Abstract
Purpose
Cks1, a conformationally heterogenous 9 kDa protein, is markedly overexpressed in cancer cells and contributes to tumor development. Cks1 is an essential component of the SCF-Skp2 ubiquitin ligase complex that targets the Cdk inhibitors p27Kip1 and p21Cip1. Cks1 is known to interact with the Hsp90-Cdc37 chaperone machinery, although whether this facilitates its conformational maturation and stability is not known. To test whether abrogating the chaperone function of Hsp90 could destabilize Cks1, we examined the effects of treating different cancer cell lines with the benzoquinone ansamycin 17-allylamino geldanamycin (17-AAG), a compound that selectively binds Hsp90 and potently inhibits its ATP-dependent chaperone activity.
Methods
The effect of Hsp90 inhibition using 17-AAG on Cks1 protein and associated cell cycle proteins including Skp2, p27Kip1, p21Cip1, and Cdk1 in cancer cells was determined by Western blotting. Ubiquitination analysis was carried out by transfecting cells with an HA-ubiquitin plasmid and specifically immunoprecipitating Cks1 to examine polyubiquitinated species. Flow cytometry was utilized to examine the effects of Hsp90 inhibition on cell cycle profiles.
Results
Here, we demonstrate for the first time that inhibition of Hsp90 utilizing 17-AAG destabilizes Cks1 in cancer cells by promoting its ubiquitination and proteasomal degradation. 17-AAG-induced Cks1 depletion was accompanied by concomitant decreases in Skp2 and Cdk1. 17-AAG treatment also induced G2/M accumulation in MCF-7 breast carcinoma cells, and G1 accumulation in the colon carcinoma lines HCT116 and SW620.
Conclusions
We conclude that perturbing the Hsp90 pathway could provide a useful therapeutic strategy in tumors driven by Cks1 overexpression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cks1 is an evolutionarily conserved 9 kDa protein first identified as a component of yeast cyclin–Cdk complexes, which has been implicated in cell cycle progression, transcriptional regulation, and growth-signaling pathways [1]. Importantly, in the past few years, several studies, including ours, have demonstrated that Cks1 is highly upregulated in tumors [2–6]. Of note, in breast cancer, it has been shown using Cox multivariate analysis that high Cks1 levels were strongly and independently associated with poor disease-free survival and poor overall survival [3]. In addition, Cks1 is also overexpressed in a number of other cancer subtypes, including colorectal carcinoma, lymphoma, lung cancer, prostate carcinoma, and multiple myeloma [2–8]. Cks1 performs multiple functions in cancer cell physiology, many of which are likely key to its roles in tumor progression. For instance, Cks1 is a crucial component of the SCF-Skp2 ubiquitin ligase complex, which is responsible for the degradation of the Cdk inhibitors p27Kip1, p21Cip1, and p130/Rb2 [9–11]. Studies from our laboratory have also shown that Cks1 regulates the expression of Cdk1 and thereby controls G2/M progress and mitotic entry [9]. Cks1 overexpression also directly contributes to tumor development by circumventing DNA damage response barriers and providing a proliferative advantage to premalignant cells [12].
Given its pleiotropic roles in the expression of tumorigenic phenotypes, inhibition of Cks1 has been suggested as a potential anticancer strategy [1]. Importantly, the therapeutic promise of such an approach has been validated in a myc-induced transgenic lymphoma model [13]. In this model, wherein high levels of Cks1 protein are present, tumor progression was severely abrogated by knockout of Cks1 [13]. Similarly, tumorigenesis is also abrogated by knockout of Cks1 in a cyclin E-induced mammary carcinoma model [12]. RNAi strategies of Cks1 inhibition using either siRNA duplexes or shRNA vectors have also been successful in vitro and have demonstrated inhibition of cancer cell proliferation and cell cycle blockade in several instances [9, 14, 15]. However, depletion of Cks1 gene expression by such means has not been demonstrated to be a feasible therapeutic strategy, either in animal models or in the clinic. Thus, from a practical standpoint, it is not entirely clear how Cks1 can be targeted in cancers. Recently, it was demonstrated that Cks1 is ubiquitinated and targeted to proteasomes for degradation [16–18]. Therefore, an alternative strategy would be to induce the depletion of Cks1 protein by inducing its proteolytic removal by proteasomes.
A potential avenue for inducing turnover of oncoproteins is to target Hsp90, a molecular chaperone that is responsible for the stability, folding, and maturation of several client proteins [19, 20]. Tumor cells are more dependent on Hsp90 than normal counterparts, and its pharmacological manipulation in order to interfere with the folding and conformational stability of client proteins has been shown to elicit their selective proteolytic degradation [21–24]. Cks proteins exist in twofolded states, a monomer or a domain-swapped dimer, and are thought to interchange between these by way of folding intermediates [25]. Cks1 has been shown to exhibit conformational heterogeneity from spontaneous unzipping of hydrogen bonds between its β4 and the β2 strands, resulting in potential intermediates in the unfolding pathway that lead to domain swapping [26]. The cellular chaperone machinery assists in the folding reactions of proteins, by helping avoid unfavorable interactions and guiding them to achieve their correct oligomeric state within the crowded environment of the cell. Chaperones are also thought to sample folding intermediates and upon certain cues transition from ‘folding facilitation mode’ to a ‘degradation mediation mode’ [23, 27]. Recent proteomic studies utilizing Ramos lymphoma cells have demonstrated that Cks1 exists in complexes with Hsp90 and its co-chaperone Cdc37 [28]. Whether these interactions stabilize Cks1 by facilitating its folding pathways within cells is not known at present. To test whether abrogating the chaperone function of Hsp90 would destabilize Cks1, we examined the effects of treating different cancer cell lines with the benzoquinone ansamycin 17-allylamino geldanamycin (17-AAG), a compound that selectively binds Hsp90 in its conserved ATP-/ADP-binding pocket and potently inhibits its ATP-dependent chaperone activity. We demonstrate that 17-AAG induces both ubiquitination and rapid proteasomal degradation of Cks1 in cancer cell lines. Loss of Cks1 was accompanied by a substantial concomitant decrease in Skp2, and also Cdk1. 17-AAG treatment also induced G2/M accumulation in MCF-7 breast carcinoma cells, and G1 accumulation in the colon carcinoma lines HCT116 and SW620. Our results suggest that Hsp90 inhibition could provide a useful therapeutic strategy in tumors driven by Cks1 overexpression.
Materials and methods
Cell lines and chemicals
MCF-7, SW620, and HCT116 cell lines were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI media (Thermo Fisher Scientific, Waltham, MA) containing 10 % FBS and 100 units/mL penicillin–streptomycin antibiotic solution (Life Technologies, Grand Island, NY). All cells were maintained at 5 % CO2 and 37 °C. Epoximicin and 17-AAG were obtained from Apex Bio (Houston, TX).
Immunoblotting
Cells harvested following each experiment were lysed with 1× lysis buffer (Cell Signaling, Danvers, MA) to obtain lysates. 25–40 μg of protein was run on Mini-PROTEANTM or CriterionTM gels (Bio-Rad, Hercules, CA) and transferred on to nitrocellulose membranes by overnight wet transfer. The membranes were blocked for 1 h with 5 % milk in 0.05 % TBST and probed with designated antibodies at the indicated dilutions—Cks1 (Life Technologies, Grand Island, NY, 1:500–1:2,000), p27Kip1 (BD Transduction Labs, San Jose CA, 1:2,000–1:3,000), p21Cip1 (BD Transduction Labs, San Jose, CA, 1:1,000–1:2,000), Skp2 (Life Technologies, Grand Island, NY, 1:500), Cdk1 (Cell Signaling, Danvers, MA, 1:3,000), ERα (SantaCruz Biotech, Dallas, TX, 1:1,000), and GAPDH Clone 14C10 (Cell Signaling, Danvers, MA, 1:20,000–1:50,000).
Ubiquitination assays
An HA-ubiquitin plasmid (obtained from Dr Harish Ramanathan, NIH, Bethesda, Maryland) was transfected into MCF-7 by lipofection. Cells were then treated with proteasomal inhibitors for the indicated times and harvested. 100–200 μg of lysate from the harvested cells was subjected to immunoprecipitation (IP) with 1–2 μg of Cks1 antibody using the Exacta CruzTM F kit (Santa Cruz Biotech, Dallas, TX) according to the manufacturer’s instructions. Normal rabbit IgG serum (Santa Cruz Biotech, Dallas, TX) was used as an isotype control for nonspecific binding during IP experiments. The IPs were then transferred to nitrocellulose by overnight wet transfer and subjected to immunoblotting to assess changes in ubiquitinated Cks1.
RNA interference
Cells plated in 60 mm dishes were transfected using DharmaFECT (GE Dharmacon, Lafayette, CO) in serum-free medium. Cells were transfected with either a Cks1-specific siRNA (Cks1 siRNA# 4, UCUGAUGUCUGAAUCUGAAUU, GE Dharmacon, Lafayette, CO) or a scrambled siRNA control duplex with no known homology to mammalian genes (nontargeting siRNA# 2, UAAGGCUAUGAAGAGAUAC). After 24 h of siRNA incubation, cultures were either harvested or re-fed with the medium and conditions indicated for each experiment and then harvested at various time points. Harvested cells were lysed for immunoblotting analysis.
Cell cycle analysis
For cell cycle studies, cells were exposed to propidium iodide. Samples were then treated with 5 ng/mL of RNase (EMD Millipore, Billerica, MA) and then analyzed on a fluorescence-activated cell sorting (FACS) scanner using laser excitation at 488 nm (Becton Dickinson, Franklin Lakes, NJ). Cell cycle analysis was done using ModFit (Verity Software House, Topsham, ME).
Results and discussion
17-AAG treatment depletes Cks1 while concomitantly decreasing Skp2 and Cdk1 in MCF-7 breast carcinoma cells
Cks1 is a conformationally heterogenous 9 kDa ‘oncoprotein’ that is overexpressed in multiple cancers [26]. Cks1 has been shown to interact in complexes with Hsp90 and its co-chaperone Cdc37 [27]. We initially compared the effects of 17-AAG treatment on MCF-7 breast carcinoma cells with cells transfected with Cks1-specific siRNA. Cks1 knockdown resulted in concomitant loss of Skp2, with modest increases in p27Kip1 and p21Cip1 (Fig. 1a). In comparison, 17-AAG treatment led to a dose-dependent decline in Cks1 protein level, resulting in a near complete loss of the protein within 48–72 h (Fig. 1b, c). In agreement with the siRNA experiments, we observed that 17-AAG-mediated treatment also resulted in decreased Skp2 in MCF-7 cells (Fig. 1b, c). This is consistent with the fact that Skp2 is autoubiquitinated in the absence of Cks1 [29]. Furthermore, treatment with 17-AAG (0.1–5 μM) for 48–72 h also resulted in stabilization of p21Cip1, a known substrate of the SCF-Skp2Cks1 ubiquitin ligase [30] (Fig. 1b, c). 17-AAG treatment also resulted in a complete loss of estrogen receptor α (ERα) and Cdk1, two recognized clients of Hsp90 (Fig. 1b, c) [31, 32]. Interestingly, we have previously reported that siRNA-mediated depletion of Cks1 does not substantially alter ERα in MCF-7 cells, suggesting that the 17-AAG effect on this protein is independent of its effect on Cks1 [9]. On the other hand, we have also previously reported that Cks1 positively regulates Cdk1 expression in MCF-7 and other cancer cells [9]. Since results herein reveal that 17-AAG induces a potent downregulation of Cdk1, we posit that Hsp90 inhibition could affect Cdk1 by both directly affecting its stability, and also indirectly by causing Cks1 downregulation. These results collectively suggest that potent depletion of Cks1 can be achieved by means of Hsp90 inhibition that can lead to downstream perturbation of other key cell cycle regulatory proteins in cancers.
Comparison of the effects of Cks1 depletion induced by either the Hsp90 inhibitor 17-AAG or by RNAi in MCF-7 breast carcinoma cells. a MCF-7 cells were transfected for 24 h with either a nontargeting or a Cks1-specific siRNA duplexes using DharmaFECT. Cells were washed and incubated for another 24 h after which they were harvested and lysed. Lysates were examined for the levels of indicated proteins by immunoblotting. b, c 17-AAG induces depletion of Cks1 and associated cell cycle proteins in a dose-dependent manner. MCF-7 cells were treated with indicated concentrations of 17-AAG for 48 h or 72 h. Cells were harvested and lysates from the harvested cells were analyzed by immunoblotting. The numbers below the immunoblots represents the changes in densitometric ratios with respect to GAPDH
17-AAG induces Cks1 polyubiquitination and proteasome-mediated degradation in MCF-7 cells
We next assessed whether Hsp90 inhibition induces Cks1 depletion by the ubiquitin-proteasome pathway. To test whether 17-AAG can trigger Cks1 ubiquitination in MCF-7, cells were transiently transfected with HA-ubiquitin. We found that immunoblots of Cks1-specific immunoprecipitates from MCF-7 cells manifest a smear of polyubiquitinated Cks1 as compared to control immunoprecipitates (Fig. 2a). Treatment with 17-AAG alone caused a marked increase in the intensity of these smears indicating that either Hsp90 inhibition increases ubiquitination of Cks1 or decreases its basal deubiquitination rates (Fig. 2a). Epoximicin alone or cotreatment with 17-AAG caused a further increase in the smear intensity, indicating that proteasomal inhibition causes a reduced clearance of polyubiquitinated Cks1 protein (Fig. 2a). We next performed a time course analysis (Fig. 2b, upper panel). Whereas epoximicin did not substantially affect constitutive Cks1 levels, it completely blocked 17-AAG-induced depletion of the protein. To assess how this correlates with its ubiquitination, we performed a time course analysis of Cks1-polyubiquitinated species under these conditions (Fig. 2b, lower panel). The data revealed that the accumulation of polyubiquitinated Cks1 upon 17-AAG treatment was a transient phenomenon with peak intensity at 4 h followed by substantial loss of the 9 kDa Cks1 band at 24 h (Fig. 2b). We postulate therefore that perturbing Hsp90 not only leads to proteasomal removal of the multiubiquitin-tagged Cks1 but could potentially induce simultaneous feedback mechanisms such as its deubiquitination, leading to the transient nature of the appearance of polyubiquitinated Cks1. Interestingly, treatment with epoximicin alone also caused a transient accumulation of polyubiquitinated Cks1, suggesting that regulation of steady-state Cks1 levels in unperturbed MCF-7 also presumably reflects a balance between proteasomal degradation and ongoing feedback deubiquitination, etc. (Fig. 2b). The overall transient pattern was maintained upon cotreatment with 17-AAG and epoximicin although the smears were more intense due to the additive effects of Hsp90 inhibition and reduced proteasomal clearance (Fig. 2b, lower panel).
17-AAG treatment induces polyubiquitination of Cks1 in MCF-7 cells. a MCF-7 cells were transfected with a plasmid expressing HA-tagged ubiquitin protein. Twenty-four-hour posttransfection, cells were treated with either vehicle (DMSO) or indicated compounds for 8 h. Cks1 was immunoprecipitated using a specific antibody. Immunoprecipitates were analyzed by anti-HA immunoblotting. Preimmune rabbit serum was used as a control. b Time course representing ubiquitination kinetics of Cks1 upon Hsp90 inhibition and proteasomal blockade in MCF-7 cells. Cells transfected with the HA-ubiquitin plasmid were treated with 17-AAG and epoximicin as indicated. Cells were harvested at indicated times, and ubiquitination of Cks1 was assessed
We next examined a time course of 17-AAG’s effects on Cks1, Skp2, p27Kip1, and p21Cip1 in MCF-7 cells for periods up to 72 h. Epoximicin treatment was, however, restricted to 24 h since longer exposures with this agent causes cytotoxicity in MCF-7 cells. As depicted in Fig. 3a, b, we found that Cks1 depletion induced by a 24-h 17-AAG treatment (either 1 or 5 μM) in MCF-7 cells was substantially reduced by simultaneous proteasomal blockade with epoximicin. However, epoximicin treatment for 24 h did not efficiently block Cks1 depletion induced by longer 17-AAG exposure (48–72 h) (Fig. 3a, lower panel). In contrast to Cks1, the decrease in Skp2 was blocked by epoximicin only at the 4 h and 8 h time points (Fig. 3a, b). This suggests that sustained inhibition of Hsp90 can overcome proteasomal blockade and can induce other mechanisms of downregulation of Cks1 and Skp2. Proteasomal blockade alone or in combination with a 24-h 17-AAG treatment led to a substantial and rapid stabilization in p21Cip1 levels (Fig. 3a, b). Interestingly, in comparison with p21Cip1, epoximicin treatment either alone or in combination with 17-AAG only induced a modest stabilization of p27Kip1 levels, which is already present at high levels in MCF-7 cells (Fig. 3a, b).
17-AAG treatment depletes Cks1 through the proteasomal degradation pathway in MCF-7 cells. a, b MCF-7 cells were either left untreated or pretreated with epoxomicin (0.5 μM) for 30 min. Cells were then treated with 17-AAG at a concentration of 1 μM (a) or 5 μM (b) in presence or absence of epoximicin. Protein lysates prepared at the indicated times were analyzed by immunoblotting. The numbers below the immunoblots represent densitometric ratios of the protein with respect to GAPDH. c MCF-7 cells were either left untreated or treated with 17-AAG at a concentration 5 μM for 24–72 h. Epoxomicin (0.5 μM) was added 24 h prior to harvest. Protein lysates prepared at the indicated times were analyzed by immunoblotting. The numbers below the immunoblots represent densitometric ratios of the protein with respect to GAPDH
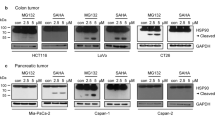
17-AAG-treated HCT116 and SW620 colon carcinoma cells exhibit proteasomal degradation of Cks1 and concomitant loss of Skp2 and Cdk1
Increased Cks1 expression is strongly correlated to tumor aggressiveness in different tumors of gastrointestinal system such as esophagus [33], stomach [34, 35], colon [5, 36, 37], and liver [14, 38]. However, whether the aberrantly high Cks1 protein found in gastrointestinal tumors can be reduced by inhibiting Hsp90 function has not been examined. Previously, Moser et al. have shown that the Hsp90 inhibitor 17-DMAG substantially inhibits phosphorylation of epidermal growth factor receptor, c-Met, and focal adhesion kinase, resulting in a significant decrease in cancer cell invasiveness in HCT116 and SW620 cells [39]. Furthermore, it was recently shown that frequent expression of hsp90alpha and hsp90N on the surface of colorectal cancer cells may enable hsp90 to promote metastasis [40]. In fact, inhibition of Hsp90 in colorectal cancer downregulates NF-κB, leading to inhibition of epithelial mesenchymal transition, motility, and invasiveness [40]. Interestingly, Cks1 supports hepatocarcinogenesis through regulation of NF-κB pathway [14]. Collectively, these studies suggest the notion that part of the decrease in tumorigenic properties of colorectal cancer cells following Hsp90 inhibition may occur through potential Cks1-dependent mechanism. These findings prompted us to investigate the consequences of Hsp90 inhibition on Cks1 protein stability in colorectal cancer cell lines.
To assess the role of 17-AAG-mediated Hsp90 inhibition on Cks1 protein stability in colorectal cancer, we analyzed two different colon cancer lines, HCT116 and SW620, cell lines that have been previously shown to exhibit a decrease in growth and invasiveness upon pharmacological inhibition of Hsp90 [39]. We initially compared RNAi-mediated depletion of Cks1 with the effects of 17-AAG on Cks1 in HCT116 cells (Fig. 4a, b). We found that 17-AAG treatment for 72 h led to a dose-dependent decrease of Cks1 and simultaneously reduced Skp2 and Cdk1 in a manner, which was largely similar to the effects elicited by Cks1 siRNA duplexes (Fig. 4a, b). 17-AAG-induced loss of Cks1 was accompanied by a substantial stabilization of p21Cip1 and p27Kip1 in HCT116 cells (Fig. 4b). Like MCF-7 cells, 17-AAG-mediated Cks1 depletion in HCT116 was blocked by epoximicin only at early time points (8–24 h) (Fig. 4c, d). On the other hand, longer exposures with 17-AAG (72 h) can overcome epoximicin and induce sustained depletion of both Cks1 and Skp2 presumably through nonproteasomal means (Fig. 4d). 17-AAG treatment also stabilized p21Cip1 and p27Kip1, which was further stabilized by simultaneous proteasomal blockade (Fig. 4d).
17-AAG treatment depletes Cks1 through the proteasomal degradation pathway and concomitantly decreases Skp2 and Cdk1 in HCT116 colon carcinoma cells. Cells were harvested and protein lysates from the harvested cells were analyzed by immunoblotting for indicated proteins following each experiment (described in a through d). a Nontargeting or Cks1-specific siRNA duplexes were applied to HCT116 cells using DharmaFECT transfection reagent for 24 h. Cells were harvested and lysed 24 and 48 h after completion of transfection. b HCT116 cells were treated with indicated dose (nM) of 17-AAG for 72 h. c HCT 116 cells were treated with 17-AAG (5 μM) in presence or absence of epoximicin (0.5 μM) and harvested at indicated times. d HCT 116 cells were treated with 17-AAG (5 μM) for 24–72 h. Epoximicin (0.5 μM) was added 24 h prior to harvest. The numbers below the immunoblots represent densitometric ratios with respect to GAPDH
We also compared the effects of RNAi-mediated depletion of Cks1 in SW620 versus the effects of 17-AAG-mediated downregulation of Cks1 in this line (Fig. 5a, b). We found that SW620 cells exhibited a sharp decline in Cks1 and Skp2 protein levels upon 17-AAG treatment (Fig. 5b). This was accompanied by accumulation of p27Kip1 as in the case of siRNA-mediated Cks1 depletion (Fig. 5a, b). Expectedly, epoximicin blocked 17-AAG-induced Cks1 depletion in SW620 at early time points (12–24 h), but less efficiently at later times (48–72 h) (Fig. 5c, d). Unlike MCF-7 and HCT116, basal p21Cip1 is nearly undetectable SW620 cells (Fig. 5c, d). However, combined Hsp90 inhibition by 17-AAG and proteasomal block by epoximicin led to a stabilization of p21Cip1 levels at least at the earlier times (Fig. 5c). On the other hand, substantial basal p27Kip1 is present in SW620 cells, which is moderately stabilized by 17-AAG alone (24–72 h) (Fig. 5c, d). Also, p27Kip1 is markedly stabilized in these cells by epoximicin or cotreatment with epoximicin and 17-AAG for up to 24 h (Fig. 5d).
17-AAG treatment depletes Cks1 through the proteasomal degradation pathway and concomitantly decreases Skp2 and Cdk1 in SW620 colon carcinoma cells. Cells were harvested and protein lysates from the harvested cells were analyzed by immunoblotting for indicated proteins following each experiment (described in a through d). a Nontargeting or Cks1-specific siRNA duplexes were applied to SW620 cells using DharmaFECT transfection reagent for 24 h. Cells were harvested and lysed 24 and 48 h after completion of transfection. b SW620 cells were treated with indicated dose (nM) of 17-AAG for 72 h. Cells were harvested and processed for immunoblotting. c SW620 cells were treated with 17-AAG (5 μM) in presence or absence of epoximicin (0.5 μM) and harvested at indicated times. d SW620 cells were treated with 17-AAG (5 μM) for 24–72 h. Epoximicin (0.5 μM) was added 24 h prior to harvest. The numbers below the immunoblots represents densitometric ratios with respect to GAPDH
17-AAG induces blockade of cell cycle progression in breast and colon cancer cells
Since our results described above suggested that Hsp90 inhibition-mediated depletion of Cks1 is accompanied by changes in other cell cycle regulatory proteins as well, we also examined the effects of 17-AAG on cell cycle distribution in MCF-7, HCT116, and SW620 cancer cell lines. In MCF-7 cells, a 24-h 17-AAG (5 μM) treatment led to a significant accumulation of cells in the G2/M phase, with a corresponding reduction in G1- and S-phase populations (Fig. 6). Epoximicin treatment alone or in combination with 17-AAG for 24 h also caused G2/M accumulation in MCF-7 although less potent, and with lesser compensatory increases in the G1 and S populations than that induced by 17-AAG alone (Fig. 6). On the other hand, HCT116 and SW620 cells responded to Hsp90 inhibition predominantly by accumulating in the G1 phase (Fig. 6). HCT116 and SW620 cells treated with epoximicin alone mainly exhibited an S-phase increase. Epoximicin in combination with 17-AAG in HCT116 and SW620 cells reduced the magnitude of the G1 accumulation caused by 17-AAG alone (Fig. 6).
17-AAG and epoximicin treatment induce cell cycle perturbations in breast and colon cancer cells. MCF-7, HCT116, or SW-620 cells were treated with 17-AAG, epoximicin, or both for 24 h. Cells were harvested and stained with propidium iodide as described in the text and analyzed by FACS scanning. Percentages of cells in G1, S, and G2/M were calculated using the ModFit software. (*Significantly different with respect to DMSO controls, p < 0.05)
In conclusion, we have identified here for the first time a potential mechanism for modulating Cks1 protein stability by perturbing Hsp90 function. The block in cell cycle progression in MCF-7 breast carcinoma cells induced by 17-AAG is likely to be a result of both reduction in key cell cycle proteins such as Cks1 and Cdk1, as well as perturbation of other estrogen-mediated responses through ERα. In this regard, Whitesell and coinvestigators had previously demonstrated that Hsp90 inhibition in MCF-7, as well as another ERα+ breast cancer line T47D, causes destabilization of ERα and marked decline in its levels [31]. Our findings herein are also consistent with those of Watanabe et al who reported that Hsp90 inhibition induces a Cdk1-mediated cell cycle blockade [32]. The differences in the cell cycle response of the colon cancer cells to 17-AAG vis-à-vis MCF-7 cells could be due to differences in the oncogenic drivers between cell lines and how they respond to Hsp90 inhibition. Herein, we demonstrate that 17-AAG triggers ubiquitination and subsequent proteasomal turnover of Cks1, which is effectively blocked by epoximicin. Longer exposure with 17-AAG potently depletes Cks1 even in the presence of epoximicin, suggesting alternative routes of degradation can come into play when unfolded proteins accumulate for long periods of time. Mimnaugh et al. [41] have also shown that simultaneous inhibition of Hsp90 and the proteasome leads to the accumulation of detergent-insoluble aggregated proteins. In this regard, it is also important to note that Kaganovich et al have suggested that cells adopt distinct strategies for removal of proteins with soluble misfolded conformations versus terminally aggregated forms [42]. According to this model, cells partition misfolded proteins into different compartments on the basis of their ubiquitination and solubility [42]. Further mechanistic studies deciphering Hsp90-mediated regulation of Cks1 and its downstream effects will provide clues for effectively targeting Cks1 in cancers.
References
Khattar V, Thottassery JV (2013) Cks1: structure, emerging roles and implications in multiple cancers. J Cancer Ther 4(8):1341–1354
Westbrook L, Ramanathan HN, Isayeva T, Mittal AR, Qu Z, Johnson MD, Kern FG, Ponnazhagan S, Grubbs CJ, Thottassery JV (2009) High Cks1 expression in transgenic and carcinogen-initiated mammary tumors is not always accompanied by reduction in p27Kip1. Int J Oncol 34(5):1425–1431
Slotky M, Shapira M, Ben-Izhak O, Linn S, Futerman B, Tsalic M, Hershko DD (2005) The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res BCR 7(5):R737–R744
Shapira M, Ben-Izhak O, Slotky M, Goldin O, Lahav-Baratz S, Hershko DD (2006) Expression of the ubiquitin ligase subunit cyclin kinase subunit 1 and its relationship to S-phase kinase protein 2 and p27Kip1 in prostate cancer. J Urol 176(5):2285–2289
Hershko DD, Shapira M (2006) Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer 107(4):668–675
Urbanowicz-Kachnowicz I, Baghdassarian N, Nakache C, Gracia D, Mekki Y, Bryon PA, Ffrench M (1999) Ckshs expression is linked to cell proliferation in normal and malignant human lymphoid cells. Int J Cancer 82(1):98–104
Tsai YS, Chang HC, Chuang LY, Hung WC (2005) RNA silencing of Cks1 induced G2/M arrest and apoptosis in human lung cancer cells. IUBMB Life 57(8):583–589
Chang H, Jiang N, Jiang H, Saha MN, Qi C, Xu W, Reece D (2010) CKS1B nuclear expression is inversely correlated with p27Kip1 expression and is predictive of an adverse survival in patients with multiple myeloma. Haematologica 95(9):1542–1547
Westbrook L, Manuvakhova M, Kern FG, Estes NR 2nd, Ramanathan HN, Thottassery JV (2007) Cks1 regulates cdk1 expression: a novel role during mitotic entry in breast cancer cells. Cancer Res 67(23):11393–11401
Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A (2001) The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol 3(3):321–324
Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI (2001) A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell 7(3):639–650
Liberal V, Martinsson-Ahlzen HS, Liberal J, Spruck CH, Widschwendter M, McGowan CH, Reed SI (2012) Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression overrides the DNA damage response barrier triggered by activated oncoproteins. Proc Natl Acad Sci USA 109(8):2754–2759
Keller UB, Old JB, Dorsey FC, Nilsson JA, Nilsson L, MacLean KH, Chung L, Yang C, Spruck C, Boyd K, Reed SI, Cleveland JL (2007) Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J 26(10):2562–2574
Lee EK, Kim DG, Kim JS, Yoon Y (2011) Cell-cycle regulator Cks1 promotes hepatocellular carcinoma by supporting NF-kappaB-dependent expression of interleukin-8. Cancer Res 71(21):6827–6835
Wang XC, Tian LL, Tian J, Wu HL, Meng AM (2009) Overexpression of Cks1 is associated with poor survival by inhibiting apoptosis in breast cancer. J Cancer Res Clin Oncol 135(10):1393–1401
Khattar V, Thottassery JV (2015) Cks1 proteasomal turnover is a predominant mode of regulation in breast cancer cells: role of key tyrosines and lysines. Int J Oncol 46(1):395–406
Hattori T, Kitagawa K, Uchida C, Oda T, Kitagawa M (2003) Cks1 is degraded via the ubiquitin-proteasome pathway in a cell cycle-dependent manner. Genes Cells 8(11):889–896
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (2004) Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428(6979):190–193
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5(10):761–772
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956):407–410
Hohfeld J, Cyr DM, Patterson C (2001) From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep 2(10):885–890
McClellan AJ, Tam S, Kaganovich D, Frydman J (2005) Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol 7(8):736–741
Krishnamoorthy GP, Guida T, Alfano L, Avilla E, Santoro M, Carlomagno F, Melillo RM (2013) Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J Biol Chem 288(24):17481–17494
Rousseau F, Schymkowitz JW, Wilkinson HR, Itzhaki LS (2004) Intermediates control domain swapping during folding of p13suc1. J Biol Chem 279(9):8368–8377
Seeliger MA, Spichty M, Kelly SE, Bycroft M, Freund SM, Karplus M, Itzhaki LS (2005) Role of conformational heterogeneity in domain swapping and adapter function of the Cks proteins. J Biol Chem 280(34):30448–30459
Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU (1996) Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA 93(25):14536–14541
Radulovic M, Crane E, Crawford M, Godovac-Zimmermann J, Yu VP (2010) CKS proteins protect mitochondrial genome integrity by interacting with mitochondrial single-stranded DNA-binding protein. Mol Cell Prote 9(1):145–152
Wang W, Ungermannova D, Jin J, Harper JW, Liu X (2004) Negative regulation of SCFSkp2 ubiquitin ligase by TGF-beta signaling. Oncogene 23(5):1064–1075
Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278(28):25752–25757
Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Akinaga S, Whitesell L (2001) Destabilization of steroid receptors by heat shock protein 90-binding drugs: a ligand-independent approach to hormonal therapy of breast cancer. Clin Cancer Res 7(7):2076–2084
Watanabe G, Behrns KE, Kim JS, Kim RD (2009) Heat shock protein 90 inhibition abrogates hepatocellular cancer growth through cdc2-mediated G2/M cell cycle arrest and apoptosis. Cancer Chemother Pharmacol 64(3):433–443
Wang JJ, Fang ZX, Ye HM, You P, Cai MJ, Duan HB, Wang F, Zhang ZY (2013) Clinical significance of overexpressed cyclin-dependent kinase subunits 1 and 2 in esophageal carcinoma. Dis Esophagus 26(7):729–736
Lee SW, Kang SB, Lee DS, Lee JU (2013) Akt and Cks1 are related with lymph node metastasis in gastric adenocarcinoma. Hepatogastroenterology 60(124):932–937
Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, Utsunomiya T, Mori M (2003) Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res 9(15):5693–5698
Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD (2005) The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer 103(7):1336–1346
Shapira M, Ben-Izhak O, Bishara B, Futerman B, Minkov I, Krausz MM, Pagano M, Hershko DD (2004) Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer 100(8):1615–1621
Calvisi DF, Ladu S, Pinna F, Frau M, Tomasi ML, Sini M, Simile MM, Bonelli P, Muroni MR, Seddaiu MA, Lim DS, Feo F, Pascale RM (2009) SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology 137(5):1816–1826 e1811-1810
Moser C, Lang SA, Kainz S, Gaumann A, Fichtner-Feigl S, Koehl GE, Schlitt HJ, Geissler EK, Stoeltzing O (2007) Blocking heat shock protein-90 inhibits the invasive properties and hepatic growth of human colon cancer cells and improves the efficacy of oxaliplatin in p53-deficient colon cancer tumors in vivo. Mol Cancer Ther 6(11):2868–2878
Milicevic Z, Bogojevic D, Mihailovic M, Petrovic M, Krivokapic Z (2008) Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int J Oncol 32(6):1169–1178
Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L (2004) Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther 3(5):551–566
Kaganovich D, Kopito R, Frydman J (2008) Misfolded proteins partition between two distinct quality control compartments. Nature 454(7208):1088–1095
Acknowledgments
JS is a fellow in the Howard Hughes Medical Institute Graduate Fellowship Program at UAB. Work in the authors’ laboratories was supported by grants from the Komen Breast Cancer Foundation grant (BCTR00-456, JVT), NCI Breast SPORE Developmental grant (JVT), and NIH grants ES016354 (BX) and CA133093 (BX).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khattar, V., Fried, J., Xu, B. et al. Cks1 proteasomal degradation is induced by inhibiting Hsp90-mediated chaperoning in cancer cells. Cancer Chemother Pharmacol 75, 411–420 (2015). https://doi.org/10.1007/s00280-014-2666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2666-7