Abstract
HSP90, one of the molecular chaperones, contributes to protein stability in most living organisms. Previously, we found cleavage of HSP90 by caspase 10 in response to treatment with histone deacetylase inhibitor or proteasome inhibitor in leukemic cell lines. In this study, we investigated this phenomenon in various cell lines and found that HSP90 was cleaved by treatment with SAHA or MG132 in 6 out of 16 solid tumor cell lines. To further investigate the effects of HSP90 cleavage on cells, we introduced mutations to the potential cleavage sites of HSP90β and found that the 294th aspartic acid residue of the protein was mainly cleaved. In the K562 and Mia-PaCa-2 cell lines expressing HSP90β D294A, the cleavage of HSP90 by the treatment with SAHA or MG132 was reduced compared with the K562 and Mia-PaCa-2 cell lines expressing HSP90β WT. Accordingly, cell growth and survival were enhanced by HSP90β D294A expression. Therefore, we suggest that HSP90 cleavage widely occurs in several cell lines, and cleavage of HSP90 may have a potential for one of the mechanisms involved in the anti-tumor effects of known drugs and novel anti-tumor drug candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock protein 90 (HSP90), one of the heat shock protein family members, is found in most organisms and induced by exposure of various stresses (Welch 1993). HSP90 is an ATP-dependent molecular chaperone required for cell growth, survival, and other signal transductions (Jackson 2013; Obermann et al. 1998; Schopf et al. 2017). Proteins for which the stability and folding can be increased by HSP90 are called client proteins, and the client proteins include tumor protein p53, a fusion protein of the breakpoint cluster region (BCR) with tyrosine protein kinase ABL1 expressed from the Philadelphia chromosome (BCR-ABL), protein kinase B (Akt), glycogen synthase kinase 3 beta (GSK3β), and hypoxia-inducible factor 1 alpha (HIF1α), etc. (Neckers and Workman 2012). HSP90 can be divided into five domains: N-terminal domain, charged domain 1, middle domain, charged domain 2, and C-terminal domain (Chen et al. 2005; Obermann et al. 1998). The N-terminal domain has an ATP binding site and ATPase activity (Prodromou et al. 1997). The 79th aspartic acid and 47th glutamic acid residues of N-terminal domain are required for ATP binding and ATP hydrolysis, respectively (Obermann et al. 1998). HSP90 forms a flexible dimer by dimerization of the C-terminal domain, which is required for the functionality of HSP90. The client proteins bind to the middle domain (Sato et al. 2000; Wandinger et al. 2008; Wayne and Bolon 2007).
Because HSP90 is highly expressed in tumor cells more than normal cells and the client proteins of HSP90 are considered as biological hallmarks of cancer (Ferrarini et al. 1992; Powers and Workman 2006), HSP90 has been investigated as a target of anti-cancer therapy, and various HSP90 inhibitors have been studied as anti-cancer drugs (Park et al. 2019a). The HSP90 expression level is also related with survival time of cancer patients, and HSP90 is also involved in the development of a tumor becoming malignant (Boltze et al. 2004; Gallegos Ruiz et al. 2008; Pick et al. 2007). In addition, tumor cells have a high-affinity conformation of HSP90 complexes compared with the latent, uncomplexed form in normal cells (Kamal et al. 2003).
Various reagents are known to suppress the HSP90 activity. The HSP90 inhibitors can be divided into ansamycins and non-ansamycins depending on their structure, and the inhibitors generally inhibit the ATP binding of HSP90. In addition to this traditional HSP90 inhibition, it is reported that cleavage of HSP90 is induced by various materials, and the possibility of HSP90 cleavage to inhibit HSP90 activity has been suggested (Beck et al. 2009; Beck et al. 2012; Castro et al. 2019; Chen et al. 2009; Park et al. 2015; Park et al. 2017; Park et al. 2019a). The cleavage of HSP90 can be divided into enzymatic and non-enzymatic cleavage, which occur by caspase 10 activation and by reactive oxygen species (ROS) through chemical degradation, respectively, and each cleavage of HSP90 produces fragments of different size (Beck et al. 2009; Beck et al. 2012; Castro et al. 2019; Chen et al. 2009; Park et al. 2015; Park et al. 2017; Park et al. 2019a).
Histone deacetylase (HDAC) inhibitors induce the acetylation of histone and thereby increase the expression of genes associated with apoptosis and cell cycle arrest, which blocks cell growth and increases apoptosis (Kornberg 1999; Richon et al. 2000; Ruefli et al. 2001). The activity of HSP90 is controlled by acetylation/deacetylation of the 294th (HSP90α)/287th (HSP90β) lysine residue of the M-domain (Scroggins et al. 2007). For HSP90 to act as a molecular chaperone, K294/K287 of HSP90 is deacetylated by histone deacetylase (HDAC) 6 (Bali et al. 2005). Therefore, the HDAC inhibitors can down-regulate HSP90 activity by inhibiting HDAC 6 and contribute to cell death by the degradation of client proteins (Bali et al. 2005; Scroggins et al. 2007).
Proteasome is a protein complex that breaks down ubiquitinated proteins and is being studied as one of the targets for cancer therapy (Crawford et al. 2011a). Proteasome inhibitors induce pro-apoptotic protein expression, cell cycle arrest, and ER stress, inhibit angiogenesis and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and down-regulate glutathione followed by ROS generation (Crawford et al. 2011a; Han et al. 2009; Shirley et al. 2005). Considering that proteasome and HSP90 are major players in proteostasis network (Jayaraj et al. 2020), there can be a regulatory link between proteasome and HSP90. In fact, ROS generation by proteasome inhibitors induces the cleavage of HSP90 (Park et al. 2015; Park et al. 2017).
In our previous studies, we found that HDAC inhibitors and proteasome inhibitors induce the cleavage of HSP90 by ROS generation and caspase 10 activation in leukemia cells (Park et al. 2015; Park et al. 2017). Taken together with other reports, the phenomenon of HSP90 cleavage can be considered as a novel mechanism of HDAC inhibitor- and proteasome inhibitor-mediated cell death (Park et al. 2019a). In this study, we found the occurrence of the cleavage of HSP90 in various cell lines and investigated whether the cleavage of HSP90 directly affects cell growth and death via site-directed mutagenesis.
Materials and methods
Cell culture
K562 (human chronic myelogenous leukemia) and CFPAC-1 (human pancreatic ductal adenocarcinoma; derived from a metastatic site: liver) cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM, Hyclone, Logan, UT, USA) with 10% fetal bovine serum (FBS, Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C under a humidified atmosphere of 5% CO2. Huh-7, SNU-449, and SNU-739 (human hepatocellular carcinoma), HCT116 (human colorectal carcinoma), LoVo (human colorectal adenocarcinoma; derived from a metastatic site: left supraclavicular lymph node), ASPC-1 (human pancreatic ductal adenocarcinoma; derived from a metastatic site: ascites), Capan-1 (human pancreatic ductal adenocarcinoma; derived from a metastatic site: liver), Capan-2 (human pancreatic ductal adenocarcinoma), MCF-7 (human breast ductal carcinoma), T47D (human breast ductal carcinoma; derived from a metastatic site: pleural effusion), and ZR75.1 (human breast ductal carcinoma; derived from a metastatic site: ascites) cells were maintained in RPMI-1640 (Hyclone) with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Mia-PaCa-2, PANC-1 (human pancreatic ductal adenocarcinoma), CT26 (mouse colon carcinoma), and PANC02 (mouse pancreatic ductal adenocarcinoma) cells were maintained in DMEM (Hyclone) with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were treated with histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) or proteasome inhibitor MG132 at the indicated concentrations for the indicated periods.
Antibodies and chemicals
MG132 was purchased from ALEXIS (Lausen, Switzerland). Dimethyl sulfoxide (DMSO) and SAHA were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The monoclonal antibodies anti-HSP90α/β (sc-13119) and anti-GAPDH (sc-32233) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-Myc-tag (#2276), anti-HSP90β (#7411), and anti-GSK3β (#9315) were from Cell Signaling Technology (Beverly, MA, USA). The polyclonal antibody anti-Akt (#9272) was from Cell Signaling Technology.
Western blot analysis
Harvested cells were lysed in a lysis buffer (pH 8.0, 20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 10 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP-40, protease inhibitor cocktail, and phosphatase inhibitor). Samples were resolved by SDS-polyacrylamide gel electrophoresis and electro-transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% dry milk in phosphate-buffered saline-Tween-20 (PBS-T; 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.05% Tween-20) and probed with an appropriate primary antibody. Immunoreactive proteins were detected by horseradish peroxidase-conjugated anti-rabbit or anti-mouse (Santa Cruz Biotechnology) secondary antibodies and an ECL solution (ATTO, Tokyo, Japan).
Reverse-transcription PCR
Total RNA was isolated with the TRI Reagent® according to the manufacturer’s instructions (MRC, Cincinnati, OH, USA). Then, 2 μg of total RNA was reverse-transcribed in the first-strand synthesis buffer containing 6 μg/ml oligo(dT) primer, 50 U M-MLV reverse transcriptase, 2 mM dNTP, 10 mM DTT, and 40 U RNaseOUT™ recombinant ribonuclease inhibitor (Invitrogen, Carlsbad, CA, USA). The reaction was carried out at 37 °C for 50 min and heat inactivated at 70 °C for 15 min. One microliter of the synthesized cDNA solution was subjected to a standard PCR reaction of 35 cycles consisting of denaturation for 40 s at 95 °C, annealing for 40 s at 58 °C, and extension for 40 s at 72 °C. The primer sequences used were as follows: GAPDH, 5′-TCC ACC ACC CTG TTG CTG TA-3′ (sense) and 5′-ACC ACA GTC CAT GCC ATC AC-3′ (anti-sense) (product size 452 bp); human HSP90β, 5′-TAG AAG AGA GGC GGG TCA AA-3′ (sense) and 5′-TCC TTA CCG CTG TCA TCC TC-3′ (anti-sense) (product size 215 bp); exogenous HSP90β, 5′-TAG AAG AGA GGC GGG TCA AA-3′ (human HSP90β sense primer) and 5′-GAG ACG TGC TAC TTC CAT TTG TC-3′ (pMSCV anti-sense primer) (product size 2155 bp).
Preparation of human HSP90β mutant constructs
The human HSP90β cDNA was purchased from Korea Human Gene Bank (KRIBB, Daejeon, South Korea). The human HSP90β cDNA was amplified by PCR using the following primer set: 5′-GTT AAC ATG CCT GAG GAA GTG CAC-3′ (sense) and 5′-ATG GAA GAA GTC GAT GGA GGT GGA GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG TAG TGA AGA TCT-3′ (anti-sense). The cDNA fragments were cloned into the TA cloning vector (TA-HSP90β) using the TA Cloning Vector Kit (RBC, New Taipei City, Taiwan).
The sites of HSP90β cleaved by caspase 10, predicted by the CaspDB database (Fig. 2a), were mutated from aspartic acid (D) to alanine (A) using the Muta-Direct™ Site Directed Mutagenesis Kit (iNtRON Biotechnology, Seongnam, South Korea). PCR was performed using 20 ng of TA-HSP90β plasmid DNA as a template with each mutagenesis primer set (Supplemental Table 1), followed by digestion of non-mutated parental DNA template.
Packaging and transduction of retroviruses encoding HSP90 constructs
The HSP90β WT and D294A mutant DNA were cloned into the expression vector pMSCVneo (Clontech, Mountain View, CA, USA). GP2-293, a cell line derived from 293 cells, was obtained from Clontech and used as a packaging cell line for preparation of the retroviruses. GP2-293 cells were maintained in DMEM containing 10% FBS in 5% CO2 incubator at 37 °C. Retroviral vectors pMSCVneo-HSP90β WT or pMSCVneo-HSP90β D294A along with pVSV-G (Clontech) encoding the pseudo-envelope protein gene were transfected into the cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Twelve hours later, the medium was exchanged with fresh culture medium supplemented with 10 mM sodium butyrate (Sigma-Aldrich Co.). After 48 h, the supernatant of the culture medium was taken and filtered through a filter with a 0.45 μm pore size. The retrovirus supernatants were concentrated using Centricon centrifugal filters (EMD Millipore, Billerica, MA, USA) and stored at − 80 °C. The viral supernatant was applied to K562 and Mia-PaC-2 cells along with 8 μg/ml of polybrene (Sigma-Aldrich Co.). Twenty-four hours later, G418 (Promega, Madison, WI, USA) was added at a concentration of 1 mg/ml, and the G418-resistant K562 and Mia-PaCa-2 cells were selected.
Cell viability assay
Cell viability was measured with the CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit according to the manufacturer’s instructions (Promega). After the cells were treated with MG132 or SAHA for 24 h, the MTS solution was added to each well, and then, the plates were incubated for 2 h at 37 °C. The color development was measured using a microplate reader (BioTek, Vermont, USA) at 490 nm.
Apoptosis assay
Apoptosis was measured with the Annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s instructions (Sigma-Aldrich Co.). After the cells were treated with MG132 for 24 h, the cells were stained with annexin V-FITC and propidium iodide (PI). The fluorescent signal was detected by flow cytometry using a FACS Calibur (BD Biosciences, Franklin Lakes, NJ, USA), and the cell cycle status was analyzed using FCS express program (De Novo Software, Glendale, CA, USA).
In vitro HSP90 cleavage assay
The cDNA fragments encoding HSP90β wild type and mutants (WT, D252A, D259A, D278A, and D294A) were cloned from TA-HSP90β mutant plasmids into the bacterial expression vector pET28a, and the recombinant plasmids were transformed into BL21 (DE3) cells. The recombinant HSP90β proteins were purified using a Ni-NTA affinity column and fast protein liquid chromatography (Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocol. Each recombinant HSP90β was incubated with 2 U of active recombinant caspase 10 (BioVision, Milpitas, CA, USA) in a reaction buffer at 37 °C for 4 h. After incubation, HSP90 protein was detected by Western blot analysis.
Caspase 10 activity assay
The Caspase-10 Colorimetric Assay Kit (BioVision) was used to measure the caspase 10 activity according to the manufacturer’s instructions. Cells were untreated or treated with 1 μM of z-VAD-fmk (pan-caspase inhibitor, Sigma-Aldrich Co.) for 1 h, followed by incubation with 5 μM of MG132 for 24 h. The cell lysates were then incubated with 200 μM of the caspase 10 substrate for 2 h at 37 °C. The absorbance values were obtained with a microplate reader (BioTek, Winooski, Vermont, USA) at a wavelength of 405 nm.
Statistics
The results are shown as the mean ± standard deviation (SD) from at least three independent experiments. Statistical significance of the differences between two samples was evaluated using the Student’s t test. P < 0.05 was considered statistically significant.
Results
Proteasome inhibitor- or HDAC inhibitor-induced cleavage of HSP90 occurred in various tissue-derived cancer cell lines
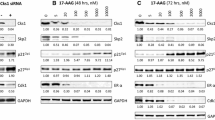
In our previous studies, we found that the cleavage of HSP90 induced by a proteasome inhibitor or HDAC inhibitor occurred in leukemia cells but not in other solid tumor cells (Park et al. 2015; Park et al. 2017). Because the experiment was performed using only a few cell lines, we questioned whether HSP90 cleavage can occur only in leukemia cells. In addition, the cleavage of HSP90 had been reported also in some solid tumor cell lines (Chen et al. 2009; Park et al. 2019b). Therefore, we checked the cleavage of HSP90 in more diverse cell lines. Sixteen solid tumor cell lines including human hepatocellular carcinoma (Huh-7, SNU-449, and SNU-739), human colon cancer (HCT116 and LoVo), mouse colon cancer (CT26), human pancreatic cancer (Mia-PaCa-2, Capan-1, Capan-2, PANC-1, AsPC-1, and CFPAC-1), mouse pancreatic cancer (PANC02), and human breast cancer (ZR-75-1, T47D, and MCF-7) cell lines were treated with the proteasome inhibitor MG132 and HDAC inhibitor SAHA. HSP90 was cleaved by the MG132 treatment in the HCT116, LoVo, CT26, Capan-1, and PANCO2 cell lines and by the SAHA treatment in the LoVo and Mia-PaCa-2 cell lines (Fig. 1 and Supplemental Fig. S1). According to these results, the cleavage of HSP90 occurs in various colon and pancreatic cancer cell lines with different sensitivity.
Cleavage of HSP90 induced by treatment with MG132 and SAHA occurs in various tissue-derived solid tumor cell lines. a Human hepatocellular carcinoma cell lines. b Human and mouse colorectal cancer cell lines. c Human pancreatic cancer cell lines. d Human breast cancer cell lines. The cells were treated with MG132 or SAHA at the indicated doses for 24 h and subjected to Western blot analysis. GAPDH was used as a loading control
Prediction of the HSP90 cleavage site and its identification by in vitro HSP90 cleavage assay
In the cytosol of human cells, HSP90 has two isoforms, HSP90α and HSP90β (Sreedhar et al. 2004). In our previous study, we confirmed that MG132-induced HSP90 cleavage occurred mainly on HSP90β and weakly on HSP90α (Park et al. 2017). SAHA-induced cleavage of HSP90β was also confirmed (Park et al. 2015). Therefore, we targeted HSP90β in this study to investigate the effect of HSP90 cleavage on cell death. We first identified the cleavage site of HSP90β. Because HSP90 was cleaved by caspase 10 (Park et al. 2015; Park et al. 2017), we predicted the potential sites of HSP90β cleaved by caspase 10 using the CaspDB database (Kumar et al. 2014). Because the size of the cleaved HSP90β fragment was approximately 50~55 kDa (Park et al. 2015), four sites (252th, 259th, 278th, and 294th aspartic acid) were selected among the predicted HSP90β cleavage sites (Fig. 2a). By the alignment for the cleavage sites of HSP90α and HSP90β, we found that sites corresponding to 252, 278, and 294th aspartic acid residues of HSP90β are conserved in HSP90α (Fig. 2b). To further specify the cleavage site, we established constructs in which a point mutation occurred at each predicted cleavage site using site-directed mutagenesis. Thereafter, using each construct (rhHSP90β WT, D252A, D259A, D278A, and D294A), a recombinant protein was produced and then directly reacted with active caspase 10 in vitro. According to the result in Fig. 2c, the cleavage of HSP90 was mainly reduced in the rhHSP90β D294A and partially in the rhHSP90β D278A. Therefore, it is likely that the cleavage of HSP90β occurs not at one site but mainly at the 294th aspartic acid residue.
Cleavage of HSP90β occurs mainly at the 294th aspartic acid residue. a The cleavage site was predicted by the CaspDB database. Thereafter, four sites (252nd, 259th, 278th, and 294th aspartic acids) were selected for potential cleavage sites based on the size of the cleaved HSP90 fragment. b Alignment for the predicted cleavage sites of HSP90α and HSP90β. c Four mutant constructs encoding recombinant human HSP90β were established (aspartic acid to alanine; D252A, D259A, D278A, D294A) by site-directed mutagenesis. The rhHSP90β mutant proteins produced in E. coli were incubated with 2 U of active recombinant caspase 10 for 4 h. The mixtures were subjected to Western blot analysis
Establishment of HSP90β WT- and HSP90β D294A-expressing cells and reduction of HSP90 cleavage by mutation at the 294th aspartic acid residue of HSP90β
To determine the change in cellular phenotype following inhibition of the HSP90 cleavage, we established K562 and Mia-PaCa-2 cell lines expressing HSP90β WT or HSP90β D294A with the Myc-tag (Fig. 3a and Supplemental Fig. S2a). To induce cleavage of HSP90, HSP90β WT, or HSP90β D294A expressed in the K562 cells, the cells were treated with MG132, whereas to induce cleavage of HSP90β WT or HSP90β D294A expressed in the Mia-PaCa-2 cells, the cells were treated with SAHA. In Fig. 3b, we used a Myc-tag for the detection of exogenous HSP90β. However, the antibody detected only the native form of exogenous HSP90β D294A but not the cleaved form. Nonetheless, when the cells were treated with MG132, the level of native exogenous HSP90β was higher in cells expressing HSP90β D294A than the cells expressing HSP90β WT (Fig. 3b). Therefore, to further determine the effect of the D294A mutation on the HSP90 cleavage, we detected HSP90 using the anti-HSP90α/β and HSP90β-specific antibodies. The amount of cleaved HSP90 fragment was smaller in HSP90β D294A-expressing K562 cells compared with HSP90β WT-expressing cells suggesting that the cleavage of the total HSP90 was suppressed by the mutation in the exogenous HSP90β (Fig. 3b). The reason why the cleavage form of exogenous HSP90β was not detected by the anti-Myc-tag antibody but detected by the anti-HSP90 antibody is unclear yet. One possibility is that the HSP90 cleavage may occur not only at the major cleavage site but also nearby at the end of the C-terminal. The epitope of the anti-HSP90α/β antibody is the C-terminal region of HSP90, and the Myc-tag was tagged at the end of the C-terminal of HSP90. Therefore, it is likely that active caspase 10 also cleaves the exogenous HSP90β somewhere between the anti-HSP90α/β antibody binding site and the Myc-tag. To exclude a possible artifact during integration of the HSP90 gene into the genome, we checked caspase 10 activity after treatment of K562 cells expressing wild-type and mutant HSP90 with MG132. As shown in Fig. 3c, there was no difference in the activation of caspase 10 induced by MG132 in both cell lines. We obtained similar results from the experiments performed with HSP90β WT- or HSP90β D294A-expressing Mia-PaCa-2 cell lines (Supplemental Fig. S2b). Therefore, we suggest that the 294th aspartic acid residue is the major site of HSP90 cleaved by active caspase 10 in cells treated with MG132 or SAHA.
MG132-mediated cleavage of HSP90 was decreased by the mutation at the 294th aspartic acid residue. a The expression of exogenous HSP90β mRNA and protein in cells was determined using RT-PCR (left) and Western blot analysis (right). Exogenous HSP90β was tagged with the Myc-tag. GAPDH was used as a loading control. b The K562-HSP90β WT and K562-HSP90β D294A cells were treated with DMSO vehicle control or indicated doses of MG132 for 24 h. The cell lysates were subjected to Western blot analysis using indicated antibodies. c The K562-HSP90β WT and K562-HSP90β D294A cells treated or untreated with z-VAD-fmk for 1 h, followed by treatment with MG132 for 24 h. The cell lysates were incubated with caspase 10 colorimetric substrate to measure the activity of caspase 10
Effect of the HSP90 cleavage site mutation on the HSP90 client protein expression and apoptosis
Because HSP90 regulates cellular activity by modulating the stability of its client proteins (Whitesell et al. 2012), the cleavage of HSP90 may reduce the amount of client proteins and inhibit cell growth. Therefore, we checked the expression levels of two HSP90 client proteins, Akt and GSK3β. As shown in Fig. 4a, the levels of Akt and GSK3β were decreased by the treatment with MG132 in HSP90β WT-expressing K562 cells, but the decreases of Akt and GSK3β were partly restored in the HSP90β D294A-expressing K562 cells. Thus, we concluded that the cleavage of HSP90 inactivates HSP90 and down-regulates HSP90 client protein expression. To identify changes in cell viability and apoptosis, we used the MTS assay and annexin V-PI staining. The cell viability of both K562 cell lines was reduced by MG132 in a concentration-dependent manner with a higher viability in HSP90β D294A-expressing cells than in the wild-type control cells (Fig. 4b). Treatment with MG132 induced a larger apoptotic population in the HSP90β WT-expressing K562 cells than in the HSP90β D294A expressing K562 cells (Fig. 4c). Similar results were obtained in the HSP90β WT- or HSP90β D294A-expressing Mia-PaCa-2 cells treated with SAHA (Supplemental Fig. S2). Therefore, the mutation at the 294th aspartic acid residue in HSP90β enhances cell viability and survival in response to HSP90 cleavage-inducing stimuli. Taken together, these data suggest that the cleavage of HSP90 induces down-regulation of client proteins, reduces cell viability, and increases apoptosis.
The effect of HSP90 cleavage on cell growth and apoptosis. a The K562-HSP90β WT and K562-HSP90β D294A cells were treated with the DMSO control or indicated doses of MG132 for 24 h. The cell lysates were subjected to Western blot analysis. The relative intensities of Akt and GSK3β bands are shown as a graph after normalization with the levels of GAPDH. P values were evaluated using t test. Values are the mean ± SD. *P < 0.05 vs DMSO control. b The cell viability of the K562-HSP90β WT and K562-HSP90β D294A cells treated with the indicated doses of MG132 was measured using the MTS assay. P values were evaluated using a ratio paired t test. Values are the means ± SD. *P < 0.05, **P < 0.01 vs. K562-HSP90β WT. c The percentages of apoptotic cells were detected by flow cytometry using annexin V-FITC and PI staining. The cells were obtained after treatment with DMSO or indicated doses of MG132 for 24 h. Values are the means ± SD. *P < 0.05 vs. K562-HSP90β WT
Discussion
Cleavage of HSP90 can occur by enzymatic cleavage and non-enzymatic cleavage (Park et al. 2019a). The enzymatic cleavage is induced by caspase 10 activation and generates approximately 55 kDa fragments (Chen et al. 2009; Park et al. 2015; Park et al. 2017), and the non-enzymatic cleavage is induced by ROS-mediated chemical degradation and generates approximately 70 kDa fragments (Beck et al. 2009; Beck et al. 2012; Castro et al. 2019). Based on the size of the HSP90 fragment produced by the enzymatic cleavage, the cleaved HSP90 fragment has a truncated N-terminus with a defect in the ATP binding pocket, and the cleavage of HSP90 may reduce the molecular chaperone activity of HSP90. Non-enzymatic cleavage is also expected to eliminate the ATPase activity of the N-terminal domain because the N-terminal region of HSP90 is physically cleaved. Therefore, it is estimated that HSP90 cleavage causes a decrease in cell growth and an increase in cell apoptosis independent of the cleavage mechanism. Previously, we found that the cleavage of HSP90 was induced by treatment with HDAC inhibitors and proteasome inhibitors by the production of ROS and activation of caspase 10 in leukemia cells (Park et al. 2015; Park et al. 2017). Here, we found that the cleavage of HSP90 is not limited in leukemic cell lines but occurs in various solid tumor cell lines. We also confirmed that the cleavage of HSP90 occurred mainly at a specific site, and inhibition of HSP90 cleavage by the site-directed mutation reduced the HDAC inhibitor- and proteasome inhibitor-mediated cell death.
Results in this study in combination with previous data reveal that the feature of HSP90 cleavage is different depending on the treated stimuli and the tested cells. Histone deacetylase inhibitor SAHA, sodium butyrate, and valproic acid caused cleavage of HSP90 but another inhibitor trichostatin A (TSA) did not induce HSP90 cleavage in K562 cells (Park et al. 2015). Importantly, it was found that TSA did not induce ROS generation while the other histone deacetylase inhibitors did (Kang et al. 2004; Kawai and Arinze 2006; Park et al. 2015; Ruefli et al. 2001; Salimi et al. 2017). HSP90 cleavage was induced by treatment of SAHA in LoVo cells unlike CT26, HCT116, Huh7, and SNU739 cells. According to our previous results, SAHA induced ROS production only in LoVo cells (unpublished data) but not in other cells (Park et al. 2015). Furthermore, SAHA and MG132-induced cleavage of HSP90 was reduced by scavenging ROS using antioxidant (Park et al. 2015; Park et al. 2017). Therefore, we suggest that HSP90 cleavage in specific cells by specific stimuli seems tightly associated with ROS production rather than the type of cells or stimuli.
HSP90 stabilizes client proteins and has a role in reducing misfolded proteins by rapid protein production in cancer cells. In addition, the expression of HSP90 is increased in response to external stimuli, acting as a defense mechanism. Reduced HSP90 activity by proteolytic cleavage results in the downregulation of its client proteins. Akt, one of the client proteins of HSP90, stimulates cell growth and suppresses cell apoptosis (Franke et al. 2003). GSK3β is also a client protein of HSP90 and involved in many cellular activities including cell growth, motility, metabolism, and apoptosis (Ali et al. 2001; Beurel et al. 2015; Woodgett 1994). GSK3β is an embryonic lethal gene, unlike GSK3α (Hoeflich et al. 2000), and studies on GSK3 as a therapeutic target are ongoing in various types of cancers (Manoukian and Woodgett 2002; McCubrey et al. 2014; Mills et al. 2011; Ougolkov and Billadeau 2006). In this study, we confirmed that the amount of Akt and GSK3β was inversely associated with the HSP90 cleavage. In addition, our previous studies have confirmed that Bcl-2 and Raf-1 also decreased (Park et al. 2015; Park et al. 2017) in a situation where HSP90 is cleaved. The reduction of the client proteins was also associated with a decrease in cell growth and increase in cell death. Because the expression of client proteins and cell survival after treatment with the inhibitors were enhanced via the mutation on the major cleavage site of HSP90, it directly supports that cleavage of HSP90 contributes to cell death by the downregulation of client proteins. Considering that HSP90 functions as a dimer and the cleavage occurs at a relatively low level, it is likely that the truncated HSP90 can associate with intact HSP90. Therefore, we speculate that relatively small amount of HSP90 cleavage contributes to detectable change of client protein expression and cell death in a similar way how dominant negative mutant works. The phenomenon that relatively small decrease of HSP90 cleavage by the mutation on the major cleavage site of HSP90 induces significant increase of cell survival can be understood in the same context.
Various HSP90 inhibitors, including 17-AAG, which entered clinical trial first, are being studied as anti-cancer drugs (Banerji et al. 2005; Park et al. 2019a). There are also many drugs that are not known to inhibit HSP90 but are being used or studied as anti-cancer drugs. HDAC inhibitors, including SAHA, suppress the HSP90 activity by inhibition of HSP90 deacetylation by HDAC6 (Bali et al. 2005; Scroggins et al. 2007). Proteasome inhibitors, including MG132, have not been identified to be directly associated with HSP90 activity. Because both HDAC inhibitors and proteasome inhibitors have an anti-tumor activity, many studies are underway to develop these as an anti-cancer drug, and several inhibitors have been tested and underwent clinical trials (Bolden et al. 2006; Crawford et al. 2011b; Curran and McKeage 2009; Cusimano et al. 2010; Dokmanovic et al. 2007; Eckschlager et al. 2017; Kelly et al. 2005; Lee et al. 2015; Ortiz-Lazareno et al. 2014). We previously confirmed that HDAC and proteasome inhibitors induce cleavage of HSP90 (Park et al. 2015; Park et al. 2017) and confirmed in this study that the cleavage of HSP90 directly contributes to the anticancer effect of the inhibitors. Therefore, we suggest that the cleavage of HSP90 may be a novel action mechanism of various drugs having anti-cancer effects and has a potential to be used as a marker for finding anti-cancer drug candidates.
Data availability
The original data are available after contact with the corresponding author.
References
Ali A, Hoeflich KP, Woodgett JR (2001) Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev 101:2527–2540
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280:26729–26734
Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I (2005) Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 23:4152–4161
Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, Raes M, Leveque P, Gallez B, Depuydt M, Collet JF, Calderon PB, Verrax J (2012) Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One 7:e40795
Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, Feron O, Calderon PB (2009) Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol 77:375–383
Beurel E, Grieco SF, Jope RS (2015) Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 148:114–131
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Boltze C, Lehnert H, Schneider-Stock R, Peters B, Hoang Vu C, Roessner A (2004) Withdrawal. HSP90 is a key for telomerase activation and malignant transition in pheochromocytoma. Endocrine 23:229–004–0002-4
Castro JP, Fernando R, Reeg S, Meinl W, Almeida H, Grune T (2019) Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: a novel gain-of-function mechanism. Redox Biol 21:101108
Chen B, Piel WH, Gui L, Bruford E, Monteiro A (2005) The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86:627–637
Chen H, Xia Y, Fang D, Hawke D, Lu Z (2009) Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol Cell Biol 29:3657–3664
Crawford LJ, Walker B, Irvine AE (2011a) Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5:101–110
Crawford LJ, Walker B, Irvine AE (2011b) Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5:101–110
Curran MP, McKeage K (2009) Bortezomib: a review of its use in patients with multiple myeloma. Drugs 69:859–888
Cusimano A, Azzolina A, Iovanna JL, Bachvarov D, McCubrey JA, D'Alessandro N, Montalto G, Cervello M (2010) Novel combination of celecoxib and proteasome inhibitor MG132 provides synergistic antiproliferative and proapoptotic effects in human liver tumor cells. Cell Cycle 9:1399–1410
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989
Eckschlager T, Plch J, Stiborova M, Hrabeta J (2017) Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci 18. https://doi.org/10.3390/ijms18071414
Ferrarini M, Heltai S, Zocchi MR, Rugarli C (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51:613–619
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Gallegos Ruiz MI, Floor K, Roepman P, Rodriguez JA, Meijer GA, Mooi WJ, Jassem E, Niklinski J, Muley T, van Zandwijk N, Smit EF, Beebe K, Neckers L, Ylstra B, Giaccone G (2008) Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One 3:e0001722
Han YH, Moon HJ, You BR, Park WH (2009) The effect of MG132, a proteasome inhibitor on HeLa cells in relation to cell growth, reactive oxygen species and GSH. Oncol Rep 22:215–221
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR (2000) Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406:86–90
Jackson SE (2013) Hsp90: structure and function. Top Curr Chem 328:155–240
Jayaraj GG, Hipp MS, Hartl FU (2020) Functional modules of the proteostasis network. Cold Spring Harb Perspect Biol 12. https://doi.org/10.1101/cshperspect.a033951
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Kang J, Chen J, Zhang D, Da W, Ou Y (2004) Synergistic killing of human leukemia cells by antioxidants and trichostatin A. Cancer Chemother Pharmacol 54:537–545
Kawai Y, Arinze IJ (2006) Valproic acid-induced gene expression through production of reactive oxygen species. Cancer Res 66:6563–6569
Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Kornberg RD (1999) Eukaryotic transcriptional control. Trends Cell Biol 9:M46–M49
Kumar S, van Raam BJ, Salvesen GS, Cieplak P (2014) Caspase cleavage sites in the human proteome: CaspDB, a database of predicted substrates. PLoS One 9:e110539
Lee P, Murphy B, Miller R, Menon V, Banik NL, Giglio P, Lindhorst SM, Varma AK, Vandergrift WA 3rd, Patel SJ, Das A (2015) Mechanisms and clinical significance of histone deacetylase inhibitors: epigenetic glioblastoma therapy. Anticancer Res 35:615–625
Manoukian AS, Woodgett JR (2002) Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res 84:203–229
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Cocco L, Cervello M, Martelli AM (2014) Multifaceted roles of GSK-3 and Wnt/beta-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 28:15–33
Mills CN, Nowsheen S, Bonner JA, Yang ES (2011) Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front Mol Neurosci 4:47
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76
Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol 143:901–910
Ortiz-Lazareno PC, Bravo-Cuellar A, Lerma-Diaz JM, Jave-Suarez LF, Aguilar-Lemarroy A, Dominguez-Rodriguez JR, Gonzalez-Ramella O, De Celis R, Gomez-Lomeli P, Hernandez-Flores G (2014) Sensitization of U937 leukemia cells to doxorubicin by the MG132 proteasome inhibitor induces an increase in apoptosis by suppressing NF-kappa B and mitochondrial membrane potential loss. Cancer Cell Int 14:13-2867-14-13
Ougolkov AV, Billadeau DD (2006) Targeting GSK-3: a promising approach for cancer therapy? Future Oncol 2:91–100
Park S, Park JA, Yoo H, Park HB, Lee Y (2017) Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS generation and caspase 10-activation in human leukemic cells. Redox Biol 13:470–476
Park S, Park JA, Kim YE, Song S, Kwon HJ, Lee Y (2015) Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones 20:149–157
Park S, Park JA, Jeon JH, Lee Y (2019a) Traditional and novel mechanisms of heat shock protein 90 (HSP90) inhibition in cancer chemotherapy including HSP90 cleavage. Biomol Ther (Seoul) 27:423–434
Park SE, Kim DE, Kim MJ, Lee JS, Rho JK, Jeong SY, Choi EK, Kim CS, Hwang JJ (2019b) Vorinostat enhances gefitinib induced cell death through reactive oxygen species dependent cleavage of HSP90 and its clients in nonsmall cell lung cancer with the EGFR mutation. Oncol Rep 41:525–533
Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM (2007) High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res 67:2932–2937
Powers MV, Workman P (2006) Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer 13(Suppl 1):S125–S135
Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75
Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A 97:10014–10019
Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW (2001) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of bid and production of reactive oxygen species. Proc Natl Acad Sci U S A 98:10833–10838
Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M (2017) Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis 16:208-017-0593-4
Sato S, Fujita N, Tsuruo T (2000) Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 97:10832–10837
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360
Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L (2007) An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell 25:151–159
Shirley RB, Kaddour-Djebbar I, Patel DM, Lakshmikanthan V, Lewis RW, Kumar MV (2005) Combination of proteasomal inhibitors lactacystin and MG132 induced synergistic apoptosis in prostate cancer cells. Neoplasia 7:1104–1111
Sreedhar AS, Kalmar E, Csermely P, Shen YF (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15
Wandinger SK, Richter K, Buchner J (2008) The Hsp90 chaperone machinery. J Biol Chem 283:18473–18477
Wayne N, Bolon DN (2007) Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers J Biol Chem 282:35386–35395
Welch WJ (1993) How cells respond to stress. Sci Am 268:56–64
Whitesell L, Santagata S, Lin NU (2012) Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med 12:1108–1124
Woodgett JR (1994) Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin Cancer Biol 5:269–275
Acknowledgments
We would like to thank Professor Kyu Lim at Chungnam National University for kindly providing the pancreatic cancer cell line PANC02.
Code availability
Not applicable.
Funding
This research was supported by a grant (2018R1A2B6002504) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and by the Basic Science Research Program (2019R1A6A3A01096936) through the NRF funded by the Ministry of Education in the Republic of Korea.
Author information
Authors and Affiliations
Contributions
Sangkyu Park and Younghee Lee conceived the study and wrote the manuscript. Sangkyu Park, Jae-Hyung Jeon, Jeong-A Park, and Jun-Kyu Choi performed the experiments. Sangkyu Park, Jae-Hyung Jeon, and Younghee Lee analyzed the data and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed the manuscript and agreed to publish the data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 251 kb)
Rights and permissions
About this article
Cite this article
Park, S., Jeon, JH., Park, JA. et al. Cleavage of HSP90β induced by histone deacetylase inhibitor and proteasome inhibitor modulates cell growth and apoptosis. Cell Stress and Chaperones 26, 129–139 (2021). https://doi.org/10.1007/s12192-020-01161-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01161-6