Abstract
The ground-based facilities 2D clinostat (CN) and Random Positioning Machine (RPM) were designed to simulate microgravity conditions on Earth. With support of the CORA-ESA-GBF program we could use both facilities to investigate the impact of simulated microgravity on normal and malignant thyroid cells. In this review we report about the current knowledge of thyroid cancer cells and normal thyrocytes grown under altered gravity conditions with a special focus on growth behaviour, changes in the gene expression pattern and protein content, as well as on altered secretion behaviour of the cells. We reviewed data obtained from normal thyrocytes and cell lines (two poorly differentiated follicular thyroid cancer cell lines FTC-133 and ML-1, as well as the normal thyroid cell lines Nthy-ori 3-1 and HTU-5). Thyroid cells cultured under conditions of simulated microgravity (RPM and CN) and in Space showed similar changes with respect to spheroid formation. In static 1g control cultures no spheroids were detectable. Changes in the regulation of cytokines are discussed to be involved in MCS (multicellular spheroids) formation. The ESA-GBF program helps the scientists to prepare future spaceflight experiments and furthermore, it might help to identify targets for drug therapy against thyroid cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown, that thyroid cells in vitro and in vivo respond to altered gravity conditions (Grimm et al. 2002; Kossmehl et al. 2002, 2003; Meli et al. 1998, 1999; Martin et al. 2000; Masini et al. 2012; Albi et al. 2011, 2012, 2014). This response might play an important role for physiological changes at the organism level during spaceflight but could also give important hints for cancer research on Earth (Becker et al. 2013; Grimm et al. 2002, 2014).
An impressive example for the response to altered gravity conditions is the spheroid formation, which was observed after exposure of thyroid cancer cells to real microgravity in Space for 10 days (Pietsch et al. 2013). This experiment was part of the Sino-German Shenzhou-8/SIMBOX-mission in 2011 (Ma et al. 2014; Pietsch et al. 2013). 3D growth and spheroid formation in Space were also observed for other cell types like chondrocytes (Freed et al. 1997; Stamenkovic et al. 2010). These multicellular spheroids mirror the alteration in cell-cell adhesion, a transition from 2- to 3-dimensional growth and might be beneficial for studies on biological processes such as metastasis or tumor-neovascularization and for pharmacological testing (Grimm et al. 2014).
In order to get a deeper insight into the molecular mechanisms behind this phenomenon, ground-based facilities (GBF) are valuable tools, as they enable a cost efficient preparation of spaceflights but also continuous research in stand-alone studies. In this context, the fast rotating clinostat (CN) and the Random Positioning Machine (RPM) have been suggested for studies with adherent mammalian cells, as they often showed similar results compared to real microgravity in earlier studies (Herranz et al. 2013). The most important findings concerning thyroid cells cultured in vitro under conditions of simulated and real microgravity (µ g), published by our group and others, as well as data obtained from Space missions from mouse thyroid glands in vivo, published by Professor Ambesi-Impiombato and coworkers, are listed in Table 1. In addition, we listed published data concerning changes in the gene expression pattern and protein content of different cell types obtained after culture under simulated and real microgravity conditions in Table 2.
This review summarizes the results from the ESA-CORA-GBF-PROJECT-2011-005 (ACRONYM DEVICE COMPARISON) and ESA-CORA-GBF-PROJECT-2013-001 (ACRONYM THYROID III). It gives an overview on the behaviour of thyroid cells under real and simulated microgravity.
Experimental Approach
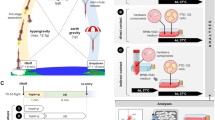
The thyroid cells were cultured in a comparative methodological approach as published in detail before (Warnke et al. 2014; Grosse et al. 2012). Static 1g-control cells were always stored together with the microgravity simulation device in the same standard cell culture incubator. We used either a fast-rotating 2D clinostat (German Aerospace Center, Cologne, Germany; Fig. 1a), operated constantly at 60 rpm, or a Random Positioning Machine (ADS, former Dutch space, the Netherlands; Fig. 1b), operated in real random speed and direction mode (60-75 ∘/s) (Fig. 1a, b). The spheroid formation was documented microscopically. Molecular biological analyses (quantitative real-time PCR, gene array, bioinformatics), Western blot technique and Multi-Analyte Profiling (MAP) as well as cytoskeletal staining were performed according to established methods (Warnke et al. 2014; Kossmehl et al. 2006; Grosse et al. 2012; Rothermund et al. 2002; Infanger et al. 2007; Pietsch et al. 2013).
The Ground-Based Facilities Random Positioning Machine and 2D Clinostat
The magnitude of the gravity vector on Earth cannot be altered, but its direction and thus its influence can be changed (Briegleb 1992; Herranz et al. 2013). This is the underlying principle for the simulation devices of interest in this review. Therefore, the term ‘simulated microgravity’ is used, as the cell might experience a condition comparable to that of real microgravity, due to a randomization of the direction of the gravity vector over time. However, device-specific side effects like centrifugal accelerations, shearing forces and vibrations remain and possibly mask the desired microgravity effects. Therefore, a careful and conscious handling and discussion of the results are suggested (Herranz et al. 2013).
Nevertheless, ground-based facilities (RPM, CN) used for the experiments have been previously described as promising candidates for microgravity simulations in adherent mammalian cells (Herranz et al. 2013; Eiermann et al. 2013; van Loon 2007; Grimm et al. 2006; Grimm et al. 2014).
The 2D clinostat (Fig. 1a) contains a horizontal rotation axis, where the sample is constantly rotated perpendicular to the gravity vector. In contrast, the RPM (Fig. 1b) contains two independently rotating frames, enabling a rotation around two axes (van Loon 2007). The RPM is operated in a random direction and random speed mode. Therefore, the influence of the gravity vector with respect to the samples is constantly changed, which assures a maximum of randomization.
The 2D clinostat is operated with a constant speed of 60 rpm, which enables highest µg-simulation quality. In addition, the radius around the rotation axis should not exceed 1-1.5 mm because of increasing centrifugal forces (Häder et al. 2005; Klaus et al. 1998). Given a speed of 60 rpm and a radius around the centre of 1.5 mm, the residual acceleration is 10 −3 g.
In case of the RPM, operated in real random mode with a highest speed of 60-75 ∘/s (which is equivalent to 12.5 rpm) and a maximum distance of 7 cm to the rotation centre, the residual acceleration over time is between 10 −4 and 10 −2 g (van Loon 2007).
Thyroid Cell Lines Cultured Under Conditions of Simulated and Real Microgravity
Nthy-ori 3-1
The cell line Nthy-ori 3-1 was derived from normal human primary thyroid follicular epithelial cells of a 35-year-old female patient. The cells were transfected with a plasmid containing an origin-defective SV40 genome for immortalization (Lemoine et al. 1989). They show thyroid epithelial functions like iodide trapping and thyroglobulin production, but are non-tumorigenic in nude mice (Lemoine et al. 1989).
HTU-5
The normal thyroid cell line HTU-5 was derived from healthy human thyroid tissue. HTU-5 thyroid cells produce thyroglobulin constitutively and exert normal diploid chromosome numbers (Curcio et al. 1994). The cells were cultured in Coon’s F-12 medium containing a mixture of growth factors as described earlier (Curcio et al. 1994).
FTC-133
The FTC-133 is classified as a poorly differentiated follicular thyroid cancer cell line. It was derived from a lymph node metastasis of a 42-year-old male patient (Goretzki et al. 1990). Nevertheless, the cells show thyroglobulin immunoreactivity, response to thyroid-stimulating hormone and epidermal growth factor receptors in the membrane (Goretzki et al. 1990).
ML-1
The human thyroid carcinoma cell line ML-1 originates from a dedifferentiated follicular thyroid carcinoma relapse of a 50-year-old female patient (Schönberger et al. 2000). The tumour progressed despite previous surgery and two radioiodine therapies. The cells are able to take up iodine and/or glucose in vitro and in vivo. Furthermore, they express and secrete thyroglobulin. Xenotransplantation in NMRI nude mice showed tumourigenic capacity, with the formation of tumours with follicular structures, in vivo (Schönberger et al. 2000).
Biological Responses to Simulated Microgravity
RPM- and CN-Exposure Induced Spheroid Formation in Thyroid Cancer Cell Lines and Normal Thyrocytes
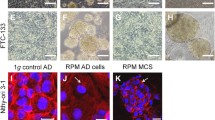
When cultured under normal 1g-conditions on Earth, thyroid carcinoma cells grew in form of an adherent monolayer. Already in 2000, Martin et al. have demonstrated that normal thyrocytes grow three-dimensionally in form of thyroid follicles, when they were cultured in a rotary cell culture system (RCCS). The cells produced thyroglobulin, when they were grown in the RCCS (Martin et al. 2000). The authors showed that these artificial human thyroid organoids generated in the RCCS and in the presence of keratinocyte growth factor structurally resembled natural thyroid tissue. Interestingly, several types of cells undergo a transition when exposed to simulated microgravity. It was shown, that an exposure to either RPM or CN lead to the detachment and formation of 3D aggregates, so-called multicellular spheroids (MCS) for some cells, while others remained adherent (AD). This transition from 2- to 3-dimensional growth is of high interest for tissue engineering but also for possible cancer therapy (Grimm et al. 1997, 2014). In 2002 we demonstrated for the first time that ML-1 thyroid cancer cells changed their growth behaviour and form MCS on the 3D clinostat (Grimm et al. 2002) (Table 1).
When FTC-133 thyroid carcinoma cells were cultured on the RPM for 24 h, one part of the cells started to form MCS, which increased in size up to 72 h, while another part remained adherent (Grosse et al. 2012). These changes in growth behaviour were observed in each experiment (Grosse et al. 2012), but also in normal thyrocytes, which had been cultured for 7 d on the RPM (Wuest et al. 2015).
FTC-133 investigated at early time points: 4 h, 24 h and 72 h showed an early onset of spheroid formation after 24 h but numerous and much larger spheroids after 72 h (Warnke et al. 2014). No spheroids were visible in 1g control cultures.
This spheroid formation is of special interest, as it occurs in a scaffold-free manner and is therefore a very promising approach for tissue engineering. The 3D structures resemble the in vivo situation much better than conventional cell culture in 2D cell monolayer. Studies on molecular mechanisms, tumor cell apoptosis and the angiogenesis process in co-cultures will be of high interest in future studies (Grimm et al. 2014, Grimm et al. 1997). In a proteomic study to analyse the spheroid formation of two human thyroid cell lines cultured on a RPM, Pietsch et al. found that FTC-133 cells express surface proteins that bind fibronectin, strengthening the 3D cell cohesion (Pietsch et al. 2011).
The Cytoskeleton as a Possible Gravisensor
The cytoskeleton is a dynamic structure, which gives shape and mechanical strength to cells but also enables the adaption to external stimuli. This phenomenon can be nicely visualized by the transformation from 2D to 3D growth as observed under real and simulated microgravity conditions (Ma et al. 2014). The underlying biological and molecular mechanisms remain mostly unclear, while it is obvious, that the physical force of gravity needs to be translated into a biochemical signal. The cytoskeleton is therefore suggested to play a role as “gravisensor” in cells lacking a distinct and so far known mechanism for gravity perception (Vorselen et al. 2014).
When follicular thyroid cancer cells were investigated during parabolic flight manoeuvres (Ulbrich et al. 2011), it was shown that the response to altered gravity conditions of ML-1 cells occurred very early, within the first few seconds. After 22 s of microgravity, the F-actin and cyto-keratin cytoskeleton was altered, and in parallel ACTB and KRT80 mRNAs were significantly up-regulated after the first parabola (Ulbrich et al. 2011). Studies performed on sounding rockets revealed that the F-actin content increased in A431 epidermoid carcinoma cells after 7 min under microgravity (Boonstra 1999), leading to the suggestion that the actin microfilament system is sensitive to changes in gravity and that remodelling of actin microfilaments may affect signal transduction. Another interesting finding was the detection of septin-11 (SEPT11) in HTU-5 cells (Pietsch et al. 2010). The proteomic discovery of SEPT11 accumulation in HTU-5 cells indicates a role for this cytoskeleton-associated protein in thyrocyte biology. The impact of microgravity on SEPT11 will be studied in detail in future studies.
A 7-day-exposure of Nthy-ori-3-1 cells induced a clear elevation of β-actin protein in AD and MCS cells as measured by Western blot analysis (Fig. 2a). In addition, we detected a significant increase in β-tubulin protein in AD and MCS after a 7-day-culture on the RPM (Fig. 2b). In contrast, β-actin remained unchanged in FTC-133 cells cultured for 7 d on the RPM and β-tubulin was significantly elevated in MCS compared with corresponding 1 g−controls (Fig. 2c, d).
Expression of Growth Factors in Microgravity
Measurements of Vascular Endothelial Growth Factor (VEGF) in the supernatants after a 3-day-exposure of FTC-133 to either CN or RPM showed a reduced (but not significant) secretion of VEGF (Warnke et al. 2014) (Table 2). In contrast, in Space after 10 d no change in the VEGF release was measured, whereas after 10 d on the RPM the cells released a significantly decreased amount of the cytokine (Ma et al. 2014). In general, VEGF is known to promote neoangiogenesis and is therefore an important player for growth and metastasis of tumours (Grimm et al. 2009). A target-based therapy is already of special interest in tumour therapy (Wehland et al. 2012).
No MCS had been detected on both ground-based facilities, CN and RPM, after 4 h, but a change in the gene expression of several cytokines was expected. The Connective Tissue Growth Factor (CTGF) mRNA was significantly enhanced on both devices (Warnke et al. 2014). After 72 h, CTGF mRNA was still elevated in AD cells on the RPM, but normalized in MCS (Warnke et al. 2014). On the CN there was only a moderate, but not significant elevation, whereas the CTGF mRNA was significantly down-regulated in MCS (Warnke et al. 2014). In Space, the CTGF gene expression was up-regulated in AD and MCS, whereas the CTGFelevation was more pronounced in AD (Pietsch et al. 2013). A clear up-regulation of Epidermal Growth Factor (EGF) mRNA in AD and MCS was found in Space and on the RPM compared with controls (Pietsch et al. 2013). Interestingly, EGF remained static in FTC-133 cells grown on the CN, whereas EGF was up-regulated in MCS on the RPM after a time-period of 72 h (Warnke et al. 2014). These data suggest that EGF and CTGF play a key role in the 3D formation of thyroid cancer cells when they are grown in Space and on the RPM.
Martin et al. have demonstrated in 2000 that recombinant human keratinocyte growth factor facilitated 3D growth of human thyrocytes.
Caveolins are integral membrane proteins and components of caveolae membranes. A higher caveolin expression results in an inhibition of cancer-related pathways (growth factor signaling). We have shown that in MCS engineered in the CN or RPM the CAV1gene expression is down-regulated in FTC-133 thyroid cancer cells after a 72-hour-exposure (Warnke et al. 2014; Table 2). An up-regulation of caveolin-1 was found in mouse thyroid glands after a three-month-spaceflight (Masini et al. 2012), a similar result was found when mice were exposed to hypergravity (Albi et al. 2014, Table 1).
Involvement of Il-6 and Il-8 During Gravisensitive Signalling
Since the discovery of IL-6 in 1986, the knowledge on this cytokine for immune homeostasis and its pathophysiology has rapidly increased (Rath et al. 2015). IL-6 is a key cytokine for linking chronic inflammation to cancer development (Rath et al. 2015). IL-6 is a multifunctional cytokine and is expressed by human thyrocytes (Grubeck-Loebenstein et al. 1989; Aust and Scherbaum 1996). It induces the production of VEGF and is involved in neoangiogenesis (Tartour et al. 2011) and thus, may be involved in 3D formation in Space or in simulated microgravity using a RPM or 2D CN. IL-6 plays an important role in modifying various tumour characteristics, such as proliferation, migration, differentiation, apoptosis, angiogenesis, invasion and adhesion thus promoting tumour growth and metastasis (reviewed by Tartour et al. 2011). Mechanical stress or stretching enhances IL-6 production in human lung epithelial cells and smooth muscle cells via NF- κB (Copland and Post 2007, Zampetaki et al. 2005). FTC-133 cells showed an enhanced IL6 gene expression in AD cells and no change in MCS when they were cultured on the RPM for 24 h (Grosse et al. 2012). A similar finding was observed after a 4-hour- and 72-hour-exposure of FTC-133 cells on the RPM, but no change was found for the IL6 gene expression on the 2D CN (Fig. 3a-d) (Warnke et al. 2014). Using MAP technology, a significantly reduced release of IL-6 protein in the supernatant was found in CN, but an increase in RPM samples (Warnke et al. 2014). We recently showed that a PKCa-independent mechanism of IL6 gene activation is very sensitive to physical forces in thyroid cells cultured in vitro as monolayers under conditions of vibration or hypergravity (Ma et al. 2013).
These findings nicely correspond to earlier data, suggesting an involvement of IL-6 in gravity-sensitive signalling for spheroid formation (Ma et al. 2013, 2014). In a new study we could show for the first time that both cytokines IL-6 and IL-8 induced the formation of MCS in ML-1 and UCLA RO82-W-1 cells using the liquid-overlay technique under 1g-conditions (Svejgaard et al. 2015). These investigations support the hypothesis that IL-6 is one of the key factors inducing spheroid formation in Space and on the RPM and CN.
Earlier studies with FTC-133 cells suggest that gravitational unloading leads to an initiation of an early phase of apoptosis. An escape from the late phase then leads to the transition from 2D to 3D growth (Grosse et al. 2012; Grimm et al. 2014).
Interleukin-8 is a chemokine produced by a variety of cell types. In humans the interleukin-8 protein is encoded by the IL8 gene. This cytokine is a known strong promoter of angiogenesis. A recent study demonstrated that NF- κB signalling is a key regulator of angiogenesis and growth in thyroid cancer, and that IL-8 may be an important downstream mediator of NF- κB signalling in advanced thyroid cancer growth and progression (Bauerle et al. 2014). FTC-133 cells cultured for 72 h on the CN showed a significant reduction of the IL8 gene expression in AD cells and MCS (Warnke et al. 2014). Interestingly, we did not observe significant changes of the IL8 mRNA after RPM exposure of the FTC-133 cells (Warnke et al. 2014). The secretion of IL-8 protein in the medium of FTC-133 cells cultured on the CN was significantly reduced, whereas a different result was found in the RPM cultures, which exerted an increase (Warnke et al. 2014, Table 2). Interestingly, a different gene expression of IL8 and differences in the IL-8 secretion behaviour of the cells were found. Reasons for this may be the different culture chambers, which had to be used due to the geometry of the devices. We have used slide flasks on the CN and T 75 cm 2 cell culture flasks on the RPM (Warnke et al. 2014). In a planned future study we will use slide flasks for both devices. Another aspect may be the impact of vibrations, which are critical for the release of cytokines by human cells. The controls were stored next to the device in the same incubator so that the influence should be minimal but this has to be investigated in the future in more detail.
Eiermann et al. (2013) had also found significant differences in the gene expression in cells located at a further distance from the CN rotation axis. These cells are exposed to higher accelerations. Therefore, only cells within the inner 6 mm of the slide flasks were collected. A problem is that the supernatant consists of released proteins from all cells. This means that also the release of cells exposed to higher accelerations (though still less than 0.036g) was measured. This problem might explain some of the differences.
Conclusions and Recommendations
Taken these data together, microgravity induces a variety of changes in thyroid cells. The thyroid cancer cells revealed signs of apoptosis (Grimm et al. 2002; Kossmehl et al. 2003), changed their growth behaviour, differentiation, migration and cell adhesion. Interestingly, already after a short-term sounding rocket flight rat FRTL-5 thyrocytes showed an increase in Bax as well as an irregular shape (Albi et al. 2011).
Spheroid formation was detected in several cell lines in ground-based facilities and also in real microgravity. It demonstrated the good practicability of ground-based devices like RPM and CN for scaffold-free tissue engineering of multicellular spheroids.
It is important to keep in mind that each device affects the cells not only by randomization of the gravity vector but also by device-specific artefacts like vibration, centrifugal accelerations and shearing forces. The susceptibility of cells to gravity alterations but also to these artefacts might vary broadly between different cell lines. A careful and conscious handling of ground-based devices is therefore suggested, with real microgravity experiments as an indispensable tool for validation to identify gravity-related effects (Herranz et al. 2013).
Changes in cytoskeletal proteins were found very early in real and simulated microgravity (Ulbrich et al. 2011; Grosse et al 2012; Pietsch et al. 2011). These observations nicely fit to early studies, reporting a cytoskeletal involvement in the transition from 2D to 3D growth behaviour (Grimm et al. 2014). So far all investigations had been made after termination of the experiments. The cells were fixed with paraformaldehyde and then stained by immunofluorescence. Great new insights are expected by the German national DLR project FLUMIAS in which a Fluorescence Microscopic Analysis System for biological and biomedical research in Space has been developed, enabling in-vivo 3D fluorescence analyses of biological samples in microgravity. FLUMIAS developed by ADS, Bremen, Germany had been successfully flown on TEXUS 52 sounding rocket in April 2015, launch site Esrange, Kiruna, Sweden. Here, FTC-133 poorly differentiated follicular thyroid cancer cells together with other cells were investigated online during a 6-min-exposure to real microgravity allowing visualization of dynamics and adaption of the cytoskeleton. These data will be published soon.
The fast rotating 2D Clinostat and the Random Positioning Machine are important ground-based devices for tissue engineering of spheroids which can be used in cancer research to study drug effects and to spare animal tests. In addition, these devices can be applied for the preparation of a future spaceflight. It is important to know when and how spheroid formation occurs and the mechanisms behind 3D growth. We become able to answer questions like, how big are the spheroids, how many are formed, do they have an impact on the operational capability of the hardware, are filters necessary to avoid that the spheroids block the tubes of the hardware, what happens to the cells when the launch is delayed, or simply to test a newly constructed hardware container under simulated microgravity conditions or check the influence of temperature changes on the cells. The ESA-CORA-Ground-based facility program has supported us to answer these questions and to prepare the SIMBOX/Shenzhou-8 in 2011 and the Cellbox-1 Space missions in 2014 (Pietsch et al. 2013, Ma et al. 2014, Riwaldt et al. 2015).
Abbreviations
∘ /s: Degrees per Second
2D: Two-dimensional
3D: Three-dimensional
ACTB: β-actin gene
AD: adherent cells in simulated microgravity samples
ADS: Airbus Defence and Space
Bax : Bcl-2-associated X protein gene
Bcl-2: B-cell Lymphoma 2 gene
Cav1/2: Caveolin 1/2 genes
cm: centimetre
CN: Clinostat
CORA: Continuously Open Research Announcement
CTGF: Connective Tissue Growth Factor
DLR: Deutsches Zentrum für Luft- und Raumfahrt
EGF: Epidermal Growth Factor
ERK1/2: Extracellular Signal-Regulated Kinases 1/2 genes
ESA: European Space Agency
f-actin: filamentous actin
FCS: Fetal Calf Serum
FLUMIAS: Fluorescence Microscopic Analysis System
FTC: Follicular Thyroid Carcinoma
g: Gravity
GBF: Ground-based facilities
h: hour
IL: Interleukin
ITGB1: Integrin Beta-1 gene
KRT80: Keratin 80 gene
MAP: Multi-Analyte Profiling
MCP1: Monocyte Chemotactic Protein 1
MCS: Multicellular Spheroids
min: minute
mm: millimetre
NF- κB: Nuclear Factor ’kappa-light-chain-enhancer’ of Activated B-cells
NMRI: Naval Medical Research Institute mice
OPN: Osteopontin gene
PCR: Polymerase Chain Reaction
PFC: Parabolic Flight Campaign
PKCa: Protein kinase Ca
PRKCA: Protein Kinase C alpha gene
RBM: Rules-Based Medicine
RPM: Random Positioning Machine
rpm: revolutions per minute
PCR: Polymerase Chain Reaction
s-μ g: Simulated Microgravity
SD: Standard Deviation
SV40: Simian Virus 40
VEGF: Vascular Endothelial Growth Factor
References
Albi, E., Curcio, F., Lazzarini, A., Floridi, A., Cataldi, S., Lazzarini, R., Loreti, E., Ferri, I., Ambesi-Impiombato, F. S.: How microgravity changes galectin-3 in thyroid follicles. Biomed. Res. Int. 2014, 652863 (2014)
Albi, E., Curcio, F., Lazzarini, A., Floridi, A., Cataldi, S., Lazzarini, R., Loreti, E., Ferri, I., Ambesi-Impiombato, F.S.: A firmer understanding of the effect of hypergravity on thyroid tissue: cholesterol and thyrotropin receptor. PLoS One 9, e98250 (2014b)
Albi, E., Curcio, F., Spelat, R., Lazzarini, A., Lazzarini, R., Cataldi, S., Loreti, E., Ferri, I., Ambesi-Impiombato, F. S.: Loss of parafollicular cells during gravitational changes (microgravity, hypergravity) and the secret effect of pleiotrophin. PLoS One 7, e48518 (2012)
Albi, E., Curcio, F., Spelat, R., Lazzarini, A., Lazzarini, R., Loreti, E., Ferri, I., Ambesi-Impiombato, F. S.: Observing the mouse thyroid sphingomyelin under space conditions: a case study from the MDS mission in comparison with hypergravity conditions. Astrobiology 12, 1035–41 (2012)
Albi, E., Ambesi-Impiombato, F.S., Peverini, M., Damaskopoulou, E., Fontanini, E., Lazzarini, R., Curcio, F., Perrella, G.: Thyrotropin receptor and membrane interactions in FRTL-5 thyroid cell strain in microgravity. Astrobiology 11, 57–64 (2011)
Aust, G., Scherbaum, W.A.: Expression of cytokines in the thyroid: thyrocytes as potential cytokine producers. Exp. Clin. Endocrinol. Diabetes 104, 64–67 (1996)
Bauerle, K.T., Schweppe, R.E., Lund, G., Kotnis, G., Deep, G., Agarwal, R., Pozdeyev, N., Wood, W.M., Haugen, B.R.: Nuclear factor κB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J. Clin. Endocrinol. Metab 99, E1436–E1444 (2014)
Becker, J.L., Souza, G.R.: Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 13, 315–327 (2013)
Boonstra, J.: Growth factor-induced signal transduction in adherent mammalian cells is sensitive to gravity. FASEB J. 13, 35–42 (1999)
Briegleb, W.: Some qualitative and quantitative aspects of the fast-rotating clinostat as a research tool. ASGSB Bull. 5, 23–30 (1992)
Copland, I.B., Post, M.: Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J. Cell Physiol. 10, 133–143 (2007)
Curcio, F., Ambesi-Impiombato, F.S., Perrella, G., Coon, H.G.: Long-term culture and functional characterization of follicular cells from adult normal human thyroids. Proc. Natl. Acad. Sci. 91, 9004–9008 (1994)
Eiermann, P., Kopp, S., Hauslage, J., Hemmersbach, R., Gerzer, R., Ivanova, K.: Adaptation of a 2D clinostat for simulated microgravity experiments with adherent cells. Microgravity Sci. Tech. 25, 153–159 (2013)
Freed, L.E., Langer, R., Martin, I., Pellis, N.R., Vunjak-Novakovic, G.: Tissue engineering of cartilage in space. Proc. Natl. Acad. Sci. USA 94, 13885–13890 (1997)
Goretzki, P.E., Frilling, A., Simon, D., Roeher, H.D.: Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 118, 48–63 (1990)
Grimm, D., Bauer, J., Hofstädter, F., Riegger, G.A., Kromer, E.P.: Characteristics of multicellular spheroids formed by primary cultures of human thyroid tumor cells. Thyroid 7, 859–865 (1997)
Grimm, D., Bauer, J., Kossmehl, P., Shakibaei, M., Schönberger, J., Pickenhahn, H., Schulze-Tanzil, G., Vetter, R., Eilles, C., Paul, M., Cogoli, A.: Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 16, 604–606 (2002)
Grimm, D., Bauer, J., Infanger, M., Cogoli, A.: The use of the random positioning machine for the study of gravitational effects on signal transduction in mammalian cells. Signal Transduct. 6, 388–396 (2006)
Grimm, D., Bauer, J., Schoenberger, J.: Blockade of neoangiogenesis, a new and promising technique to control the growth of malignant tumors and their metastases. Curr. Vasc. Pharmacol. 7, 347–357 (2009)
Grimm, D., Wehland, M., Pietsch, J., Aleshcheva, G., Wise, P., van Loon, J., Ulbrich, C., Magnusson, N.E., Infanger, M., Bauer, J.: Growing tissues, in real and simulated microgravity: new methods for tissue engineering. Tissue Eng. Part B Rev. 20, 555–566 (2014)
Grosse, J., Wehland, M., Pietsch, J., Schulz, H., Saar, K., Hübner, N., Eilles, C., Bauer, J., Abou-El-Ardat, K., Baatout, S., Ma, X., Infanger, M., Hemmersbach, R., Grimm, D.: Gravity-sensitive signaling drives 3-dimensional formation of multicellular thyroid cancer spheroids. FASEB J. 26, 5124–5140 (2012)
Grubeck-Loebenstein, B., Buchan, G., Chantry, D., Londei, M., Turner, M., Pirich, K., Roka, R., Niederle, B., Kassal, H.: Analysis of intrathyroidal cytokine production in thyroid autoimmune disease: thyroid follicular cells produce interleukin-1 alpha and interleukin-6. Clin. Exp. Immunol. 77, 324–330 (1989)
Häder, D., Hemmersbach, R., Lebert, M.: Gravity and the behaviour of unicellular organisms. Cambridge University Press, New York (2005)
Herranz, R., Anken, R., Boonstra, J., Braun, M., Christianen, P.C., de Geest, M., Hauslage, J., Hilbig, R., Hill, R.J., Lebert, M., Medina, F.J., Vagt, N., Ullrich, O., van Loon, J.J., Hemmersbach, R.: Ground-based facilities for simulation of microgravity: organism-specific recommend-dations for their use, and recommended terminology. Astrobiology 13, 1–17 (2013)
Infanger, M., Ulbrich, C., Baatout, S., Wehland, M., Kreutz, R., Bauer, J., Grosse, J., Vadrucci, S., Cogoli, A., Derradji, H., Neefs, M., Küsters, S., Spain, M., Grimm, D.: Modeled gravitational unloading induced downregulation of endothelin-1 in human endothelial cells. J. Cell. Biochem. 101, 1439–1455 (2007)
Klaus, D.M., Todd, P., Schatz, A.: Functional weightlessness during clinorotation of cell suspensions. Adv. Space Res. 21(8-9), 1315–1318 (1998)
Kossmehl, P., Cogoli, A., Shakibaei, M., Pickenhahn, H., Paul, M., Grimm, D.: Simulated microgravity induces programmed cell death in human thyroid carcinoma cells. J. Gravit. Physiol. 9, 295–296 (2002)
Kossmehl, P, Shakibaei, M, Cogoli, A, Infanger, M, Curcio, F, Schönberger, J, Eilles, C, Bauer, J, Pickenhahn, H, Schulze-Tanzil, G, Paul, M, Grimm, D: Weightlessness induced apoptosis in normal thyroid cells and papillary thyroid carcinoma cells via extrinsic and intrinsic pathways. Endocrinology 144 (9), 4172–4179 (2003)
Kossmehl, P., Kurth, E., Faramarzi, S., Habighorst, B., Shakibaei, M., Wehland, M., Kreutz, R., Infanger, M., Danser, A.H., Grosse, J., Paul, M., Grimm, D.: Mechanisms of apoptosis after ischemia and reperfusion: role of the renin angiotensin system. Apoptosis 11, 347–358 (2006)
Lemoine, N.R., Mayall, E.S., Jones, T., Sheer, D., Mcdermid, S., Kendalltaylor, P., Wynfordthomas, D.: Characterisation of human thyroid epithelial-cells immortalised in vitro by simian-virus 40-DNA transfection. Br. J. Cancer 60, 897–903 (1989)
Ma, X., Wehland, M., Aleshcheva, G., Hauslage, J., Wasser, K., Hemmersbach, R., Infanger, M., Bauer, J., Grimm, D.: Interleukin-6 expression under gravitational stress due to vibration and hypergravity in follicular thyroid cancer cells. PLoS One 8, e68140 (2013)
Ma, X., Pietsch, J., Wehland, M., Schulz, H., Saar, K., Hübner, N., Bauer, J., Braun, M., Schwarzwälder, A., Segerer, J., Birlem, M., Horn, A., Hemmersbach, R., Wasser, K., Grosse, J., Infanger, M., Grimm, D.: Differential gene expression profile and altered cytokine secretion of thyroid cancer cells in space. FASEB J. 28, 813–835 (2014)
Martin, A., Zhou, A., Gordon, R.E., Henderson, S.C., Schwartz, A.E., Schwartz, A.E., Friedman, E.W., Davies, T.F.: Thyroid organoid formation in simulated microgravity: influence of keratinocyte growth factor. Thyroid 10, 481–7 (2000)
Masini, M. A., Albi, E., Barmo, C., Bonfiglio, T., Bruni, L., Canesi, L., Cataldi, S., Curcio, F., D’Amora, M., Ferri, I., Goto, K., Kawano, F., Lazzarini, R., Loreti, E., Nakai, N., Ohira, T., Ohira, Y., Palmero, S., Prato, P., Ricci, F., Scarabelli, L., Shibaguchi, T., Spelat, R., Strollo, F., Ambesi-Impiombato, F.S.: The impact of long-term exposure to space environment on adult mammalian organisms: a study on mouse thyroid and testis. PLoS One 7, e35418 (2012)
Meli, A., Perrella, G., Curcio, F., Hemmersbach, R., Neubert, J., Impiombato, F. A.: Response to thyrotropin of normal thyroid follicular cell strain FRTL5 in hypergravity. Biochimie 81, 281–5 (1999)
Meli, A., Perrella, G., Curcio, F., Ambesi-Impiombato, F. S.: Response to hypogravity of normal in vitro cultured follicular cells from thyroid. Acta Astronaut 42, 465–72 (1998)
Pietsch, J., Kussian, R., Sickmann, A., Bauer, J., Weber, G., Nissum, M., Westphal, K., Egli, M., Grosse, J., Schönberger, J., Wildgruber, R., Infanger, M., Grimm, D.: Application of free-flow IEF to identify protein candidates changing under microgravity conditions. Proteomics 10, 904–13 (2010)
Pietsch, J., Sickmann, A., Weber, G., Bauer, J., Egli, M., Wildgruber, R., Infanger, M., Grimm, D.: A proteomic approach to analysing spheroid formation of two human thyroid cell lines cultured on a random positioning machine. Proteomics 11, 2095–2104 (2011)
Pietsch, J., Sickmann, A., Bauer, J., Weber, G., Nissum, M., Westphal, K., Egli, M., Grosse, J., Schönberger, J., Eilles, C., Infanger, M., Grimm, D.: Proteome analysis of thyroid cancer cells after long-term exposure to simulated microgravity. Microgravity Sci. Technol. 23, 381–390 (2011)
Pietsch, J., Ma, X., Wehland, M., Aleshcheva, G., Schwarzwälder, A., Segerer, J., Birlem, M., Horn, A., Bauer, J., Infanger, M., Grimm, D.: Spheroid formation of human thyroid cancer cells in an automated culturing system during the Shenzhou-8 Space mission. Biomaterials 34, 7694–7670 (2013)
Pietsch, J., Riwaldt, S., Bauer, J., Sickmann, A., Weber, G., Grosse, J., Infanger, M., Eilles, C., Grimm, D.: Interaction of proteins identified in human thyroid cells. Int. J. Mol. Sci. 14, 1164–1178 (2013)
Rath, T., Billmeier, U., Waldner, M.J., Atreya, R., Neurath, M.F.: From physiology to disease and targeted therapy: interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch. Toxicol. 89, 541–554 (2015)
Riwaldt, S., Pietsch, J., Sickmann, A., Bauer, J., Braun, M., Segerer, J., Schwarzwälder, A., Aleshcheva, G., Corydon, T.J., Infanger, M., Grimm, D.: Identification of proteins involved in inhibition of spheroid formation under microgravity. Proteomics 15, 2945–2952 (2015)
Rothermund, L., Kreutz, R., Kossmehl, P., Fredersdorf, S., Shakibaei, M., Schulze-Tanzil, G., Paul, M., Grimm, D.: Early onset of chondroitin sulfate and OPN expression in angiotensin II-dependent left ventricular hypertrophy. Am. J. Hypertens. 15, 644–652 (2002)
Schönberger, J., Bauer, J., Spruß, T., Weber, G., Chahoud, I., Eilles, C., Grimm, D.: Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J. Mol. Med. 78, 102–110 (2000)
Stamenković, V., Keller, G., Nesic, D., Cogoli, A., Grogan, S.P.: Neocartilage formation in 1 g, simulated, and microgravity environments: implications for tissue engineering. Tissue Eng. Part A 16, 1729–1736 (2010)
Svejgaard, B., Wehland, M., Ma, X., Kopp, S., Sahana, J., Warnke, E., Aleshcheva, G., Hemmersbach, R., Hauslage, J., Grosse, J., Bauer, J., Corydon, T.J., Islam, T., Infanger, M., Grimm, D.: Common effects on cancer cells exerted by a Random Positioning Machine and a 2D clinostat. PLoS One 10, e0135157 (2015)
Tartour, E., Pere, H., Maillere, B., Terme, M., Merillon, N., Taieb, J., Sandoval, F., Quintin-Colonna, F., Lacerda, K., Karadimou, A., Badoual, C., Tedgui, A., Fridman, W.H., Oudard, S.: Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 30, 83–95 (2011)
Ulbrich, C., Pietsch, J., Grosse, J., Schulz, H., Saar, K., Hübner, N., Hemmersbach, R., Braun, M., van Loon, J. J. W. A., Vagt, N., Egli, M., Richter, P., Einspanier, R.: Differential gene regulation under altered gravity conditions in follicular thyroid cancer cells: relationship between the extracellular matrix and the cytoskeleton. Cell Physiol. Biochem. 28, 185–198 (2011)
Van Loon, J.J.: Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 39, 1161–1165 (2007)
Vorselen, D., Roos, W.H., MacKintosh, F.C., Wuite, G. J., van Loon, J.J.: The role of the cytoskeleton in sensing changes in gravity by non-specialised cells. FASEB J 28, 536–547 (2014)
Warnke, E., Pietsch, J., Wehland, M., Bauer, J., Infanger, M., Görög, M., Hemmersbach, R., Braun, M., Ma, X., Sahana, J., Grimm, D.: Spheroid formation of human thyroid cancer cells under simulated microgravity: a possible role of CTGF and CAV1. Cell Commun. Signal 12, 32 (2014)
Wehland, M., Bauer, J., Infanger, M., Grimm, D.: Target-based anti-angiogenic therapy in breast cancer. Curr. Pharm. Des. 18, 4244–4257 (2012)
Wuest, S.L., Richard, S., Kopp, S., Grimm, D., Egli, M.: Simulated microgravity: critical review on the use of Random Positioning Machines for mammalian cell culture. BioMed Res. Int. 2015, 971474 (2015)
Zampetaki, A., Zhang, Z., Hu, Y., Xu, Q.: Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-kappaB signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 288, H2946–H2954 (2005)
Acknowledgments
The authors would like to thank the European Space Agency (ESA-CORA-GBF-PROJECT-2011-005 (ACRONYM DEVICE COMPARISON), ESA-CORA-GBF-PROJECT-2013-001 (ACRONYM THYROID III), D.G.) and the German Space Administration (DLR; BMWi grants 50WB1124/50WB1524; D.G.). Elisabeth Warnke is a doctoral candidate of the Helmholtz Space Life Sciences Research School, German Aerospace Center Cologne, Germany and was further funded by the German only one space Aviation and Space Medicine (DGLRM; Young Fellow Program).
Author Contributions
This review is based on data of the the ESA-CORA-GBF-PROJECT-2011-005 (ACRONYM DEVICE COMPARISON) and ESA-CORA-GBF-PROJECT-2013-001 (ACRONYM THYROID III).
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warnke, E., Kopp, S., Wehland, M. et al. Thyroid Cells Exposed to Simulated Microgravity Conditions – Comparison of the Fast Rotating Clinostat and the Random Positioning Machine. Microgravity Sci. Technol. 28, 247–260 (2016). https://doi.org/10.1007/s12217-015-9456-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12217-015-9456-7