
The author would like to thank ICAR- National Dairy Research Institute, Karnal, Haryana, India, for providing financial support as senior research fellowship.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
UP; data analysis, data curation, investigation, conceptualization, validation, methodology writing- original draft preparation. SP; data interpretation, writing- reviewing and editing. SV; work management, writing- reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pal, U., Pal, S. & Vij, S. Kluyveromyces marxianus MTCC 1389 Augments Multi-stress Tolerance After Adaptation to Ethanol Stress. Indian J Microbiol 63, 483–493 (2023). https://doi.org/10.1007/s12088-023-01102-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-023-01102-8