Abstract
It was discovered over 60 years ago that the mitochondrial respiratory chain is constituted of a series of protein complexes imbedded in the inner mitochondrial membrane. Experimental evidence has more recently ascertained that the major respiratory complexes involved in energy conservation are assembled as supramolecular units (supercomplexes, SCs) in stoichiometric ratios. The functional role of SCs is less well defined, and still open to discussion. Several lines of evidence favour the concept that electron transfer from Complex I to Complex III operates by channelling of electrons through Coenzyme Q molecules bound to the SC I1III2IV n , in contrast with the previously accepted hypothesis that the transfer of reducing equivalents from Complex I to Complex III occurs via random diffusion of the Coenzyme Q molecules in the lipid bilayer. On the contrary, electron transfer from Complex III to Complex IV seems to operate, at least in mammals, by random diffusion of cytochrome c molecules between the respiratory complexes even if assembled in SCs. Furthermore, another property provided by the supercomplex assembly is the control of generation of reactive oxygen species by Complex I, that might be important in the regulation of signal transduction from mitochondria. This review discusses physiological and pathological implications of the supercomplex assembly of the respiratory chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The advancement of Science is long and tortuous: it often happens that novel discoveries, rather than implementing the background of established knowledge, introduce uncertainties and controversies. This is exactly the case of the discovery and function of respiratory supercomplexes (SCs). To this purpose it is useful to perform a brief historical outline of the respiratory chain, since some discoveries of the past have anticipated some of the present knowledge, although they were surprisingly neglected at the time.
1 The discovery of the respiratory chain
The first real understanding of the respiratory chain is due to Britton Chance at the beginning of the 50’s of the last century. It had just been discovered that respiration takes place in mitochondria, where also the enzymes of fatty acid oxidation and Krebs cycle are localized (Kennedy and Lehninger 1949), when Chance and Williams (1955) on the basis of sophisticated spectroscopic studies depicted the respiratory chain as a solid-state assembly of flavins and cytochromes in a protein matrix. Though aware that respiration relies on enzymes, they formulated the following respiratory chain for substrate oxidation [note the unusual order of the components in the sequence (1) originally published by the authors, stressing oxidation of cytochromes a3/a, c, b, flavoprotein (fp) and nicotinamide adenine dinucleotide, formerly known as diphosphopyridine nucleotide (DPN), rather than substrate dehydrogenation]:
Hatefi et al. (1962a) in David Green’s laboratory in Wisconsin, accomplished the systematic resolution and reconstitution of four respiratory complexes from mitochondria, thus leading Green (Green and Tzagoloff 1966) to postulate that the overall respiratory activity (Fig. 1) is the result of both intra-complex electron transfer in the ‘solid’ state of redox components (e.g. flavins, FeS clusters, cytochromes) having fixed steric relation and, in addition, of inter-complex electron transfer ensured by rapid diffusion of mobile components acting as co-substrates, i.e. ubiquinone (Coenzyme Q, CoQ), a lipophilic quinone inserted in the membrane lipid bilayer (cf. below), and cytochrome c (cyt. c), a hydrophilic heme protein confined to the water phase of the mitochondrial intermembrane space, in close contact with the external surface of the inner membrane.

Reprinted from Green and Tzagoloff (1966) with permission from Elsevier
Scheme of the respiratory chain. Sequence, composition, and stoichiometry of the four complexes of the mitochondrial electron transfer chain, as originally drawn by Green and Tzagoloff (1966). nHFe, nonheme iron; Fd, acid-extractable flavin; Fs, acid-nonextractable flavin.
In fact, Hatefi et al. (1962a) had been able to isolate, from mitochondria of beef heart, four primary enzyme complexes that are operative in the oxidation of succinate and NADH, namely NADH-Coenzyme Q reductase (Complex I, CI), succinate-Coenzyme Q reductase (Complex II, CII), ubiquinol-cytochrome c reductase (Complex III, CIII or cytochrome bc1 Complex) and cytochrome c oxidase (Complex IV, CIV).
These enzyme complexes are functionally connected by their partner substrates, CoQ and cyt. c, acting as two mobile redox-active molecules.
Indeed, Coenzyme Q was discovered by Fred Crane (Crane et al. 1957) in the laboratory of David Green: its redox activity made it a plausible candidate for a respiratory chain component, as also demonstrated by Crane in extraction/reconstitution studies in bovine mitochondria.
About the same period Morton (1958) in UK isolated from mitochondria a lipophlic quinone and named it ubiquinone. According to Redfearn and Pumphrey (1960), the kinetics of CoQ reduction in the respiratory chain seemed too slow to account for a central position. Nevertheless, Crane et al. (1959) demonstrated that CoQ extraction from mitochondria abolishes respiration, that can be restored by CoQ addition. In Crane’s studies, the essentiality of CoQ for electron transfer was limited to succinate oxidation, since organic solvents destroyed NADH oxidation activity. Only almost 10 years later Ludmilla Szarkowska, visiting professor in Madison, demonstrated the role of CoQ in NADH oxidation using lyophilized pentane-extracted mitochondria (Szarkowska 1966).
In the current view, the respiratory chain functions with the operation of the respiratory enzyme complexes in the following sequence of redox reactions between the partner components:
or
The best fit unit stoichiometry between complexes in bovine heart mitochondria is: 1:1.3:3:6.7 for CI:CII:CIII:CIV (Schägger and Pfeiffer 2001).
The random distribution of the respiratory complexes embedded in the lipid bilayer of the inner mitochondrial membrane was reinforced over the following years, thanks to the careful kinetic analysis of Kröger and Klingenberg (1973a, b) (cf. Sect. 3), and the reconstitution and freeze-fracture electron microscopy studies of Hackenbrock et al. (1986) leading this latter author to postulate the Random Collision Model of Electron Transfer. According to Hackenbrock, electron transport is a diffusion-coupled kinetic process. Electron transport is a multicollisional, obstructed, long-range diffusional process. Moreover, the rates of diffusion of the redox components have a direct influence on the overall kinetic process of electron transport and can be rate limiting, as in diffusion control. In particular, it was the lateral diffusion of CoQ to be considered the rate-limiting step in substrate oxidation up to CIII.
At the same time, our research group, though not contrasting the role of CoQ diffusion in establishing useful interactions between CI and CIII or between CII and CIII, provided experimental evidence that CoQ diffusion is not rate-limiting in mitochondrial electron transfer (Lenaz and Fato 1986).
2 An unexpected shift in understanding the respiratory chain: respiratory supercomplexes
The organization of the respiratory chain has represented a major research subject in the 1970–1980’s, culminating with acceptation of the random collision model (Hackenbrock et al. 1986) by the majority of investigators in the field.
The accumulation of recent experimental evidence obtained with newly developed techniques and the instauration of a holistic approach has led to the proposal of a different model of supramolecular organisation of the respiratory chain based upon specific, though dynamic, interactions between individual respiratory components, and leading to the acquisition of new properties (substrate channelling, assembly, morphological organisation) that were unpredictable in the previous reductionist approach.
Paradoxically, evidence against a random distribution of respiratory complexes also derived from the same early investigations in Green’s laboratory, reporting isolation of CI + CIII units (Hatefi et al. 1962b), indicating that such units may be preferentially associated in the native membrane. The authors of these studies were well aware that these preferential associations were physiological and indicated the existence of supramolecular units of electron transfer. Hatefi et al. (1962b) in their paper on the reconstitution of the electron transfer system stated that
“the four primary enzyme systems may be suitably combined to form secondary units capable of catalysing the sums of the reactions catalysed by the respective subunits”.
A careful examination of these pioneering papers allows one to conclude that respiratory SCs, as well as the entire DPNH oxidase (the respirasome, see later) had been isolated and reconstituted. The appearance of the fluid mosaic model of membranes (Singer and Nicolson 1976) strongly influenced the researchers involved in the study of mitochondrial membranes, and this is probably the reason why the random collision model of Hackenbrock (Hackenbrock et al. 1986) was so well and uncritically accepted, obscuring in such way Hatefi’s (1962b) concept of intercomplex specific associations.
There were, however, a few reports before the year 2000 on the possible presence of specific associations between respiratory complexes, either fixed (Ozawa et al. 1987) or dynamic (Hochman et al. 1985).
A real breakthrough occurred only in 2000 when Schägger applied the previously introduced technique of Blue-Native Polyacrylamide Gel Electrophoresis (BN-PAGE) to digitonin-solubilized yeast and mammalian mitochondria (Schägger and Pfeiffer 2000). The newly discovered associations were considered to represent the physiological state of the respiratory complexes. In the same paper, the authors also described a dimeric state for the ATP-synthase complex.
The structural evidence of supercomplex association of the respiratory chain is nowadays well consolidated (Wittig and Schägger 2005; Enríquez 2016; Lenaz et al. 2016) and also supported by X-ray analysis (cf. Letts and Sazanov 2017 for recent review), although its functional role is still debated and a possible relation with a random distribution of the individual complexes is not completely clarified (Acin-Perez and Enriquez 2014; Genova and Lenaz 2013, 2014; Milenkovic et al. 2017).
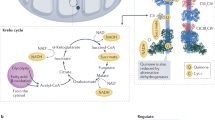
Most evidence presently favours the view that SCs are formed by the three “core” complexes of the respiratory chain, i.e. complexes I, III, and IV (Fig. 2), whereas the other respiratory enzymes may be free in the lipid bilayer (Genova and Lenaz 2010). These “core” complexes have the common feature of carrying out proton translocation and of having subunits encoded by mitochondrial DNA.

Reprinted from Letts and Sazanov (2017) with permission from Springer Nature
The supercomplexes (SCs) of the mitochondrial respiratory chain. Left, BN–PAGE analysis of digitonin-extracted mitochondrial membranes from ovine heart. Assignment of bands is based on molecular weight and comparison to proteomic and western blot, according to Letts and Sazanov (2017). The experimentally determined structural models of the different respiratory complexes and SCs, with their approximate molecular weights and architecture, are indicated at right. The atomic structures of complex I [CI, ovine, PDB 5LNK (Fiedorczuk et al. 2016)], complex II [CII, porcine, PDB 1ZOY (Sun et al. 2005)]. Complex III [CIII, bovine, PDB 1NTM (Gao et al. 2003)], complex IV [CIV, bovine, PDB 5B1A (Yano et al. 2016)] and the medium-resolution structure of complex V [CV, bovine, PDB 5ARA (Zhou et al. 2015)] are shown shaded by subunit with CI dark blue to light blue, CII cyan to light green, CIII2 green to yellow, CIV magenta to red and CV red to yellow. Atoms from the models are shown as spheres.
3 The role of supercomplexes: substrate channelling?
After the discovery of respiratory SCs it was proposed that the natural consequence of such assemblies is substrate channelling or enhanced catalysis in inter-complex electron transfer. Substrate channelling is the direct transfer of an intermediate between the active sites of two enzymes catalysing consecutive reactions (Ovádi 1991); in the case of electron transfer, this means direct transfer of electrons between two consecutive enzymes by successive reduction and re-oxidation of the intermediate without its diffusion in the bulk medium. In such a case, inter-complex electron transfer becomes indistinguishable from intra-complex electron transfer, so that ubiquinone and cyt.c (i.e. the so-called mobile intermediates predicted to exhibit substrate-like behaviour in the classic view of the random collision model, Hackenbrock et al. 1986) would rather be buried with restraints between two consecutive components of the SC.
The view that electron transfer occurs by channelling of CoQ between adjacent complexes I and III was opposed to the previous concept of CoQ pool behaviour. The functional significance of a random distribution of mitochondrial complexes connected by CoQ was supported in the early times by the kinetic analysis of Kröger and Klingenberg (1973a); they showed that steady-state respiration in submitochondrial particles from beef heart, could be modelled as a simple two-enzyme system, the first causing reduction of ubiquinone (Vred) and the second causing oxidation of ubiquinol (Vox), behaving kinetically as a homogeneous pool. According to this assumption the total CoQ molecules must effectively link any number of the dehydrogenase with any other number of the oxidase, and the activities of all the reducing and oxidizing enzymes determine the overall steady-state activity (Vobs) that is related to Vred and Vox as indicated in the so-called pool equation (Eq. 4).
The view that SCs enhance mitochondrial respiration by channelling the intermediate substrates is still challenged by some debated structural and functional evidence, as we discuss in the following sections.
3.1 Structural evidence for channelling: Molecular structure of supercomplexes
If CoQ-channelling occurs between CI and CIII, the redox groups involved in CoQ reduction by CI and CoQH2 re-oxidation in CIII must be located in such way to provide a pre-defined conducting pathway that contains CoQ itself. To ascertain this condition, it is required to have a high resolution knowledge of the molecular structure of the SCs.
At first, some SCs have been purified and analysed by negative-stain electron microscopy (Schäfer et al. 2006) and single-particle cryo-EM (Althoff et al. 2011; Dudkina et al. 2011). Pseudo-atomic models of the 1.7 MDa bovine respirasome consisting of one copy of CI, one CIII dimer and one CIV monomer were produced by fitting the X-ray structures of the component complexes to the 3D maps of the respirasome (Fig. 3).

Reprinted from Letts and Sazanov (2017) with permission from Springer Nature
Model of electron flow in the respirasome. Breaking of the inherent symmetry of CIII2 through interactions with CI and CIV may result in the formation of a CI-proximal QH2-oxidation cavity and a CI-distal Q-reduction cavity. The presence of CIV capping the Q-reduction cavity may help shield the Q· intermediate from reacting with oxygen. One possibility for symmetry breaking is steric hindrance of motion of the distal UQCRFS1 subunit (indicated by yellow star; FeS cluster shown in the distal position and colored red)34,36. Both proximal (orange FeS cluster) and distal (red FeS cluster) are shown for the proximal UQCRFS1 indicating free motion (double-headed arrow) of this subunit.
Some early evidence for possible channelling came from the observation of a unique arrangement of the three component complexes showing that the CoQ-binding sites in CI and in CIII face each other and are separated by a gap within the membrane core of the SC. In 2011, Althoff et al. (2011) proposed that CoQ is likely to run a trajectory through such a 13 nm-long gap, which lies within the membrane imbedded domains of the SC and is, therefore, most likely filled with lipids.
The recent high resolution studies by cryo-EM of mammalian mitochondrial SCs (Letts et al. 2016; Letts and Sazanov 2017; Wu et al. 2016; Sousa et al. 2016) allow to make some new predictions on the pathway taken by CoQ between CI and CIII, as shown in Fig. 3. However, understanding the mechanism of CoQ-channelling still requires more detailed knowledge of the molecular structure of the interacting sites of CI and CIII in the SC.
In theory, within the SC we may have the extremes from close docking of the active sites with real interprotein tunnelling of substrates, up to relatively long distances that may be covered either by important conformation changes of the protein itself or by restricted diffusion (microdiffusion) of the substrates within the space between the two active sites; all of these alternatives have in common obligate channelling between two fixed sites of the same SC, so that even microdiffusion would be kinetically distinguishable from random collisions as exploited through bulk diffusion, i.e. where the interaction of the substrate molecules may stochastically occur with a great number of possible sites in distinct respiratory complexes/SCs reached by random diffusion in the membrane bilayer. However, steady state kinetic analysis cannot distinguish among the above mentioned three possible mechanisms of channelling.
Notably, the respirasome structure recently published by Letts et al. (2016) provides specific information about the protein contacts in the SC, but also indicates that the CoQ reduction site in CI and the CoQH2 oxidation site in CIII are separated and both easily accessible to the membrane, thus the same authors interpreted that no limit to free diffusion of CoQ in the bilayer exists. On the contrary, the group of Kuhlbrandt (Sousa et al. 2016) recently took in account the dimeric structure of CIII by showing that the CoQ reduction site of CI in the SC is separated by 11 nm from the proximal CIII monomer, and 18 nm from the distal one, thus suggesting that the latter is functionally inactive whereas the linear arrangement of CI with the active monomer of CIII and also with CIV is strongly in the favour of channelling for electron transfer between complexes by substrate microdiffusion.
In fact, information about the molecular structure of the SC must be compatible with the recognized mechanisms of CoQ reduction by CI and its re-oxidation by dimeric CIII. In particular, the latter occurs via a complicated mechanism known as the Q-cycle (Mitchell 1975; Sarewicz and Osyczka 2015). In the Q-cycle there are two sites in CIII where CoQ interacts, one located near the intermembrane space (Qo) where ubiquinol is sequentially oxidized by the Rieske iron-sulphur protein and by the bL heme, and one located near the matrix space (Qi) where ubiquinone is reduced. Apparently if CoQ channelling occurs in the SC by microdiffusion, there should exist at least two CoQ molecules trapped in the interstices between CI and CIII to fulfil the Q-cycle mechanism. However, considering the functional asymmetry of the CIII dimer (Covian et al. 2007), it is also suggestive to consider that in the bound CIII dimer (Fig. 3) only one monomer is active for NADH-dependent respiration and CoQ-channelling due to neighbourhood of its (proximal) Qo cavity and the CoQ-binding site in CI whereas the other monomer, not having such constrictions, might be easily available to interact with the CoQ pool at the (distal) Qi site.
3.2 Dynamic nature of supercomplexes: the plasticity model
In 2003, when we first provided kinetic evidence by flux control analysis in favour of metabolic channelling in the CoQ region, we also postulated (Bianchi et al. 2003) that the SC I1III2 may be in equilibrium with free complexes, depending on metabolic conditions, to explain the presence of NADH-cyt. c reductase activity both in the absence and presence of channelling, depending on the protein to phospholipid ratio of the examined proteoliposome samples.
Acín-Pérez et al. (2008) demonstrated that purified respirasomes (i.e. SC I1III2IV1) are able to respire in a Clark’s electrode, and they also observed other types of associations, as I + III or III + IV, thus concluding that a variety of associations between respiratory complexes likely co-exists in vivo with free complexes. In this context, they proposed an integrated model, the plasticity model, for the organization of the mitochondrial electron transport chain. The previous opposed models, solid vs. fluid (cf. Sect. 1), would be two extreme and functional situations of a dynamic range of molecular associations between respiratory complexes (Acín-Pérez et al. 2008). Intrinsic to its dynamic nature, the plasticity model proposes that segregation of both CoQ and cyt. c into distinct sub-domains is incomplete and that their respective functional pools are promiscuous (i.e. the intermediate substrates are both trapped into SCs and freely diffusible). According to Enríquez (2016) this promiscuity can be generated either by the dissociation of SCs or by the escape of CoQ or cyt. c from the supramolecular assembly. As also discussed by Milenkovic et al. (2017), the different crystal structures of the respirasome (see before) may depict the respirasome in various stages of disassembly, either following its extraction from the membrane or resembling different physiological conditions with respirasomes constantly dissociating, reforming and reorganizing in the membrane.
The plasticity model and the dynamics of mitochondrial SCs (Acin-Perez and Enriquez 2014) are still debated in the literature. Possible factors involved in association/dissociation of SCs, such as membrane potential and post-translational changes, are reviewed by Genova and Lenaz (2014). However, despite the popularity of the model and the indirect evidence supporting it, no direct demonstration exists that SCs are cyclically formed and dissociated during physiological events.
3.3 Evidence for channelling by metabolic flux control analysis
By exploiting the flux control analysis (Kholodenko and Westerhoff 1993) using specific inhibitors to define the extent of metabolic control exerted by each individual complex over the entire NADH-dependent respiration in isolated bovine heart mitochondria, our laboratory achieved the first demonstration that CI and CIII behave as a single enzymatic unit (e.g. SC I1III2) and that electron transfer through CoQ is accomplished by channelling between the two redox enzymes (Bianchi et al. 2004). Flux control analysis is based on the principle that the sum of the flux control coefficients (FCCs) of the individual enzymes in an integrated pathway must equal 1 unless these enzymes form supramolecular units and establish substrate channelling. In the latter case, these enzymes would be all equally rate-limiting and the sum of the control coefficients would be higher than 1 (Kholodenko and Westerhoff 1993).
Using the same method for succinate-dependent respiration in the same bovine heart mitochondria samples we found that CII, but not CIII, is rate-limiting, supporting the notion that CII does not form SCs and that the oxidation of succinate follows pool behaviour (cf. below).
Ten years later, Blaza et al. (2014) criticized the evidence for channelling in the respiratory chain deriving from flux control analysis (Bianchi et al. 2004): they reasoned that rotenone is competitive with CoQ and, therefore, the extent of its inhibition of CI is affected by the additional presence of the exogenous quinone employed in CI assay but absent in the assay of NADH aerobic oxidation. Indeed, using rotenone and CoQ1, Blaza et al. (2014) find a FCC for CI that is certainly not valid, since it exceeds 1. However, the discrepancy may be ascribed to the different exogenous substrates used for CI assay in the two studies, i.e. CoQ1 and decylubiquinone (DB), respectively. In fact, DB has much lower affinity than CoQ1 for CI (Fato et al. 1996), thus exerting lower competition with rotenone and lower influence on the inhibition efficiency measured by Bianchi et al. (2004). Moreover, we note that the FCC values found by Blaza et al. (2014) using alternative inhibitors of CI (e.g. piericidin and diphenyleneiodonium, not competitive with CoQ) are oddly low and it is likely that such low FCC for CI is caused by the limiting amount of cyt.c in their samples, that would shift the major control of the chain to the cyt.c region. Significantly, in Blaza’s study, addition of exogenous cyt.c to the mitochondrial samples raised the FCC from 0.19 to 0.67.
Finally, we emphasize that the major point discussed by Bianchi et al. (2004) to demonstrate the existence of a SC I1III2IV n was not so much the high FCC of CI but the high FCCs of both CI and CIII in NADH oxidation (the latter not measured, on the contrary, by Blaza et al. 2014), which is incompatible with a CoQ-pool model postulating CI and CIII as independent molecules in the membrane. Such high FCC value of CIII over NADH-dependent respiration, as calculated by inhibitor titration with mucidin, is clearly not suspect of artefact because the corresponding FCC of CIII measured using the same inhibitor in succinate oxidation is low (as expected from the rate-limiting role of CII and the lack of CII-containing SCs). In other words, the FCC of CIII can be close to 1 if CIII is assembled together with the quinone reductase that precedes in the electron pathway and, in our hands, this condition is experimentally observed only for the NADH-dependent pathway involving CI whereas it does not occur in the case of CII in succinate-dependent respiration. In addition, the high FCC that CIII shows over NADH-cyt.c oxidoreductase activity in reconstituted proteoliposomes is drastically decreased after dissociation of the SC I1III2 (Genova et al. 2008) by reconstitution in excess phospholipids or in peroxidized phospholipids.
Very few other studies were addressed to the functional aspects of SCs using metabolic control analysis (Quarato et al. 2011; Kaambre et al. 2012, 2013); these studies confirmed that the respiratory chain is organized in functionally relevant supramolecular structures.
3.4 Separate compartments of Coenzyme Q?
The effect on respiration of the simultaneous addition of NADH and succinate was examined in an early study (Gutman and Silman 1972) showing incomplete additivity and partial mutual competition between succinate oxidation and NADH oxidation; this effect was considered unlikely to be due to competition of the dehydrogenases for a single CoQ pool. Also on the basis of other observations (Gutman et al. 1971), a compartmentalization of the CoQ pool was suggested, in which two sub-pools were able to partially interact through a spill-over diffusion-mediated mechanism.
Gutman (1985) investigated the properties of the NADH and succinate oxidation in submitochondrial particles in relation to the rates of energy-dependent reverse electron transfer from succinate to NAD+ and of forward electron transfer from NADH to fumarate, concluding that the electron flux from succinate dehydrogenase to oxygen (i.e. forward electron transfer towards CIII) or to NADH dehydrogenase (i.e. reverse electron transfer from CII to CI, excluding CIII) employs the same carrier (i.e. CoQ pool) and is controlled by the same reaction whereas the electron transfer from NADH to oxygen (i.e. forward electron transfer from CI to CIII) does not share the same pathway through which electrons flow in the NADH-fumarate reductase. In other words, CI and CII are linked by a different CoQ-mediated pathway with respect to CI and CIII. The non-homogeneity of the ubiquinone pool with respect to succinate and NADH oxidation may be interpreted today in terms of compartmentalization of CoQ trapped in the SC I1III2IV n in contrast with the free CoQ molecules (i.e. the membrane CoQ pool, properly speaking) used for connecting CII and CIII.
Several other reports have suggested in the past that the CoQ pool is not homogeneous, thus raising doubts on its universal validity (Ragan and Cottingham 1985); even Kröger and Klingenberg (1973a) observed that an aliquot of CoQ cannot be reduced by either NADH or succinate. Later, Jørgensen et al. (1985) noticed that three pools of ubiquinone appeared to be present in heart mitochondria: a metabolically inactive pool consisting of reduced as well as oxidized ubiquinone, a pool coupled to oxidation of added (cytoplasmic) NADH, and the well-known pool coupled to citric acid cycle oxidations. The latter pool, however, could not distinguish NADH-dependent from succinate-dependent reduction of CoQ.
Benard et al. (2008) described the existence of three different pools of CoQ during succinate-dependent steady-state respiration in rat liver and muscle mitochondria: one pool is directly utilised, another (approx. 8% in muscle and 23% in liver) is mobilized as a reserve in case of a perturbation to maintain the energy fluxes at normal values (e.g. due to inhibition of the respiratory complexes or in case of mitochondrial diseases), and a third one (approx. 79% in muscle and 21% in liver) cannot be mobilized at all. These results are compatible with CoQ compartmentalization, although similar results with NADH oxidation were not provided to functionally prove that the fraction of CoQ that is not utilisable for succinate oxidation is channelled within SC I1III2IV n .
Lapuente-Brun et al. (2013) demonstrated that the physical assembly between CI and CIII determines a preferential pathway for electrons mediated by a dedicated subset of CoQ molecules. According to their results, this compartmentalization prevents significant cross talk between NADH oxidation (CI-dependent) and succinate oxidation (dependent on CII) or other flavoenzyme-dependent oxidations. Moreover, CIII molecules bound in the SC I1III2IV n and free CIII molecules are, respectively, and exclusively dedicated to oxidation of NADH and other substrates using the free CoQ pool.
On the contrary, the already quoted paper by Blaza et al. (2014) showed that the steady-state rates of aerobic NADH and succinate oxidation were not additive in bovine heart submitochondrial particles (cf. Sect. 3.3); moreover, the extents of cytochromes bH, bL, c and c1 reduction in the same cyanide-inhibited particles were similar if the reductant was either NADH or succinate or a mixture of the two substrates. Blaza et al. interpreted the results as a demonstration that a single homogeneous pool of CoQ molecules exists that receives electrons indifferently from CI and CII. Consequently, free CIII and SC-bound CIII are able to equally receive electrons from the CoQ pool. However, on the basis of those mere data, which considered the whole pathway of electrons from ubiquinol to oxygen and, therefore, comprised the redox cycling of cyt.c and CIV, it is not possible to discriminate whether the pool behaviour is due to the homogeneity of the CoQ-pool, as claimed by the same authors, or to the homogeneity of the cyt.c pool. In fact, under such conditions, cyt.c may be rate-limiting provoking a bottleneck step both in NADH and in succinate oxidation when electrons compete for a common pool of cyt.c and CIV molecules in free form. Indeed, most cyt.c and CIV are free and able to receive electrons from both NADH and succinate with only a small portion of CIV dedicated to NADH (i.e. forming the respirasome I1III2IV), as demonstrated by our flux control analysis data (Bianchi et al. 2004) that showed no channelling in the cyt.c region.
To overcome the problem of possible kinetic interferences due to the presence of the cyt. c pool, we recently performed a study of succinate and NADH oxidation by excess exogenous cyt.c in KCN-inhibited mitochondria (Lenaz et al. 2016); this experimental approach shortens the electron transfer pathway for both oxidation reactions by including only CoQ and CIII as redox partners, and thus excluding cyt. c re-oxidation kinetics by CIV. Under such condition, we found that NADH and succinate oxidation by cyt.c are completely or almost completely additive and close to the theoretical summation of the two single activities (Table 1), once again suggesting the existence of two separate CoQ-compartments.
Interestingly, similar results were also obtained in preliminary experiments from our laboratory (Tioli G, Falasca AI, Lenaz G and Genova ML, data communicated at EBEC2016-Session P4) using proteoliposomes where the additivity of NADH and succinate oxidation decreased when CIII was progressively inhibited with mucidin. This is in accordance with the idea that the increasing number of inactive CIII units forces ubiquinol produced by succinate and CII-reduction to access the binding sites of residual active units of CIII even if assembled in the CI-containing SC, thus implying that the CoQ molecules in the SC I1III2IV n are in a dissociation equilibrium with the molecules in the membrane pool (Lenaz and Genova 2007). The recent structural model of Letts et al. (2016) strongly supports our kinetic observations, since the neighbourhood of the CoQ sites in CI and CIII to one another, as mentioned in Sect. 3.1 and Fig. 3, is consistent with preferred channelling of reduced CoQ within the SC I1III2IV n itself, while the exposure of the same sites to the membrane lipids supports diffusion in the pool. To this purpose it is suggestive to consider that in the bound CIII dimer only one monomer is active for NADH oxidation due to the neighbourhood of the active sites (Sousa et al. 2016), whereas the other monomer not having such constrictions might be easily available to receive electrons from the CoQ pool.
It is intuitive that channelling should be largely predominant during high turnover of the respiratory chain, whereas CoQ diffusion in the pool would preferentially take place at low turnover.
4 The role of the Coenzyme Q pool
4.1 Dissociation equilibrium of bound Coenzyme Q
As described in the previous sections of this chapter, CI is almost totally associated in a SC with CIII, and CoQ channelling is likely to occur in the boundary between the two complexes. However, this does not exclude that free CoQ in the pool is also necessary for proper channelling. In fact, there is evidence suggesting that the bound inter-complex quinone that may allow electron flow directly from CI to CIII within the SCI1III2IV n is in dissociation equilibrium with the membrane CoQ pool, so that the amount of bound CoQ, at steady state, would be dictated by the size of the pool itself. In particular, the existence of this equilibrium is suggested by the saturation kinetics for total ubiquinone exhibited by the integrated activity of CI and CIII (NADH-cyt.c oxidoreductase) (Estornell et al. 1992) and by the decrease of respiratory activity in mitochondria fused with phospholipids causing subsequent dilution of the CoQ pool (Schneider et al. 1982). To be in agreement with the experimental observations in the favour of channelling, this proposition requires that the dissociation rate constants (koff) of bound CoQ be considerably slower than the rates of inter-complex electron transfer via the same bound quinone molecules.
By this way, free CoQ acts as the diffusible substrate of various redox enzymes not involved in respiratory supercomplex organization (cf. next section) and at the same time as a reservoir for binding to the SC I1III2IV n . A different question is whether electron transfer between CI and CIII can occur via the CoQ pool when in the absence of any supercomplex organization; reconstitution studies (Ragan and Heron 1978; Heron et al. 1978; Genova et al. 2008) indicated that electron transfer is still possible. In a proteoliposome system where CI was reconstituted together with an alternative oxidase and CoQ10 in the absence of CI-containing SCs, Jones et al. (2016) also found high rates of NADH oxidation, which demonstrates that CoQ10 was able to shuttle electrons from CI by following pool behaviour.
4.2 Electron transfer between individual complexes not involved in supercomplex organization
The CoQ pool is required for electron transfer from CII to CIII. Indeed, CII kinetically follows pool behaviour after extraction and reconstitution (Kroger and Klingenberg 1973a, b) and in intact mitochondria (Stoner 1984) in accordance with the lack of SCs found by both BN-PAGE and flux control analysis as described in the previous sections.
Other enzymes such as glycerol-3-phosphate dehydrogenase, ETF dehydrogenase, dihydroorotate dehydrogenase, choline dehydrogenase, sulphide dehydrogenase, that are likely to be in minor amounts and strongly rate-limiting in integrated electron transfer, are probably inserted in the respiratory chain by interaction through the CoQ pool (Genova and Lenaz 2010).
Since no clear association was demonstrated between CI and CII and most authors agree that CII is not a significant part of SCs, also reverse electron transfer from succinate to NAD+, involving sequential interaction of CII and CI by means of CoQ, must take place by collisional interactions with molecules in the membrane CoQ pool. The hyperbolic relation experimentally found by Gutman (1985) between the rate of reverse electron transfer and succinate oxidase is in complete accordance with the pool equation.
This observation poses the puzzling question about how ubiquinol reduced in the membrane pool by CII interacts with the CoQ binding site in CI, since most CI is engaged in the SC with CIII and the interaction of CoQ in the pool with the quinone-binding site in common between the two enzymes is necessarily slow. In view of the recent progress in the knowledge of the detailed atomic structure of CI (Fiedorczuk et al. 2016), the previous view that two different routes may exist for forward and reverse electron transfer within CI (Grivennikova et al. 2003) is no longer tenable as such, unless we consider two different conformations of the enzyme, of which the conformation present during reverse electron transfer makes the CoQ site more accessible to the membrane pool. It must be noted that the ATP-driven reverse electron transfer from succinate to NAD+ occurs in the presence of a high mitochondrial transmembrane protonmotive force that, according to Piccoli et al. (2006), might be the physiological signal causing the structural reorganization of the respiratory chain leading to SC dissociation and this would no longer limit the access from the CoQ pool to the binding site in CI. The already mentioned analysis of SCs by cryo-EM (Letts et al. 2016; Sousa et al. 2016) provide strong support for the accessibility of the CoQ site in CI to the free CoQ in the membrane. Such accessibility, however, would be no proof against the existence of channelling between CI and CIII.
5 Concluding evidence about channelling in the Coenzyme Q region
In briefly recapitulating on the previous sections of this chapter, we point out that the major observations supporting the notion that supercomplex association determines channelling in the CoQ region are the following: (a) rate advantage of NADH-cyt.c reductase when CI-containing SCs are present; (b) both CI and CIII are rate-limiting as measured by flux control analysis; (c) evidence for two compartments of CoQ in the experiments of competition of NADH and succinate oxidation.
The dynamic character of CoQ bound within the SC, which is in dissociation equilibrium with the free pool of CoQ in the membrane, is one main point favouring controversy. It is tempting to speculate that some CoQ molecules are trapped in a lipid micro-domain within the SC I1III2IV n and are channelled from CI to CIII during electron transfer at steady state; however, the relevance of CoQ dissociation from the SC to the membrane pool becomes significant when electron transfer in the respiratory chain is slow or blocked by an inhibitor downhill of the CoQ region. Structural evidence pertaining the relevant sites where CoQ is reduced and re-oxidized strongly supports this idea. To this respect, the plasticity model (Acín-Pérez et al. 2008) would be a functional rather than structural feature of the respiratory chain, at least in the CoQ region, a possibility advanced by Enriquez himself (Enríquez 2016). In other words, the SCs be stable supramolecular assemblies that do not readily dissociate, whereas CoQ behaves in a highly dynamic fashion in which channelling and diffusion take place in proportions depending on the turnover rates of the respiratory chain.
6 Supercomplexes and reactive oxygen species
The capacity of mitochondria to produce reactive oxygen species (ROS) depends on the type of tissue as well as on the metabolic conditions of the cell.
The redox potential of the NAD+/NADH couple and the mitochondrial proton-motive force act as powerful regulators of the steady-state concentration of redox species responsible for electron leaking and ROS generation in the respiratory chain (Jezek and Hlavatá 2005; Lenaz 2001). In turn, these forces are regulated by the redox supply (i.e. NADH/NAD molar ratio) to the respiratory chain, by the degree of coupling and/or by physio-pathological constraints to electron transfer, such as enzyme phosphorylation, cyt.c removal, CIV inhibition and oxygen concentration. (cf. Lenaz 2012 for extensive review).
The “Redox-Optimized ROS Balance hypothesis” (R-ORB) described by Aon et al. (2010) attempts to explain at a mechanistic level the link between mitochondrial respiration and ROS emission. The hypothesis is based on the observation that ROS levels (as the net result of production and scavenging) attain a minimum when mitochondria maximize their energetic output (i.e. maximal state-3 respiration) at intermediate values of the redox environment between fully oxidized and fully reduced redox couples such as NADH/NAD+ and GSH/GSSG. In state-3 respiration, these redox carriers are more oxidized than the corresponding values in state-4 respiration. On the other hand, ROS overflow will occur at both highly reduced or highly oxidized redox environment, albeit governed by the extent of proton leak and by the compromised scavenging capacity, respectively. By assessing mitochondrial respiration (i.e. forward electron transport), ROS emission and redox environment in isolated guinea pig heart mitochondria, Cortassa et al. (2014) confirmed that mitochondria are able to keep ROS emission to a minimum likely compatible with signalling, while maximizing their energetic output.
In addition, mitochondrial ROS release is modulated by a series of nuclear encoded proteins such as by p53, p66Shc, the Bcl-2 family and Romo-1 and in response to external stimuli, such as TNFα, hypoxia, serum deprivation, oxidative stress (the so-called ROS-induced ROS release) (cf. Genova and Lenaz 2015).
ROS production in mitochondria is a by-product of core metabolism of the cell that cannot be easily down-regulated without under-mining cell function (Andreyev et al. 2015).
An implication of supercomplex organization as the missing link between oxidative stress and energy failure was first suggested by our speculation (Lenaz and Genova 2007) that dissociation of SC I1III2 occurs under conditions of oxidative stress, with loss of facilitated CoQ channelling, thus causing electron transfer to depend upon diffusive collisional encounters of the free ubiquinone molecules with their binding partner complexes, which is a random process and, therefore, much less efficient. As a consequence, the alteration of electron transfer may elicit high reducing pressure and further induction of ROS generation. Following this line of thought, the different susceptibility of different types of cells and tissues to ROS damage (Dencher et al. 2007) may be interpreted in terms of (1) extent and tightness of supercomplex organization in their respiratory chains and, related to the former, (2) capability of the respiratory enzymes to hinder reaction of their auto-oxidizable prosthetic groups with oxygen. Disorganization of the supramolecular architecture of the respiratory chain would both decrease NAD-linked respiration and ATP synthesis and increase the capacity of producing superoxide by destabilised CI and CIII.
On the basis of their studies on rat brain mitochondria oxidizing different substrates, Panov et al. (2007) suggested that the assembly of CI into SCs prevents excessive superoxide production during oxidation of NAD-linked substrates because efficient CoQ channelling helps maintaining the chain in the oxidized state (see also below).
Maranzana et al. (2013) obtained a direct demonstration that loss of SC I1III2 organization causes a dramatic enhancement of ROS generation by CI. In our model system of reconstituted binary CI/CIII proteoliposomes at high lipid to protein ratio (30:1), where formation of the SC I1III2 is prevented, the generation of superoxide is several-fold higher than in the same binary system reconstituted at a 1:1 ratio, which is conversely rich in SC I1III2 (Fig. 4). In agreement with this finding, dissociation of the SC I1III2 after treatment with the detergent DDM induces a strong enhancement of ROS generation both in proteoliposomes and in mitochondrial membranes. It is worth noting that, in all these experiments, the production of ROS is investigated in the presence of inhibitors (mucidin and rotenone) that prevent electron transfer to any respiratory intermediate substrate, therefore, the redox centres in CI are maximally reduced both in the presence and in the absence of the supercomplex assembly. Consequently, the above mentioned reasoning by Panov et al., giving emphasis to the relief of the redox pressure in the super-assembled respiratory chain, cannot be taken as the only explanation of the role of SC I1III2 in regulating ROS formation by CI.

Reprinted from Maranzana et al. (2013) with permission from Mary Ann Liebert, Inc.
Production of ROS by mitochondrial Complex I in different situations where supercomplexes are maintained or disassembled. Data of ROS production, measured in the presence of 1.8 μM mucidin and 4 μM rotenone and shown as percentage values of the corresponding reference samples as described in Maranzana et al. (2013), are plotted in the graph against the corresponding ratio of free Complex I vs total Complex I. The statistical analysis of the data using the Pearson’s parametric test indicates a positive correlation (r = 0.884, p < 0.05) between ROS generation and relative amount of free Complex I in proteoliposomes and submitochondrial particles (SMP) (black symbols). The BHM samples (gray symbols) were not included in the correlation analysis because the existence of endogenous antioxidant systems operating to reduce ROS levels in the mitochondria might have counteracted the dramatic effects of the complete dissociation of Complex I, thus leading to a two-fold only increase of the measured ROS production.
If we consider the structure of Complex I, the destabilization of CI in the absence of SC observed by Maranzana et al. (2013) may render the 51 kDa subunit containing the FMN more prone to interact with oxygen. According to Milenkovic et al. (2017), however, the localization of the 51 kDa subunit in the matrix, far from the protein interactions at the basis of SC association, seems incompatible with this assumption.
The established architecture of respiratory SCs may help in controlling ROS production also because, due to the high oxygen-consuming activity of CIV, the local concentration of O2 would be low near the cavity in CIII adjacent to CIV, an ideal condition for shielding a reactive semiquinone intermediate (Q·), and thus preventing ROS generation from CIII (Letts et al. 2016).
Several observations in cellular and animal models also link together SC dissociation and enhanced ROS production.
A strong decrease of high molecular weight SC correlating with higher ROS generation was observed in mouse fibroblasts expressing the activated form of the k-ras oncogene, in comparison with wild type fibroblasts (Baracca et al. 2010; Lenaz et al. 2010).
Diaz et al. (2012) showed that diminished stability of SC is associated with increased levels of ROS in mouse lung fibroblasts lacking the Rieske iron-sulphur protein of CIII and hence devoid of CI-containing SC. Enriquez’ group (Guarás et al. 2016) suggested that the saturation of CoQ re-oxidation capacity by FAD-linked substrates induces reverse electron transport from reduced CoQ to CI, and the resulting local generation of superoxide oxidizes specific CI subunits, triggering their degradation and the disintegration of the complex. For this reason, the SCI1III2IV n can be reconfigured through the degradation of respiratory CI, thus loosing CIII molecules in the membrane to facilitate electron flux via FAD-dependent substrates at the expense of NAD-dependent activity.
The cardiolipin defect in Barth syndrome, a cardio-skeletal myopathy with neutropenia which is characterized by respiratory chain dysfunction, results in destabilization of SC by weakening the interactions between CIII and CIV (McKenzie et al. 2006). Remarkably, hydroethidine staining revealed higher basal levels of superoxide production in lymphoblasts from patients, compared to control cells (McKenzie et al. 2006; Gonzalvez et al. 2013). The availability of cardiolipin-deficient yeast mutants, although constitutively lacking CI, provided the opportunity to demonstrate alterations in the stabilization of SC III2IV n ; similar to those found in Barth syndrome and exhibited increased protein carbonylation, an indicator of ROS (Chen et al. 2008). The increase in ROS is most likely not due to defective oxidant defence systems, since the CL mutants do not display sensitivity to other oxidants such as paraquat, menadione or hydrogen peroxide (H2O2).
The proposal that CI-containing SCs may physiologically exist in dynamic equilibrium with isolated CI (cf. Sect. 3.2) raises the puzzling suspect that the production of ROS may consequently be subjected to physiological oscillations. It is tempting to suggest that these changes may be aimed to controlling ROS levels in the cell, in view of their well-documented role in cellular redox signalling.
References
Acin-Perez R, Enriquez JA (2014) The function of the respiratory supercomplexes: the plasticity model. Biochim Biophys Acta 1837:444–450
Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32:529–539
Althoff T, Mills DJ, Popot JL, Kühlbrandt W (2011) Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30:4652–4664
Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA (2015) Mitochondrial ROS metabolism: 10 years later. Biochem (Mosc) 80:517–531
Aon MA, Cortassa S, O’Rourke B (2010) Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta 1797:865–877
Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G (2010) Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta 1797:314–323
Benard G, Faustin B, Galinier A, Rocher C, Bellance N, Smolkova K, Casteilla L, Rossignol R, Letellier T (2008) Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int J Biochem Cell Biol 40:1543–1554
Bianchi C, Fato R, Genova ML, Parenti Castelli G, Lenaz G (2003) Structural and functional organization of Complex I in the mitochondrial respiratory chain. BioFactors 18:3–9
Bianchi C, Genova ML, Parenti Castelli G, Lenaz G (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279:36562–36569
Blaza JN, Serreli R, Jones AJ, Mohammed K, Hirst J (2014) Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc Natl Acad Sci USA 111:15735–15740
Chance B, Williams GR (1955) A method for the localization of sites for oxidative phosphorylation. Nature 176:250–254
Chen S, He Q, Greenberg ML (2008) Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol 68:1061–1072
Cortassa S, O’Rourke B, Aon MA (2014) Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim Biophys Acta 1837:287–295
Covian R, Zwicker K, Rotsaert FA, Trumpower BL (2007) Asymmetric and redox-specific binding of quinone and quinol at center N of the dimeric yeast cytochrome bc1 complex. Consequences for semiquinone stabilization. J Biol Chem 282:24198–24208
Crane FL, Hatefi Y, Lester RL, Widmer C (1957) Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta 25:220–221
Crane FL, Widmer C, Lester RL, Hatefi Y (1959) Studies on the electron transport system. XV. Coenzyme Q (Q275) and the succinoxidase activity of the electron transport particle. Biochim Biophys Acta 31:476–489
Dencher NA, Frenzel M, Reifschneider NH, Sugawa M, Krause F (2007) Proteome alterations in rat mitochondria caused by aging. Ann N Y Acad Sci 1100:291–298
Diaz F, Enríquez JA, Moraes CT (2012) Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol 32:415–429
Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ (2011) Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc Natl Acad Sci USA 108:15196–15200
Enríquez JA (2016) Supramolecular organization of respiratory complexes. Annu Rev Physiol 78:533–561
Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G (1992) Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett 311:107–109
Fato R, Estornell E, Di Bernardo S, Pallotti F, Parenti Castelli G, Lenaz G (1996) Steady-state kinetics of the reduction of coenzyme Q analogs by complex I (NADH:ubiquinone oxidoreductase) in bovine heart mitochondria and submitochondrial particles. Biochemistry 35:2705–2716
Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA (2016) Atomic structure of the entire mammalian mitochondrial complex I. Nature 538:406–410
Gao X, Wen X, Esser L, Quinn B, Yu L, Yu CA, Xia D (2003) Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry 42:9067–9080
Genova ML, Lenaz G (2010) Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal 12:961–1008
Genova ML, Lenaz G (2013) A critical appraisal of the role of respiratory supercomplexes in mitochondria. Biol Chem 394:631–639
Genova ML, Lenaz G (2014) Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 1837:427–443
Genova ML, Lenaz G (2015) The interplay between respiratory supercomplexes and ROS in aging. Antioxid Redox Signal 23:208–238
Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M, Falasca AI, Formiggini G, Sgarbi G, Solaini G, Lenaz G (2008) Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim Biophys Acta 1777:740–746
Gonzalvez F, D’Aurelio M, Boutant M, Moustapha A, Puech JP, Landes T, Arnauné-Pelloquin L, Vial G, Taleux N, Slomianny C, Wanders RJ, Houtkooper RH, Bellenguer P, Møller IM, Gottlieb E, Vaz FM, Manfredi G, Petit PX (2013) Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim Biophys Acta 1832:1194–1206
Green DE, Tzagoloff A (1966) The mitochondrial electron transfer chain. Arch Biochem Biophys 116:293–304
Grivennikova VG, Roth R, Zakharova NV, Hägerhäll C, Vinogradov AD (2003) The mitochondrial and prokaryotic proton-translocating NADH: ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Biochim Biophys Acta 1607:79–90
Guarás A, Perales-Clemente E, Calvo E, Acín-Pérez R, Loureiro-Lopez M, Pujol C, Martínez-Carrascoso I, Nuñez E, García-Marqués F, Rodríguez-Hernández MA, Cortés A, Diaz F, Pérez-Martos A, Moraes CT, Fernández-Silva P, Trifunovic A, Navas P, Vazquez J, Enríquez JA (2016) The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep 15:197–209
Gutman M (1985) Kinetic analysis of electron flux through the quinones in the mitochondrial system. In: Lenaz G (ed) Coenzyme Q. Wiley, Chichester, pp 215–234
Gutman M, Silman N (1972) Mutual inhibition between NADH oxidase and succinoxidase activities in respiring submitochondrial particles. FEBS Lett 26:207–210
Gutman M, Kearney EB, Singer TP (1971) Control of succinate dehydrogenase in mitochondria. Biochemistry 10:4763–4770
Hackenbrock CR, Chazotte B, Gupte SS (1986) The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr 18:331–368
Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962a) Studies on the electron transfer system. XLII. Reconstitution of the electron transfer system. J Biol Chem 237:2661–2669
Hatefi Y, Haavik AG, Griffiths DE (1962b) Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem 237:1676–1680
Heron C, Ragan CI, Trumpower BL (1978) The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J 174:791–800
Hochman J, Ferguson-Miller S, Schindler M (1985) Mobility in the mitochondrial electron transport chain. Biochemistry 24:2509–2516
Jezek P, Hlavatá L (2005) Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 37:2478–2503
Jones AJ, Blaza JN, Bridges HR, May B, Moore AL, Hirst J (2016) A self-assembled respiratory chain that catalyzes NADH oxidation by ubiquinone-10 cycling between complex I and the alternative oxidase. Angew Chem Int Ed Engl 55:728–731
Jørgensen BM, Rasmussen HN, Rasmussen UF (1985) Ubiquinone reduction pattern in pigeon heart mitochondria. Identification of three distinct ubiquinone pools. Biochem J 229:621–629
Kaambre T, Chekulayev V, Shevchuk I, Karu-Varikmaa M, Timohhina N, Tepp K, Bogovskaja J, Kütner R, Valvere V, Saks V (2012) Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. J Bioenerg Biomembr 44:539–558
Kaambre T, Chekulayev V, Shevchuk I, Tepp K, Timohhina N, Varikmaa M, Bagur R, Klepinin A, Anmann T, Koit A, Kaldma A, Guzun R, Valvere V, Saks V (2013) Metabolic control analysis of respiration in human cancer tissue. Front Physiol 4:151. https://doi.org/10.3389/fphys.2013.00151
Kennedy EP, Lehninger AL (1949) Oxidation of fatty acids and tricarboxylic acid intermediates by isolated rat liver mitochondria. J Biol Chem 179:957–972
Kholodenko BN, Westerhoff HV (1993) Metabolic channelling and control of the flux. FEBS Lett 320:71–74
Kröger A, Klingenberg M (1973a) The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem 34:358–368
Kröger A, Klingenberg M (1973b) Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem 39:313–323
Lapuente-Brun E, Moreno-Loshuertos R, Acín-Pérez R, Latorre-Pellicer A, Colás C, Balsa E, Perales-Clemente E, Quirós PM, Calvo E, Rodríguez-Hernández MA, Navas P, Cruz R, Carracedo Á, López-Otín C, Pérez-Martos A, Fernández-Silva P, Fernández-Vizarra E, Enríquez JA (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340:1567–1570
Lenaz G (2001) The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52:159–164
Lenaz G (2012) Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol 942:93–136
Lenaz G, Fato R (1986) Is ubiquinone diffusion rate-limiting for electron transfer? J Bioenerg Biomembr 18:369–401
Lenaz G, Genova ML (2007) Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol 292:C1221–C1239
Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del Sole M, Faccioli M, Falasca A, Fato R, Genova ML, Sgarbi G, Solaini G (2010) Mitochondrial respiratory chain super-complex I–III in physiology and pathology. Biochim Biophys Acta 1797:633–640
Lenaz G, Tioli G, Falasca AI, Genova ML (2016) Complex I function in mitochondrial supercomplexes. Biochim Biophys Acta 1857:991–1000
Letts JA, Sazanov LA (2017) Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol 24:800–808
Letts JA, Fiedorczuk K, Sazanov LA (2016) The architecture of respiratory supercomplexes. Nature 537:644–648
Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML (2013) Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19:1469–1480
McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 361:462–469
Milenkovic D, Blaza JN, Larsson NG, Hirst J (2017) The enigma of the respiratory chain supercomplex. Cell Metab 25:765–776
Mitchell P (1975) The protonmotive Q cycle: a general formulation. FEBS Lett 59:137–139
Morton RA (1958) Ubiquinone. Nature 182:1764–1767
Ovádi J (1991) Physiological significance of metabolic channelling. J Theor Biol 152:1–22
Ozawa T, Nishikimi M, Suzuki H, Tanaka M, Shimomura Y (1987) Structure and assembly of mitochondrial electron-transfer complexes. In: Ozawa T, Papa S (eds) Bioenergetics: structure and function of energy-transducing systems. Japan Science Society Press, Tokyo, pp 101–119
Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, Rosenfeld J (2007) Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol 292:C708–C718
Piccoli C, Scrima R, Boffoli D, Capitanio N (2006) Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem J 396:573–583
Quarato G, Piccoli C, Scrima R, Capitanio N (2011) Variation of flux control coefficient of cytochrome c oxidase and of the other respiratory chain complexes at different values of protonmotive force occurs by a threshold mechanism. Biochim Biophys Acta 1807:1114–1124
Ragan CI, Heron C (1978) The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Evidence for stoicheiometric association. Biochem J 174:783–790
Ragan CI, Cottingham IR (1985) The kinetics of quinone pools in electron transport. Biochim Biophys Acta 811:13–31
Redfearn ER, Pumphrey AM (1960) The kinetics of ubiquinone reactions in heart-muscle preparations. Biochem J 76:64–71
Sarewicz M, Osyczka A (2015) Electronic connection between the quinone and cytochrome C redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol Rev 95:219–243
Schäfer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, Vonck J (2006) Architecture of active mammalian respiratory chain supercomplexes. J Biol Chem 281:15370–15375
Schägger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19:1777–1783
Schägger H, Pfeiffer K (2001) The ratio of oxidative phosphorylation complexes I–V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem 276:37861–37867
Schneider H, Lemasters JJ, Hackenbrock CR (1982) Lateral diffusion of ubiquinone during electron transfer in phospholipid- and ubiquinone-enriched mitochondrial membranes. J Biol Chem 257:10789–10793
Singer SJ, Nicolson GL (1976) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Sousa JS, Mills DJ, Vonck J, Kühlbrandt W (2016) Functional asymmetry and electron flow in the bovine respirasome. eLife 5:e21290
Stoner CD (1984) Steady-state kinetics of the overall oxidative phosphorylation reaction in heart mitochondria. Determination of the coupling relationships between the respiratory reactions and miscellaneous observations concerning rate-limiting steps. J Bioenerg Biomembr 16:115–141
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z (2005) Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121:1043–1057
Szarkowska L (1966) The restoration of DPNH oxidase activity by coenzyme Q (ubiquinone). Arch Biochem Biophys 113:519–525
Wittig I, Schägger H (2005) Advantages and limitations of clear-native PAGE. Proteomics 5:4338–4346
Wu M, Gu J, Guo R, Huang Y, Yang M (2016) Structure of mammalian respiratory supercomplex I1III2IV1. Cell 167:1598–1609
Yano N, Muramoto K, Shimada A, Takemura S, Baba J, Fujisawa H, Mochizuki M, Shinzawa-Itoh K, Yamashita E, Tsukihara T, Yoshikawa S (2016) The Mg2+-containing water cluster of mammalian cytochrome c oxidase collects four pumping proton equivalents in each catalytic cycle. J Biol Chem 291:23882–23894
Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, Grigorieff N, Rubinstein JL (2015) Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife 4:e10180
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that no conflict of interest exists.
Additional information
This paper belongs to a series of peer-reviewed contributions coordinated by Guest Editor Ferdinando Palmieri on the theme “Current topics in biology”.
Rights and permissions
About this article
Cite this article
Lenaz, G., Tioli, G., Falasca, A.I. et al. Coenzyme Q and respiratory supercomplexes: physiological and pathological implications. Rend. Fis. Acc. Lincei 29, 383–395 (2018). https://doi.org/10.1007/s12210-018-0689-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-018-0689-4