Abstract
The methods of preparation of fatty acids from brewer’s yeast and its use in production of biofuels and in different branches of industry are described. Isolation of fatty acids from cell lipids includes cell disintegration (e.g., with liquid nitrogen, KOH, NaOH, petroleum ether, nitrogenous basic compounds, etc.) and subsequent processing of extracted lipids, including analysis of fatty acid and computing of biodiesel properties such as viscosity, density, cloud point, and cetane number. Methyl esters obtained from brewer’s waste yeast are well suited for the production of biodiesel. All 49 samples (7 breweries and 7 methods) meet the requirements for biodiesel quality in both the composition of fatty acids and the properties of the biofuel required by the US and EU standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some biofuels use as input raw materials food crops such as camelina, canola, coconut, corn, jatropha, palm, rapeseed, safflower, soybean, sunflower oils, etc. All these plants contain readily available saccharides including starch or are producers of plant oils. Obtaining biofuel from them is thus relatively simple since fermentation of the saccharides gives rise to bioethanol (used as gasoline replacement) while transesterification of triacylglycerols yields biodiesel.
These known oilseed plants contain mostly five major fatty acids (FAs), i.e., saturated palmitic and stearic acid, and unsaturated acids, namely oleic, linoleic, and α-linolenic acid. Another, less common but industrially important fatty acid biosynthesized in various plants is palmitoleic acid (Po, Δ9-16:1 or 16:1n-7), which is a valuable renewable resource for the production of industrially important chemicals such as 1-octene, a highly valued material in the production of linear so-called low-density polyethylene, and can be advantageously used also for the production of biodiesel (Wu et al. 2012), which is defined as a mixture of mono-alkyl esters of fatty acids originating mainly from vegetable oils or animal fats. While biodiesel has many advantages because it is biodegradable, is made from renewable raw materials, and has low toxicity in most ecosystems, technical problems facing biodiesel include its low oxidation stability and poor filterability at low temperatures. Methyl palmitoleate is considered the ideal candidate to solve these problems, because it has a low melting point (about −34 °C) (Murray et al. 2010) and at the same time an acceptable oxidative stability. Its cetane number is 54.
A common negative feature of all commercially grown and available oilseed plants is that they either do not contain palmitoleic acid or its content is a mere 1–2 % of the total fatty acids. As palmitoleic acid cannot be produced from natural sources in large amounts and at a reasonably low price, interest has focused on metabolic engineering of oilseed plants for the production of palmitoleic acid. In recent years, research in this area focused on two main topics, a genetic manipulation in order to increase the content of Po, which most often involves transferring a gene encoding Δ9desaturase from Saccharomyces yeast. The other option is to search for alternative natural sources of Po, i.e., organisms producing 10 % or more of Po based on the total fatty acid content (Khoomrung et al. 2012).
As mentioned above, low concentrations of Po are virtually ubiquitous, but few plants produce oils containing more than 10 relative percent of palmitoleic acid. In fact, there are only two plants available with this property, i.e., Macadamia integrifolia nuts (Knothe 2010) and sea buckthorn Hippophae rhamnoides (Rüsch et al. 2004). Other sources (see, e.g., Table 1) are very exotic, so even though the oil from the Kermadecia sinuate seeds contains up to 70 % palmitoleic acid, this plant cannot be used for commercial purposes, as it is an endemic flowering plant of the Proteaceae family growing only in New Caledonia.
Biofuels of the next generation are produced by microorganisms from lignocellulosic materials that include agro-wastes such as corn stalks (cellulose) or waste effluent from the production of cellulose (lignin) to monomeric compounds, which are subsequently fermented. It should be noted, however, that the conversion of this biomass to fermentable low molecular compounds requires costly technologies including preprocessing with special enzymes, and this generation of biofuels cannot be at present produced on a large scale (Brennan and Owende 2010).
The attention has therefore been focusing on the next generation of biofuels that are considered as a viable energy source free of the main drawbacks associated with the previous generations of biofuels (Nigam and Singh 2011; Chisti 2007; Li et al. 2008).
Yeast are unduly neglected in this regard, if only because most genetic experiments (Seip and Zhu 2011; Brillhart 2001) allowing expression of Δ9desaturase make use of genetic material from yeasts of the genus Saccharomyces. The variability of fatty acids in yeast, specifically in Saccharomyces ones, is relatively large (Rezanka et al. 2013a). Nevertheless, one can say that it features five major fatty acids, i.e., palmitic, palmitoleic, stearic, oleic, and linoleic acid, which make up to 90 % of the total acids (Rezanka et al. 2013b; Sarris et al. 2011; Sarris et al. 2013). Many other minor acids present include medium length acids, e.g., caprylic, capric, lauric, and myristic acids. Arachidic and α-linolenic acids and very long chain acids, i.e., behenic, lignoceric, cerotic, and many others have been identified as minority acids (see, e.g., Welch and Burlingame 1973). Among other constituents are also acids with an odd number of atoms, such as pentadecanoic or pentadecenoic (Bravi et al. 2009).
It is known that certain yeasts, e.g., Rhodosporidium toruloides, Cryptococcus curvatus, Lipomyces starkey, and Yarrowia lipolytica accumulate lipids to more than 20 % dry weight (Feofilova et al. 2010; Papanikolaou and Aggelis 2010; Thiru et al. 2011; Wild et al. 2010; Wu et al. 2011). However, the industrial use of lipids produced by these yeasts encounters the problem of low productivity (Feofilova et al. 2010). Oleaginous yeast growth is much slower compared with Saccharomyces yeast and production of lipids by oleaginous yeasts therefore takes a long time to reach the maximum of produced lipids.
Since palmitoleic acid (Po) cannot as yet be produced from natural sources in large quantities and at low cost, the need has arisen to find such resources that would enable its production, for instance brewer’s yeast, especially the top-fermenting Saccharomyces cerevisiae (top fermentation) and the bottom fermenting Saccharomyces pastorianus. Within the genus Saccharomyces, S. pastorianus and S. cerevisiae belong to the species complex Saccharomyces sensu stricto. The waste from a brewery with the production of 1 million hectoliters of beer per year includes 1000 t of brewer’s yeast paste (15–17 % dry weight) which contains up to about 50 % of Po in the lipid fraction (Sigler and Matoulková 2013). The ratio of palmitoleic acid to total lipids is thus much higher than with commercially used oils and is comparable to commercial oils derived from sea buckthorn or macadamia.
The amount of palmitoleic acid is to some extent influenced by the technological process used for beer production (e.g., top vs. bottom fermentation (see Experimental)) and yeast strain. Fermentation temperature also has a significant effect on the production of palmitoleic acid. Bottom and top brewer’s yeast differ in their flocculation properties, and in the final stage of fermentation, bottom yeast forms flocks and sediments at the bottom of the fermentation vessel, top yeast after fermentation rises to the surface where it forms thick foam. Bottom fermentation takes place at a temperature of 8–15 °C. S cerevisiae tolerates higher temperatures than S. pastorianus; top fermentation is usually carried out at 18–22 °C (Boulton and Quain 2001). Separation of yeast biomass after fermentation is already well resolved in the context of brewing technology. Subsequent centrifugation can be used for greater reduction of its water content. Even after lipid extraction, the biomass still contains up to 48 % protein and can therefore be used as livestock feed.
Yeast is commonly used for industrial production of ergosterol, i.e., provitamin D2 (Buehler 1958; Feeney 1956). The most important step in the process of isolation is either mechanical or chemical disruption of cell walls followed by further processing. Moreover, in terms of economy, brewer’s yeast is in fact waste material and the cost of its cultivation is part of the cost of beer production.
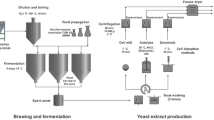
In this study, we compare different types of disintegration of yeast cells used in the manufacture of beer and subsequent extraction of lipids, including the calculation of important characteristics of the biodiesel produced from the waste yeast, i.e., average unsaturation, viscosity, density, cloud point, and cetane number.
Results and discussion
The effect of fatty acids produced by yeast on the quality of the biofuel has been known for some years (Knothe 2008, 2009). Gas chromatography–mass spectrometry analysis of yeast samples from individual breweries revealed relatively small differences in the proportions of FAs (see below). The major FAs in many strains of brewer’s yeast are mostly C16 and C18 FAs, particularly P, Po, S, O, and L (Rezanka et al. 2013a) (for abbreviations see Table 2). Other FAs including both short-chain (Cy, C, La, and M) and longer-chain FAs with more than 20 carbon atoms (A, G, B, E, Lg, N, and Ce) are present in very small amounts far below 10 relative percent of the total FAs (Rezanka et al. 2013b). It should be noted that both these groups have positive effect on the properties of biodiesel. Shorter-chain FAs (i.e., C8–C14) lower the cloud point; saturation is more important for the value of the cloud point than unsaturation, see Tables 3, 4, 5, 6, 7, 8, and 9. FAs with chains longer than C18 raise the cetane number. For instance, the methyl ester of behenic acid has a cetane number of 106 (Anitescu and Bruno 2012).
The lipid production and proportions of individual fatty acids in waste yeast from individual breweries are given in Tables 3, 4, 5, 6, 7, 8, and 9. As a reference method for lipid extraction, we used the commonly used procedure according to Bligh and Dyer (1959), which, for walled cells like yeast, has to be complemented by cell disruption with glass beads in liquid nitrogen (Uppuluri et al. 2007). This procedure is the most gentle of all methods in use but it cannot be used for biodiesel production on industrial scale due to a high cost. Its usefulness as a reference method is demonstrated by the data it yielded on the amount of α-linolenic acid in waste yeast from brewery D (for the list of breweries see Table 10), which was up to 1.4 % total FAs (Table 3). With all other methods, the content of this acid was somewhat lower. A similar drop in the efficiency of fatty acid extraction was noted also with other unsaturated FAs, especially with linoleic acid. Extraction with boiling NaOH solution (method C) caused its degradation in all seven yeast strains (Table 5).
Another two methods, i.e., B and C, are relatively drastic since they include extraction with butanol or water under reflux at a high pH. They were originally developed for an industrial acquisition of ergosterol (provitamin D2) from yeast (Feeney 1956; Buehler 1958). As seen in Tables 4 and 5, they cause a complete degradation of α-linolenic acid, method C brings about also the degradation of linoleic acid. On the other hand, the removal of fatty acids with two or three double bonds does not appreciably affect the quality of biodiesel (see Tables 4 and 5), since acids with multiple double bonds, or their methyl esters, have very low cetane numbers (cetane number is 42 for L and 22 for Ln, respectively) (Anitescu and Bruno 2012). The use of these two methods offers the advantage that the technological problems of processing large volumes of material (thousands of liters) have already been solved as part of ergosterol production while their drawback is the presence of high salt content after acidification of hydrolyzed yeast. When using hydrochloric acid, the salt is sodium chloride, which prevents the further use of the waste yeast as livestock feed.
Method D is based on yeast extraction by petroleum ether (Tarasova et al. 1976) and has been originally developed for the production of ergosterol and also 9-ubiquinone. The results of the extraction given in Table 6 show that the method provides satisfactory recovery of fatty acids and the properties of the biodiesel meet the required standards.
Methods E, F, and G (Green and Edwin 1961) use for cell disruption the fact that water-soluble amino compounds having a dissociation constant between 10−2 and 10−6 (e.g., aqueous ammonia, aliphatic alkyl derivatives such as diethylamine, and cyclic amine compounds, such as piperidine or cyclohexylamine) cause degradation of cell walls.
Based on the patent, we chose as most convenient three nitrogenous compounds, i.e., cyclohexylamine (method E), ammonia solution (method F), and diethylamine (method G). The resulting differences in the composition of fatty acids and in the biodiesel properties were minor (Tables 7, 8, and 9). A considerable advantage of these methods is the fact that the amount of the nitrogenous compound added to the yeast is tenths to units of percent relative to the biomass, which positively affects the overall economy. Moreover, as pointed out in Conclusion section, the waste biomass can be further used as livestock feed.
The only difference in the content of FAs was in the brewery using top-fermenting yeast S. cerevisiae, not the bottom-fermenting S. pastorianus used by the remaining six breweries.
To determine various properties of biodiesel, i.e., relevant values characterizing its properties, it is necessary to have considerable amounts of lipids or methyl esters, and also special devices, which causes considerable difficulties. Mathematical methods were therefore developed for identifying important physical quantities (Ramirez-Verduzco et al. 2012; Hoekman et al. 2012; Knothe and Steidley 2011). The mathematical method was also successfully used for various types of oleaginous yeast (Tanimura et al. 2014). By using these methods, we calculated and characterized the relevant properties of waste yeast lipids from the breweries under study. Our calculations were based on six values, viz. average unsaturation, viscosity, density, cloud point, cetane number, and iodine value. The resulting values of the yeast fatty acid content from the seven breweries and seven kinds of disintegration and extraction are presented in Tables 3, 4, 5, 6, 7, 8, and 9. All our results appear to be in the range of allowed values of EU and US standards (ASTM 6751; EN 14214).
Conclusion
We have concluded that fatty acids in the form of methyl esters obtained from brewer’s waste yeast are well suited for the production of biodiesel. All 49 samples meet the requirements for biodiesel quality in both the composition of fatty acids (Knothe 2009) and the properties of the biofuel (e.g., viscosity, density, cloud point, cetane number, etc.) required by the US and EU standards.
After lipid extraction, yeast can be used as fodder (cell envelopes and other constituents) since its constituents contain about 40 % of digestible proteins, saccharides and especially the vitamin B complex.
Materials and methods
Yeast
Top brewer’s yeast S. cerevisiae and bottom brewer’s yeast S. pastorianus were obtained from selected breweries (see Table 10).
Method A
Ten grams of yeast dry matter content of 15–17 % was mixed with 2 mL of 0.1 M Na2CO3 and the mixture was briefly triturated with glass beads (diameter 0.2 mm) in a mortar, covered with liquid nitrogen, and triturated again. This process was repeated three times and 50 mL of 0.1 M Na2CO3 was finally added (Uppuluri et al. 2007). Crushed yeast was extracted with chloroform to methanol ratio of 1:2 (Bligh and Dyer 1959), centrifuged, and the lower phase was evaporated and saponified with 10 % KOH solution in methanol at room temperature overnight. The aqueous solution of fatty acids was acidified to pH 2 and the free acids were extracted into hexane.
Method B
Ten grams of yeast was extracted with 3 × 60 mL of butanol containing 200 mg of KOH. The mixture was heated to boiling with stirring for 1 h, filtered hot, diluted with water (150 mL), and then concentrated to 50 % (Feeney 1956) of the original volume. The aqueous solution was acidified to pH 2 and further processed according to method A.
Method C
A mixture containing 10 g of yeast was mixed with 10 % NaOH solution and refluxed for 1 h, filtered hot, and then processed as in method B (Buehler 1958).
Method D
Lipid extraction was carried out with petroleum ether. Seven grams of yeast was suspended in 30 mL of petroleum ether and extracted for 7 h (Tarasova et al. 1976). Filtration and evaporation in vacuo at a temperature not exceeding 40 °C were followed by further processing as in method A.
Method E
Ten grams of yeast was mixed with 2 mL of 10 % aqueous solution of cyclohexylamine and the mixture was heated at 50 ° C for 1 h (Green and Edwin 1961). After cooling, 40 mL of methanol was added and the mixture was filtered. Yeast was washed with 3 × 30 mL of methanol, and the combined liquors were evaporated to dryness and further processed according to method A.
Method F
Ten grams of yeast was mixed with 4 mL of ammonia solution obtained by fivefold dilution of commercially available solution (25 % solution) (Green and Edwin 1961). The mixture was heated with stirring for 1 h and further processed as in method A.
Method G
Ten grams of yeast was mixed with 40 mL of diethylamine (diluted five times in water), the mixture was allowed to stand at 20 °C for 24 h (Green and Edwin 1961) and was then treated according to method A.
FAMEs analysis
Fatty acid fractions (~5 mg) obtained by methods A–G were dissolved in hexane and were methylated using BF3/MeOH (Vancura et al. 1987, 1988).
Gas chromatography–mass spectrometry of FAME was done on a GC-MS system consisting of Varian 450-GC, Varian 240-MS ion trap detector with electron impact ionization, and CombiPal autosampler (CTC, USA). Splitless injection was at 100 °C, and a fused silica capillary column (Supelcowax 10; 60 m × 0.25 mm i.d., 0.25 mm film thickness; Supelco, Prague) was used. The temperature program was as follows: 100 °C for 1 min, subsequently increasing at 20 °C/min to 180 °C and at 2 °C/min to 280 °C, which was maintained for 1 min. The carrier gas was helium at a linear velocity of 60 cm/s. The structures of FAMEs were confirmed by comparison of retention times and fragmentation patterns with those of the standard FAMEs (Supelco, Prague) (Juzlova et al. 1996).
Concentrations of individual fatty acids produced by yeast from different breweries are given in Tables 3, 4, 5, 6, 7, 8, and 9.
Calculation of biodiesel properties
The biodiesel properties (i.e., average unsaturation, viscosity, density, cloud point, cetane number, and iodine value) were estimated based on the equations of Hoekman et al. (2012). Average unsaturation (AU) was calculated from the compositional profiles in Tables 3, 4, 5, 6, 7, 8, and 9 as AU = ΣN × Ci, where N is the number of carbon-carbon double bonds of unsaturated fatty acids and Ci is the concentration (mass fraction) of the component.
Each property was calculated using the following equations (Hoekman et al. 2012).
References
Anitescu G, Bruno TJ (2012) Biodiesel fuels from supercritical fluid processing: quality evaluation with the advanced distillation curve method and cetane. Energy Fuels 26:5256–5264
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Boulton C, Quain D (2001) Brewing yeast and fermentation, 1st edn. Blackwell Science Ltd., London, England
Bravi E, Perretti G, Buzzini P, Sera RD, Fantozzi P (2009) Technological steps and yeast biomass as factors affecting the lipid content of beer during the brewing a process. J Agric Food Chem 57:6279–6284
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 14:557–577
Brillhart DD (2001) Monounsaturated fatty acid compositions and method of making. US Patent 6:183,796
Buehler HJ (1958) Extraction of sterols. US Patent 2:837,540
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 5:294–306
Feeney RL (1956) Recovery of ergosterol. US Patent 2:730,536
Feofilova EP, Sergeeva YE, Ivashechkin AA (2010) Biodiesel-fuel content, production, producers, contemporary biotechnology. Appl Biochem Microbiol 46:369–378
Green J, Edwin EE (1961) Production of ergosterol from yeast. US Patent 3:006,932
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169
Juzlova P, Rezanka T, Martinkova M, Kren V (1996) Long-chain fatty acids from Monascus purpureus. Phytochemistry 43:151–153
Khoomrung S, Chumnanpuen P, Jansa-ard S, Nookaew I, Nielsen J (2012) Fast and accurate preparation fatty acid methyl esters by microwave-assisted derivatization in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 94:1637–1646
Knothe G (2008) Designer biodiesel optimizing fatty ester composition to improve fuel properties. Energy Fuels 22:1358–1364
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766
Knothe G (2010) Biodiesel derived from a model oil enriched in palmitoleic acid, macadamia nut oil. Energy Fuels 24:2098–2103
Knothe G, Steidley KR (2011) Kinematic viscosity of fatty acid methyl esters. Prediction, calculated viscosity contribution of esters with unavailable data, and carbon-oxygen equivalents. Fuel 90:3217–3224
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae Biotechnol Prog 24:815–820
Murray SM, O’Brien RA, Mattson KM, Ceccarelli C, Sykora RE, West KN, Davis JH (2010) The fluid-mosaic model, homeoviscous adaptation, and ionic liquids dramatic lowering of the melting point by side-chain unsaturation. Angew Chem Int Ed 49:2755–2758
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37:52–68
Papanikolaou S, Aggelis G (2010) Yarrowia lipolytica a model microorganism used for the production of tailor-made lipids. Eur J Lipid Sci Technol 112:639–654
Ramirez-Verduzco LF, Rodriguez-Rodriguez JE, Jaramillo-Jacob AD (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111
Rezanka T, Kolouchova I, Cejkova A, Cajthaml T, Sigler K (2013a) Identification of regioisomers and enantiomers of triacylglycerols in different yeasts using reversed- and chiral-phase LC–MS. J Sep Sci 36:3310–3320
Rezanka T, Matoulkova D, Kolouchova I, Masak J, Sigler K (2013b) Brewer’s yeast as a new source of palmitoleic acid-analysis of triacylglycerols by LC-MS. J Am Oil Chem Soc 90:1327–1342
Rüsch G, Klaas M, Meurer PU (2004) A palmitoleic acid ester concentrate from sea buckthorn pomace. Eur J Lipid Sci Technol 106:412–416
Sarris D, Galiotou-Panayotou M, Koutinas AA, Komaitis M, Papanikolaou S (2011) Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J Chem Technol Biotechnol 86:1439–1448
Sarris D, Giannakis M, Philippoussis A, Komaitis M, Koutinas AA, Papanikolaou S (2013) Conversions of olive mill wastewater-based media by Saccharomyces cerevisiae through sterile and non-sterile bioprocesses. J Chem Technol Biotechnol 88:958–969
Seip JE, Zhu QQ (2011) Δ9 desaturase and its use in making polyunsaturated fatty acids. US Patent 7:923,223
Sigler K, Matoulková D (2013) Waste brewer’s yeast as a source of nutritionally valuable palmitoleic acid. Kvasný prům 59:329–330
Tanimura A, Takashima M, Sugita T, Endoh R, Kikukawa M, Yamaguchi S, Sakuradani E, Ogawa J, Shima J (2014) Selection of oleaginous yeasts with high lipid productivity for practical biodiesel production. Bioresour Technol 153:230–235
Tarasova NV, Obolnikova EA, Gololobov AD, Samokhvalov GI, Chepigo SV, Ivanova GI, Imshenetsky VV, Kulikova VM (1976) Method for preparing ergosterol and ubiquinone-9 in a single process. US Patent 3:965,130
Thiru M, Sankh S, Rangaswamy V (2011) Process for biodiesel production from Cryptococcus curvatus. Bioresour Technol 102:10436–10440
Uppuluri P, Perumal P, Chaffin WL (2007) Analysis of RNA species of various sizes from stationary-phase planktonic yeast cells of Candida albicans. FEMS Yeast Res 7:110–117
Vancura A, Rezanka T, Marsalek J, Vancurova I, Kristan V, Basarova G (1987) Effect of ammonium-ions on the composition of fatty-acids in Streptomyces fradiae, producer of tylosin. FEMS Microbiol Letts 48:357–360
Vancura A, Rezanka T, Marsalek J, Melzoch K, Basarova G, Kristan V (1988) Metabolism of L-threonine and fatty-acids and tylosin biosynthesis in Streptomyces fradiae. FEMS Microbiol Lett 49:411–415
Welch JW, Burlingame AL (1973) Very long chain fatty acids in yeast. J Bacteriol 115:464–466
Wild R, Patil S, Popovic M, Zappi M, Dufreche S, Bajpai R (2010) Lipids from Lipomyces starkeyi. Food Technol Biotech 48:329–335
Wu S, Zhao X, Shen H, Wang Q, Zhao ZK (2011) Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour Technol 102:1803–1807
Wu Y, Li R, Hildebrand DF (2012) Biosynthesis and metabolic engineering of palmitoleate production, an important contributor to human health and sustainable industry. Prog Lipid Res 51:340–349
Acknowledgments
The research was supported by the project GACR P503/11/0215 and by the Institutional Internal Projects RVO61388971 and RO1914 (Ministry of Agriculture of the Czech Republic).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 35.5 kb)
Rights and permissions
About this article
Cite this article
Řezanka, T., Matoulková, D., Kolouchová, I. et al. Extraction of brewer’s yeasts using different methods of cell disruption for practical biodiesel production. Folia Microbiol 60, 225–234 (2015). https://doi.org/10.1007/s12223-014-0360-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0360-0