Abstract
Betaine is a methyl derivative of glycine first isolated from sugar beets. Betaine consumed from food sources and through dietary supplements presents similar bioavailability and is metabolized to di-methylglycine and sarcosine in the liver. The ergogenic and clinical effects of betaine have been investigated with doses ranging from 500 to 9,000 mg/day. Some studies using animal models and human subjects suggest that betaine supplementation could promote adiposity reductions and/or lean mass gains. Moreover, previous investigations report positive effects of betaine on sports performance in both endurance- and resistance-type exercise, despite some conflicting results. The mechanisms underlying these effects are poorly understood, but could involve the stimulation of lipolysis and inhibition of lipogenesis via gene expression and subsequent activity of lipolytic-/lipogenic-related proteins, stimulation of autocrine/endocrine IGF-1 release and insulin receptor signaling pathways, stimulation of growth hormone secretion, increased creatine synthesis, increases in protein synthesis via intracellular hyper-hydration, as well as exerting psychological effects such as attenuating sensations of fatigue. However, the exact mechanisms behind betaine action and the long-term effects of supplementation on humans remain to be elucidated. This review aims to describe evidence for the use of betaine as an ergogenic and esthetic aid, and discuss the potential mechanisms underlying these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Betaine is a neutral zwitterionic compound, a methyl derivative of glycine, and is a naturally occurring byproduct of sugar beet refinement extracted from molasses (Craig 2004). In addition to sugar beets, betaine is high in other foods such as wheat bran (1,330 mg/100 g), wheat germ (1,241 mg/100 g), spinach (600 mg/100 g), beets (250 mg/100 g), and wheat bread (200 mg/100 g), although exact values vary highly with different sources and cooking methods (Zeisel et al. 2003). Betaine serves dual roles in human physiology: as a methyl donor in the transmethylation of homocysteine (Hcy) and as an osmolyte maintaining fluid balance. The ergogenic potential of betaine was first proposed by Borsook et al. (1952) after poliomyelitis patients supplemented with betaine–guanidinoacetate experienced improvements in general strength and endurance. Improvements in lactate metabolism and hydration reported in horses exercising to fatigue (Warren et al. 1999) and later human studies demonstrating improvements in anaerobic metabolism (Armstrong et al. 2008) have led to several studies investigating the ergogenic effects of betaine on strength and power. Although the emerging body of evidence demonstrates the potential, the application to specific performance tasks requires further examination. The use of betaine in animal feeds to improve lean meat yield in pigs and chickens (Eklund et al. 2005) has also led some researchers to investigate the potential of betaine to improve body composition in humans; however, data limited and the results are equivocal. Therefore, the present article aims to first review the effects of betaine on performance and body composition, and second to identify and discuss potential physiological mechanisms underlying such outcomes.
Ingestion and absorption
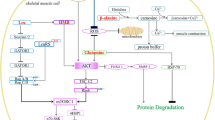
Average betaine intakes in adult humans are approximately 100–400 mg/day (Cho et al. 2006). When betaine is ingested by healthy subjects it is freely filtered in the kidney, reabsorbed into the circulation, and either catabolized via transmethylation or taken up and stored in tissues as an organic osmolyte (Lever et al. 2004). Betaine consumed from food sources and through dietary supplements has similar bioavailability, although supplemental betaine is absorbed approximately 33 % more rapidly (Lever and Slow 2010). Plasma betaine concentrations at rest are individualized, higher in men than in women, ranging from 20 to 70 mmol/l, and are mainly eliminated via metabolism versus excretion in healthy subjects (Lever et al. 1994; Lever and Slow 2010; Schwahn et al. 2003). Concentrations of plasma betaine increase in a dose-related manner, and reach new steady states within a few days following changes in dietary intake (Atkinson et al. 2009). Betaine excretion via urine is minimal and does not correlate with plasma concentrations (Lever et al. 1994); however, substantial betaine can be lost in sweat (Craig et al. 2010). Catabolism of betaine occurs in hepatic and renal mitochondria, and involves a series of reactions resulting in the transmethylation of Hcy to methionine (Met) via betaine-homocysteine S-methyltransferase (BHMT), and the subsequent generation of di-methylglycine (DMG: Williams and Schalinske 2007). By transmethylating Hcy betaine conserves Met for protein synthesis, detoxifies Hcy, and supplies the universal methyl donor S-adenylmethionine (SAM: Fig. 1). Betaine has been used to treat genetic homocystinuria (Lever and Slow 2010), and has been shown to attenuate a rise in plasma Hcy following a Met load (Lever et al. 2004). Reported values for decreases in plasma Hcy and increases in DMG demonstrate that while a small portion of supplemental betaine is metabolized to transmethylate Hcy most is absorbed by the tissues (Lever et al. 2004).
Metabolic pathways of betaine catabolism. Met metabolism begins with its adenylation, via S-adenosylmethionine synthase (reaction 2), to form S-adenosylmethionine (AdoMet), which donates its methyl group to substrates via numerous methyltransferases. Phosphatidylethanolamine N-methyltransferase (reaction 4) catalyzes the synthesis of PC from phosphatidylethanolamine (PE) and consumes 3 AdoMet molecules in the process. Guanidinoacetate (GAA), formed in the kidney via l-arginine:glycine N-methyltransferase (reaction 1), is transported to liver and methylated by guanidinoacetate methyltransferase (reaction 3) to form creatine. All methyltransferases produce adenosylhomocysteine (AdoHcy), which is cleaved by S-adenosylhomocysteine hydrolase (reaction 5) to produce adenosine and homocysteine (Hcy). Hcy remethylation to methionine occurs via methionine synthase (reaction 7) with the use of 5-methyltetrahydrofolate [synthesized by methylenetetrahydrofolate reductase (reaction 6)] as the methyl donor or via betaine-homocysteine methyltransferase (reaction 8), which uses betaine as the methyl donor. Hcy may also be exported to the extracellular space. Orn ornithine, Arg arginine, Gly glycine, Ser serine, THF tetrahydrofolate, DAG diacylglycerol, DMG di-methylglycine. Adapted from: Stead et al. (2006) with permission
The ergogenic and clinical effects of betaine have been investigated with doses ranging from 500 to 20,000 mg/day (Atkinson et al. 2008; Abdelmalek et al. 2009; Bloomer et al. 2011; Craig 2004; Schwab et al. 2002). Several researchers have reported that 2.5–5 g/day is sufficient to increase plasma betaine values (Bloomer et al. 2011; Schwab et al. 2002) and 2.5 g has been shown to positively affect performance (Armstrong et al. 2008; Apicella et al. 2012; Cholewa et al. 2013; Hoffman et al. 2009; Lee et al. 2010; Pryor et al. 2012). Based on rat hematology results a supplemental betaine to dietary protein ratio as high as .15:1 (~12 g/d betaine in humans) may be projected as a safe range for human intake (Hayes et al. 2003).
Performance
Aerobic endurance exercise
Research investigating the effects of betaine on endurance-based performance is limited. Betaine may improve the NAD+:NADH ratio by accepting nucleophilic H+, yielding DMG, CH4, and the oxidation of NADH to NAD+, thereby attenuating cellular acidosis and improving glycolytic metabolism (Ghyczy and Boros 2001). Armstrong et al. (2008) investigated the effects of acute betaine supplementation on running performance during mild dehydration in a hot environment in competitive distance runners. Subjects were actively dehydrated by 2.7 % body mass then given 1,000-ml rehydration fluid (with or without 5 g/day betaine) 45 min prior to running a performance trial consisting of 75 min of treadmill running at 65 % VO2PEAK followed by a run to exhaustion at 84 % VO2PEAK. Betaine treatment increased plasma betaine; however, there were no differences in heart rate, body or skin temperature, sweat rate, or body mass loss. Betaine increased VO2 consumption and plasma lactate during the final sprint, and although the difference in sprint time to exhaustion did not reach statistical significance, a trend for an increase in sprint duration was observed. While betaine increased plasma lactate it is indeterminable whether this was due to an increase in glycolysis or enhanced lactate clearance. Thus, the osmolytic effects of betaine may have optimized the cellular state by increasing biopolymer hydration and cytoplasmic osmolarity, ultimately leading to enhanced glycolytic flux.
The increased VO2 consumption during the final sprint in the betaine group may have been attributed to increased protein stability and muscle oxygen consumption. Trepanowski et al. (2011) demonstrated 14 days of betaine ingestion resulted in greater post-exercise muscle tissue oxygen saturations compared to placebo. Betaine has been shown to defend citrate synthase against thermodenaturation, and thereby may have enhanced Kreb’s cycle efficiency (Caldas et al. 1999). Therefore, the osmoprotectant properties of betaine may enhance high-intensity fatiguing aerobic performance in thermally challenging environments by extending glycolytic flux and improving tissue oxygen consumption; however, more research is needed to evaluate this hypothesis.
Strength-based exercise
Betaine has been suggested to enhance strength-based performance (Craig 2004); however, investigations into the effects of betaine are limited and have reported conflicting results. Hoffman et al. (2009) demonstrated improved squat repetitions to fatigue, but not bench press throw or vertical jump with 15 days of 2.5 g/day betaine supplementation. In follow-up study Hoffman et al. (2011) reported no improvements in isokinetic peak force output, mean force output, and change in force output following an identical supplementation protocol. In contrast, Lee et al. (2010) demonstrated improvements in vertical jump power output, bench press throw power output, and force production in the isometric back squat and bench press with 12 days of betaine supplementation. Our lab (Cholewa et al. 2013) demonstrated a trend for improved vertical jump with 6 weeks of betaine supplementation, and Pryor et al. (2012) reported 7 days of betaine supplementation resulted in increases in mean and peak power during four 12-s resisted cycle sprints. Unlike Hoffman et al. (2011), the subjects in Cholewa et al. (2013) and Lee et al. (2010) were assigned standardized resistance training between testing sessions. Detections in power improvements are compromised when power movements are not a regular part of training (Hoffman et al. 2006); therefore, it is possible that the lack of robust improvements in muscular power may in part be attributed to the absence of power-specific training. These studies, however, provide evidence that betaine supplementation may improve performance during high-intensity resistance exercise protocols.
On the other hand, Del Favero et al. (2011) reported no improvements in power output, strength, or body composition with betaine treatment. The authors suggested that differences in fitness status (inactive vs. recreationally trained), dose (2 vs. 2.5 g), and duration (10 vs. 15 days) may have been a factor in the outcome. This differs from previous reports (Apicella et al. 2012; Hoffman et al. 2009) where increases in muscular strength were observed following 14 days of betaine treatment. The sequence of testing in Del Favero et al. (2011) may have also influenced the results. Strength and power testing was performed 3 and 6 days, respectively, following the cessation of betaine treatment in comparison to other studies (Apicella et al. 2012; Cholewa et al. 2013; Hoffman et al. 2009; Lee et al. 2010) where performance was tested on the final day of supplementation and betaine was found to be ergogenic; however, further research is needed to evaluate the sustainability of adaptations following cessation of betaine treatment.
The effects of betaine on resistance-based work capacity are also conflicting. Betaine did not improve single-set repetitions to fatigue at 75 % (Hoffman et al. 2009) or 3 sets of repetitions to fatigue at 85 % 1 RM (Lee et al. 2010); however, 14 days of betaine supplementation increased repetitions and volume during 10 sets of bench press to fatigue at 50 % 1 RM (Trepanowski et al. 2011). Our group demonstrated that betaine supplementation improved bench press work capacity during cycles of high (2 weeks, each of 3 sets of 8–10 and 12–15 repetitions, respectively), but not low (4 sets of 4–6 repetitions) volume training (Cholewa et al. 2013). The discrepancy in findings may have been due to the sets and intensity prescribed: betaine improved work capacity when multiple sets to fatigue with <75 % 1 RM were prescribed (Cholewa et al. 2013; Trepanowski et al. 2011), whereas no improvements were seen with 1 and 3 sets to fatigue at 75 and 85 % 1 RM, respectively (Hoffman et al. 2009; Lee et al. 2010). Betaine may, therefore, pose the most ergogenic potential in exercise protocols generating high levels of metabolic stress, such as those employing shorter rest periods and higher volumes.
In contrast to the improvements in upper body work capacity, we observed that betaine did not improve work capacity in free-weight back squats when compared to placebo (Cholewa et al. 2013). These lack of differences in back squat volume were similar to other studies (Hoffman et al. 2009, 2011) whereby no differences in repetitions to fatigue with three sets of 85 % of 1 RM, and no improvements in mean or peak isokinetic power during 5 sets of 6 repetitions at 80 % peak force were reported. Increases in upper body muscle mass and strength during a 12-week training protocol have been shown to occur at a greater magnitude and earlier than do the lower extremities (Abe et al. 2000). Thus, it is possible that improvements in lower body muscular work capacity may have occurred with longer study durations.
The mechanisms of action whereby betaine improves performance have also yet to be identified. Due to the increases in power and force production during short duration exercises requiring high rates of phosphagen metabolism and because dietary betaine supplementation increases serum SAM availability in healthy men (Craig 2004), several researchers have suggested betaine may improve performance via enhanced creatine synthesis (Fig. 1, Hoffman et al. 2009, 2011; Lee et al. 2010; Trepanowski et al. 2011). Betaine supplementation increases muscle PCr in animals (Wise et al. 1997); however, 10 days of 2 g/day betaine supplementation did not enhance muscle PCr content in humans (Del Favero et al. 2011). The subjects in Del Favero et al. were untrained and instructed not to exercise, and it was likely that muscle PCr metabolism and, therefore, the demand for additional SAM to synthesize creatine were minimal. It is therefore possible that the results reported by Del Favero et al. may have been different had the subjects been prescribed an exercise program relying on the phosphagen energy system, thereby increasing creatine synthesis and ultimately resulting in greater muscle PCr content.
The osmolytic effects of betaine may also be ergogenic as an optimally hydrated cellular state can support enhanced glycolytic flux during high-volume resistance training. Metabolic stress results in the cellular accumulation of inorganic ions and organic osmolytes including betaine (Häussinger 1996). Betaine accumulation in cells results in increased cytoplasmic osmolality, redistribution of cellular water, biopolymer hydration, and has been shown to help maintain biochemical function during stressful conditions (Brigotti et al. 2003). Ion influx is limited, however, due to the destabilization of protein structure, enzyme function, and polarization (Petronini et al. 1992). In contrast, betaine does not affect protein structure or compromise enzymatic function, and can stabilize cellular metabolic function under osmotic stress (Dragolovich 1994). Betaine has also been labeled a “counteracting” solute for its role in enhancing protein stability and countering the denaturing effect of urea (Gilles 1997). Betaine has been shown to protect myosin ATPase and myosin heavy-chain proteins against denaturation by urea (Ortiz-Costa et al. 2008) and attenuate the reduction in the affinity of troponin for Ca2+ by defending against muscle protein dehydration (Suarez et al. 2003). Therefore, betaine may be ergogenic, in part, by facilitating a hospitable environment for excitation contraction coupling in the face of increasing osmotic stress. Because betaine defends citrate synthase against thermodenaturation (Caldas et al. 1999) betaine may have also increased work capacity by improving recovery between sets via enhanced aerobic energy production.
Another possible explanation for the enhanced performance with betaine supplementation may have been psychological. Armstrong et al. (2008) reported that betaine reduced thirst and thermal sensations during the final sprint, and Hoffman et al. (2011) reported that betaine supplementation reduced perceptions of fatigue during high-volume upper body exercise. Sensations of thirst, perceived exertion, and thermal conditions have all been shown to impact performance by influencing motivation (Knicker et al. 2011). Phosphatidylcholine (PDC) supplementation has been shown to improve reaction time and focus the following intense exercise (Hoffman et al. 2010). Because betaine donates SAM in the synthesis of PDC (Fig. 1) (Stead et al. 2006), betaine may has also been suggested to reduce perceptions of fatigue via an elevation in free choline and a subsequent increase in motor neuron acetylcholine synthesis.
Body composition
The effects of betaine supplementation on body composition and hypertrophy in humans are limited. Schwab et al. (2002) investigated the effect of betaine supplementation on body composition and resting energy expenditure in obese, sedentary subjects free of liver, kidney, or thyroid abnormalities. Subjects were provided with a nutrition program yielding a 500 kcal/day deficit and supplemented with 6 g/day betaine for 12 weeks. Betaine did not improve caloric restriction-induced decreases in diastolic blood pressure, waist circumference, or fat free mass in obese, sedentary subjects. Del Favero et al. (2011) supplemented untrained young men with 2 g/day betaine, employed food logs to maintain energy intake, and instructed subjects not to exercise. Ten days of betaine supplementation did not improve body composition.
Betaine is a common additive in animal feed and has been shown to increase lean mass and decrease fat mass in pigs (Fernández-Fígares et al. 2002; Huang et al. 2007, 2008; Rojas-Cano et al. 2011; Wray-Cahen et al. 2004) and chickens (Xing et al. 2011; Zhan et al. 2006). Betaine has been shown to exert the most influential effects on muscle growth under conditions of metabolic or nutritional stress (Fernández-Fígares et al. 2002). Given that the subjects in Schwab et al. (2002) and Del Favero et al. (2011) were restricted from exercising, we hypothesized that betaine may improve body composition when combined with resistance training. Experienced resistance-trained men were divided into two groups, prescribed a progressive resistance training program, and supplemented with 2.5 g/day betaine for 6 weeks. Compared with placebo, we demonstrated for the first time in humans that betaine supplementation can significantly improve body composition by both increasing lean mass and reducing fat mass (Cholewa et al. 2013). In addition, we observed an increase in arm lean cross-sectional area that corresponded to improved upper body work capacity in the betaine group. The mechanisms of action whereby betaine improves body composition require further investigation. The following is an overview of proposed mechanisms that may have led to increases in lean mass and/or decreases in fat mass.
Lean body mass
Homocysteine thiolactone (HCTL), a toxic metabolite of excess homocysteine, is an inhibitor of the insulin receptor substrate (IRS) leading to reduced Akt phosphorylation (Li et al. 2008). Ultimately, HCTL inhibits insulin/IGF-1-mediated mRNA expression and protein synthesis (Najib and Sánchez-Margalet 2005) and has also been shown to denature and inactivate the enzymes involved in protein synthesis (Jakubowski 2006). Given that betaine reduces Hcy we hypothesized that betaine supplementation may increase muscle mass by reducing HCTL. Although betaine attenuated a dietary rise in urinary HCTL, the pre-, intra-, and post-study HCTL values in both groups were not high enough to have negatively affected protein synthesis (Cholewa et al. 2013).
The accumulation of betaine in cells during conditions of metabolic and ionic stress (Courtenay et al. 2000) resulting in increased sarcoplasmic osmolality may also have contributed to increases in muscle mass. The osmoregulated betaine transporter (BTG-1) is found in liver and skeletal muscle, and readily takes up betaine to maintain higher tissue to plasma betaine concentrations thereby promoting cellular hyper-hydration (Slow et al. 2009). In hepatocytes increases in cell volume are related to increased protein synthesis and hypertrophy, whereas a decrease in cell volume signals protein degradation (Stoll et al. 1992). Häussinger et al. (1993) suggested a similar mechanism may contribute to skeletal muscle protein synthesis. Cellular swelling is sensed by sarcolemma integrins coupled to g-proteins and transduced downstream to initiate a series of cascades that result in protein synthesis, gene transcription, and proteolysis inhibition via mitogen-activated protein kinase activation (Häussinger 1996). In addition, cellular swelling also augments protein synthesis via enhanced amino acid uptake (Low et al. 1997). Although it is well established that betaine induces cellular swelling in hepatocytes (Hoffmann et al. 2013), the degree to which betaine induces cellular swelling in skeletal muscle and its influences on protein synthesis in the myofiber requires further investigation.
Betaine ingestion may influence body composition by stimulating growth hormone (GH) secretion and improving insulin and IGF-1 receptor signaling (Fig. 2). In pigs betaine ingestion increases betaine deposition in the hypothalamus. Betaine has been shown to promote growth hormone releasing hormone (GHRH) gene transcription and GHRH secretion by the hypothalamus, and ultimately results in increased plasma GH (Yan 2001). In humans Apicella et al. (2012) reported that 2.5 g/day betaine ingestion for 14 days resulted in an increase in plasma GH and IGF-1 and a decrease in exercise-induced cortisol concentrations. Because cortisol inhibits GH release by reducing the stimulator effects of GHRH (Thompson et al. 1995) and increasing somatostatin (Barbarino et al. 1990), the reduction in cortisol during and post-exercise may have also contributed to greater exercise-induced GH release. In addition, betaine has been shown to directly enhance hepatocyte IGF-1 secretion (Lee et al. 2006).
In conjunction with elevated growth factors, betaine supplementation has been shown to increase resting and post-exercise Akt phosphorylation, while attenuating the decline in P70 S6K pre- and post-exercise in humans (Apicella et al. 2012). Betaine may have improved IGF-1 signaling at the IRS via increased availability of methyl groups. Protein N-arginine methyltransferase 1 (PRMT1) catalyzes arginine methylation and has been implicated in insulin signaling in skeletal muscle cells (Iwasaki 2008). Insulin-mediated tyrosine phosphorylation and internalization of the insulin receptor are achieved when PRMT1 methylates heterogenous nuclear ribonucleoprotein, and have been shown to positively modulate the Akt-mTOR pathway of protein synthesis (Iwasaki and Yada 2007). By regenerating SAM via Hcy transmethylation, betaine may increase the availability of SAM groups for PRMT1, thus increasing IGF-1 sensitivity. Betaine has also shown to directly agonize IGF-1 receptors in vitro resulting in increased myosin heavy-chain protein synthesis and myoblast proliferation and differentiation (Senesi et al. 2013). Given that GH directly signals adipocyte lipolysis (Dietz and Schwartz 1991), betaine induced GH release and the subsequent enhancements in IGF-1 mediated protein synthesis may have also contributed to the improvements in body composition reported by Cholewa et al. (2013).
Adiposity
The results from numerous animal studies allude to additional mechanisms underlying the reductions in fat mass observed with betaine supplementation. Betaine provides a methyl group to form trimethyl-lysine in the biosynthesis of carnitine (Borum and Broquist 1977). Many researchers have reported elevated muscle carnitine following betaine supplementation (Zhan et al. 2006; Feng and Xu 2001; Wang et al. 2000). This increase in intramuscular carnitine would suggest that betaine may increase carnitine palmitoyltransferase I-mediated FFA translocation into the mitochondria and β-oxidation; however, reductions in muscle-type carnitine palmitoyltransferase I gene expression and activity have been reported with betaine supplementation in pigs (Huang et al. 2009). More research is therefore needed to clarify these responses in humans.
In addition to its lipolytic effects on adipose tissue, betaine may positively influence body composition by reducing TAG synthesis. Lipoprotein lipase (LPL) has been identified as the rate limiting enzyme in chylomicron and VLDL catabolism, and provides adipose tissue with FFA for storage (Bonen et al. 2006). In humans, a relationship exists between CpG methylation, LPL gene expression, and adipose cell differentiation (Noer et al. 2006). In broiler chickens 66 days of dietary betaine supplementation decreased mRNA expression of adipose fatty acid binding protein (A-FABP), LPL, and fatty acid synthase (FAS: Xing et al. 2011). In addition, betaine decreased the percent and change in pattern of CpG promoter methylation. This suggests betaine may improve body composition by reducing lipogenesis.
A-FABP protein transports long-chain fatty acids into the adipocyte and plays a role in adipose homeostasis. Acetyl-CoA carboxylase (ACC) produces malonyl-CoA, a critical substrate in TAG synthesis and inhibitor of lipolysis, while FAS catalyzes the final step in the lipogenic reaction (Chmurzyńska 2006). ACC is also a major regulator of fatty acid synthesis, and is the rate limiting enzyme of lipogenesis (Scott et al. 1981). Huang et al. (2008) supplemented finishing pigs feed with 1.25 g/kg betaine (yielding approximately 3.1 g/day betaine intake) for 42 days. Measurements were taken of lipogenic enzymes and FAS complex activity, as well as the mRNA expression of both enzyme systems in subcutaneous adipose tissue. Betaine supplementation significantly reduced ACC and FAS activity, and decreased FAS mRNA expression. Likewise, old broilers supplemented with betaine also presented a decreased FAS gene expression (Xing et al. 2011). Therefore, betaine supplementation may improve body composition by decreasing the ability of adipose cells to extract, uptake, and synthesize FFA into TAG; however, this hypothesis has yet to be tested in humans and requires further investigation.
Conclusion and future research
The clinical use of betaine to treat conditions related to homocysteinemia and nonalcoholic fatty liver disease is well documented in the literature (Craig 2004; Abdelmalek et al. 2009); however, less understood are the mechanisms whereby betaine influences performance and body composition. While several investigations provide evidence that 2.5 g/day of sub-chronic betaine supplementation may improve metabolically demanding strength-based performance, the effects of betaine supplementation on power-based tasks are equivocal. Given that the majority of improvements reported were in studies employing a standardized exercise protocol, future research is required to investigate the interaction between betaine supplementation and varying training protocols on performance and body composition. To the authors knowledge the studies performed to date have all utilized 2–5 g/day of betaine supplementation; therefore, additional research is required to evaluate if there is a dose–response effect. Finally, there is potential for betaine supplementation to enhance the effects of resistance training in the treatment of sarcopenia and age related functional decline; however, more research is first required to evaluate the effects of betaine supplementation on force output, body composition, and GH secretion in older adults.
References
Abdelmalek MF, Sanderson SO, Angulo P et al (2009) Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 50:1818–1826. doi:10.1002/hep.23239
Abe T, DeHoyos DV, Pollock ML, Garzarella L (2000) Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81:174–180. doi:10.1007/s004210050027
Apicella JM, Lee EC, Bailey BL et al (2012) Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol. doi:10.1007/s00421-012-2492-8
Armstrong LE, Casa DJ, Roti MW et al (2008) Influence of betaine consumption on strenuous running and sprinting in a hot environment. J Strength Cond Res 22:851
Atkinson W, Elmslie J, Lever M et al (2008) Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am J Clin Nutr 87:577–585
Atkinson W, Slow S, Elmslie J et al (2009) Dietary and supplementary betaine: effects on betaine and homocysteine concentrations in males. Nutr Metab Cardiovasc Dis 19:767–773. doi:10.1016/j.numecd.2009.01.004
Barbarino A, Corsello SM, Della Casa S et al (1990) Corticotropin-releasing hormone inhibition of growth hormone-releasing hormone-induced growth hormone release in man. J Clin Endocrinol Metab 71:1368–1374
Bloomer RJ, Farney TM, Trepanowski JF et al (2011) Effect of betaine supplementation on plasma nitrate/nitrite in exercise-trained men. J Int Soc Sports Nutr 8:1–7. doi:10.1186/1550-2783-8-5
Bonen A, Tandon NN, Glatz JF et al (2006) The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes 30:877–883. doi:10.1038/sj.ijo.0803212
Borsook ME, Billig HK, Golseth JG (1952) Betaine and glycocyamine in the treatment of disability resulting from acute anterior poliomyelitis. Ann West Med Surg 6:423–427
Borum PR, Broquist HP (1977) Purification of S-adenosylmethionine: epsilon-N-l-lysine methyltransferase. The first enzyme in carnitine biosynthesis. J Biol Chem 252:5651–5655
Brigotti M, Petronini PG, Carnicelli D et al (2003) Effects of osmolarity, ions and compatible osmolytes on cell-free protein synthesis. Biochem J 369:369–374. doi:10.1042/BJ20021056
Caldas T, Demont-Caulet N, Ghazi A, Richarme G (1999) Thermoprotection by glycine betaine and choline. Microbiology 145(Pt 9):2543–2548
Chmurzyńska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47:39–48. doi:10.1007/BF03194597
Cho E, Zeisel SH, Jacques P et al (2006) Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr 83:905–911
Cholewa JM, Wyszczelska-Rokiel M, Glowacki R et al (2013) Effects of betaine on body composition, performance, and homocysteine thiolactone. J Int Soc Sports Nutr 10:39. doi:10.1186/1550-2783-10-39
Courtenay ES, Capp MW, Anderson CF, Record MT (2000) Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 39:4455–4471
Craig SAS (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549
Craig SS, Craig SA, Ganio MS et al (2010) The betaine content of sweat from adolescent females. J Int Soc Sports Nutr 7:3. doi:10.1186/1550-2783-7-3
Del Favero S, Roschel H, Artioli G et al (2011) Creatine but not betaine supplementation increases muscle phosphorylcreatine content and strength performance. Amino Acids 42:2299–2305. doi:10.1007/s00726-011-0972-5
Dietz J, Schwartz J (1991) Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism 40:800–806
Dragolovich J (1994) Dealing with salt stress in animal cells: the role and regulation of glycine betaine concentrations. J Exp Zool 268:139–144. doi:10.1002/jez.1402680211
Eklund M, Bauer E, Wamatu J, Mosenthin R (2005) Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev 18:31–48. doi:10.1079/NRR200493
Feng J, Xu ZR (2001) Effect of betaine on muscle, liver and serum amino acid composition in finishing swine. J Zhejiang Univ Agric Life Sci 27:107–110
Fernández-Fígares I, Wray-Cahen D, Steele NC et al (2002) Effect of dietary betaine on nutrient utilization and partitioning in the young growing feed-restricted pig. J Anim Sci 80:421–428
Ghyczy M, Boros M (2001) Electrophilic methyl groups present in the diet ameliorate pathological states induced by reductive and oxidative stress: a hypothesis. Br J Nutr 85:409–414
Gilles R (1997) “Compensatory” organic osmolytes in high osmolarity and dehydration stresses: history and perspectives. Comp Biochem Physiol 117:279–290
Häussinger D (1996) The role of cellular hydration in the regulation of cell function. Biochem J 313(Pt 3):697–710
Häussinger D, Roth E, Lang F, Gerok W (1993) Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet 341:1330–1332
Hayes KC, Pronczuk A, Cook MW, Robbins MC (2003) Betaine in sub-acute and sub-chronic rat studies. Food Chem Toxicol 41:1685–1700. doi:10.1016/S0278-6915(03)00196-0
Hoffman J, Ratamess N, Kang J et al (2006) Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int J Sport Nutr Exerc Metab 16:430–446
Hoffman JR, Ratamess NA, Kang J et al (2009) Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr 6:7. doi:10.1186/1550-2783-6-7
Hoffman JR, Ratamess NA, Gonzalez A et al (2010) The effects of acute and prolonged CRAM supplementation on reaction time and subjective measures of focus and alertness in healthy college students. J Int Soc Sports Nutr 7:39. doi:10.1186/1550-2783-7-39
Hoffman JR, Ratamess NA, Kang J et al (2011) Effect of 15 days of betaine ingestion on concentric and eccentric force outputs during isokinetic exercise. J Strength Cond Res 25:2235–2241. doi:10.1519/JSC.0b013e3182162530
Hoffmann L, Brauers G, Gehrmann T et al (2013) Osmotic regulation of hepatic betaine metabolism. Am J Physiol Gastrointest Liver Physiol 304:G835–G846. doi:10.1152/ajpgi.00332.2012
Huang QC, Xu ZR, Han XY, Li WF (2007) Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs. J Anim Physiol Anim Nutr (Berl) 91:85–90. doi:10.1111/j.1439-0396.2006.00644.x
Huang Q, Xu Z, Han X, Li W (2008) Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mRNA expression in finishing pigs. Anim Feed Sci Technol 140:365–375. doi:10.1016/j.anifeedsci.2007.03.007
Huang Q-C, Han X-Y, Xu Z-R et al (2009) Betaine suppresses carnitine palmitoyltransferase I in skeletal muscle but not in liver of finishing pigs. Livest Sci 126:130–135. doi:10.1016/j.livsci.2009.06.015
Iwasaki H (2008) Involvement of PRMT1 in hnRNPQ activation and internalization of insulin receptor. Biochem Biophys Res Commun 372:314–319. doi:10.1016/j.bbrc.2008.05.051
Iwasaki H, Yada T (2007) Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem Biophys Res Commun 364:1015–1021. doi:10.1016/j.bbrc.2007.10.113
Jakubowski H (2006) Pathophysiological consequences of homocysteine excess. J Nutr 136:1741–1749
Knicker AJ, Renshaw I, Oldham ARH, Cairns SP (2011) Interactive processes link the multiple symptoms of fatigue in sport competition. Sport Med 41:307–328. doi:10.2165/11586070-000000000-00000
Lee MS, Kim M-S, Park SY, Kang C-W (2006) Effects of betaine on ethanol-stimulated secretion of IGF-I and IGFBP-1 in rat primary hepatocytes: involvement of p42/44 MAPK activation. World J Gastroenterol 12:1718–1722
Lee EC, Maresh CM, Kraemer WJ et al (2010) Ergogenic effects of betaine supplementation on strength and power performance. J Int Soc Sports Nutr 7:27. doi:10.1186/1550-2783-7-27
Lever M, Slow S (2010) The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43:732–744. doi:10.1016/j.clinbiochem.2010.03.009
Lever M, Sizeland PC, Bason LM et al (1994) Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta 1200:259–264
Lever M, Sizeland PC, Frampton CM, Chambers ST (2004) Short and long-term variation of plasma glycine betaine concentrations in humans. Clin Biochem 37:184–190. doi:10.1016/j.clinbiochem.2003.11.004
Li Y, Jiang C, Xu G et al (2008) Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57:817–827. doi:10.2337/db07-0617
Low SY, Rennie MJ, Taylor PM (1997) Signaling elements involved in amino acid transport responses to altered muscle cell volume. FASEB J 11:1111–1117
Najib S, Sánchez-Margalet V (2005) Homocysteine thiolactone inhibits insulin-stimulated DNA and protein synthesis: possible role of mitogen-activated protein kinase (MAPK), glycogen synthase kinase-3 (GSK-3) and p70 S6K phosphorylation. J Mol Endocrinol 34:119–126. doi:10.1677/jme.1.01581
Noer A, Sørensen AL, Boquest AC, Collas P (2006) Stable CpG hypomethylation of adipogenic promoters in freshly isolated, cultured, and differentiated mesenchymal stem cells from adipose tissue. Mol Biol Cell 17:3543–3556. doi:10.1091/mbc.E06
Ortiz-Costa S, Sorenson MM, Sola-Penna M (2008) Betaine protects urea-induced denaturation of myosin subfragment-1. FEBS J 275:3388–3396. doi:10.1111/j.1742-4658.2008.06487.x
Petronini PG, De Angelis EM, Borghetti P et al (1992) Modulation by betaine of cellular responses to osmotic stress. Biochem J 282:69–73
Pryor JL, Craig SA, Swensen T (2012) Effect of betaine supplementation on cycling sprint performance. J Int Soc Sports Nutr 9:12. doi:10.1186/1550-2783-9-12
Rojas-Cano ML, Lara L, Lachica M et al (2011) Influence of betaine and conjugated linoleic acid on development of carcass cuts of Iberian pigs growing from 20 to 50 kg body weight. Meat Sci 88:525–530. doi:10.1016/j.meatsci.2011.02.004
Schwab U, Törrönen A, Toppinen L et al (2002) Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr 76:961–967
Schwahn BC, Hafner D, Hohlfeld T et al (2003) Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br J Clin Pharmacol 55:6–13
Scott RA, Cornelius SG, Mersmann HJ (1981) Effects of age on lipogenesis and lipolysis in lean and obese swine. J Anim Sci 52:505–511
Senesi P, Luzi L, Montesano A et al (2013) Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J Transl Med 11:174. doi:10.1186/1479-5876-11-174
Slow S, Lever M, Chambers ST, George PM (2009) Plasma dependent and independent accumulation of betaine in male and female rat tissues. Acad Sci Bohemoslov 58:403–410
Stead LM, Brosnan JT, Brosnan ME et al (2006) Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 83:5–10
Stoll B, Gerok W, Lang F, Häussinger D (1992) Liver cell volume and protein synthesis. Biochem J 287(Pt 1):217–222
Suarez MC, Machado CJV, Lima LMTR et al (2003) Role of hydration in the closed-to-open transition involved in Ca2+ binding by troponin C. Biochemistry 42:5522–5530. doi:10.1021/bi027102h
Thompson K, Coleman ES, Hudmon A et al (1995) Effects of short-term cortisol infusion on growth hormone-releasing hormone stimulation of growth hormone release in sheep. Am J Vet Res 56:1228–1231
Trepanowski JF, Farney TM, McCarthy CG, et al (2011) The effects of chronic betaine supplementation on exercise performance, skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J Strength Cond Res 1–11. doi:10.1519/JSC.0b013e318217d48d
Wang Y, Xu Z, Feng J (2000) Study on the effect of betaine on meat quality and the mechanism in finishing pigs. Sci Agric Sin 33:94–99
Warren LK, Lawrence LM, Thompson KN (1999) The influence of betaine on untrained and trained horses exercising to fatigue. J Anim Sci 77:677–684
Williams KT, Schalinske KL (2007) New insights into the regulation of methyl group and homocysteine metabolism. J Nutr 137:311–314
Wise CK, Cooney CA, Ali SF, Poirier LA (1997) Measuring S-adenosylmethionine in whole blood, red blood cells and cultured cells using a fast preparation method and high-performance liquid chromatography. J Chromatogr 696:145–152
Wray-Cahen D, Fernández-Fígares I, Virtanen E et al (2004) Betaine improves growth, but does not induce whole body or hepatic palmitate oxidation in swine (Sus scrofa domestica). Comp Biochem Physiol 137:131–140. doi:10.1016/j.cbpb.2003.09.015
Xing J, Kang L, Jiang Y (2011) Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers. Mol Biol Rep 38:1975–1981. doi:10.1007/s11033-010-0319-4
Yan XC (2001) Effects of betaine on growth hormone releasing factor (GRF) and approach to the mechanism in the hypothalamus of finishing pig. Zhejiang University, Hangzhou
Zeisel SH, Mar M-H, Howe JC, Holden JM (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307
Zhan XA, Li JX, Xu ZR, Zhao RQ (2006) Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br Poult Sci 47:576–580. doi:10.1080/00071660600963438
Acknowledgments
The authors thank Stuart A.S. Craig for his help refining the manuscript.
Conflict of interest
No funding was obtained for the preparation of this manuscript. The authors declare no conflicts of interest relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cholewa, J.M., Guimarães-Ferreira, L. & Zanchi, N.E. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids 46, 1785–1793 (2014). https://doi.org/10.1007/s00726-014-1748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1748-5