Abstract
Neuroinflammation is closely associated with the pathophysiology of neurodegenerative diseases including Parkinson’s disease (PD). Recent evidence indicates that astrocytes also play pro-inflammatory roles in the central nervous system (CNS) by activation with toll-like receptor (TLR) ligands. Therefore, targeting anti-inflammation may provide a promising therapeutic strategy for PD. Curcumin, a polyphenolic compound isolated from Curcuma longa root, has been commonly used for the treatment of neurodegenerative diseases. However, the details of how curcumin exerts neuroprotection remain uncertain. Here, we investigated the protective effect of curcumin on 1-methyl-4-phenylpyridinium ion-(MPP+-) stimulated primary astrocytes. Our results showed that MPP+ stimulation resulted in significant production of tumor necrosis factor (TNF)-α, interleukin (IL-6), and reactive oxygen species (ROS) in primary mesencephalic astrocytes. Curcumin pretreatment decreased the levels of these pro-inflammatory cytokines while increased IL-10 expression in MPP+-stimulated astrocytes. In addition, curcumin increased the levels of antioxidant glutathione (GSH) and reduced ROS production. Our results further showed that curcumin decreased the levels of TLR4 and its downstream effectors including NF-κB, IRF3, MyD88, and TIRF that are induced by MPP+ as well as inhibited the immunoreactivity of TLR4 and morphological activation in MPP+-stimulated astrocytes. Together, data suggest that curcumin might exert a beneficial effect on neuroinflammation in the pathophysiology of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases among elderly people (Phani et al. 2012). The pathological symptoms of PD are characterized by akinesia, rest tremor, and loss of the dopaminergic neurons (DNs) in the nigra striatal (Dauer and Przedborski 2003; Noelker et al. 2013). Although the mechanisms underlying this disease remain unclear, there have been numerous studies suggesting that neuroinflammation plays a prominent role in the pathogenesis of PD (More et al. 2013; Tansey and Goldberg 2010). Neuroinflammation is triggered by activation of glial cells. The activated cells produce a variety of inflammatory mediators such as inflammatory cytokines, which in turn act on these cells and result in further expression of inflammatory cytokines (Hirsch and Hunot 2009).
Astrocytes are the most abundant glial cell type in the brain, and they exert dual roles in regulating brain immune reactivity (Farina et al. 2007; Parpura et al. 2012). On the one hand, astrocytes possess a variety of physiological roles in brain functions, such as nutritional support for neurons and tissue repair (Rossi 2015). On the other hand, overactive astrocytes also play a pro-inflammatory role in the central nervous system (CNS) through production of a number of inflammatory mediators including cytokines, chemokines, and reactive oxygen intermediates (Farina et al. 2007; Hirsch and Hunot 2009).
Toll-like receptors (TLRs) are a class of pattern recognition receptors (PRRs) that interact with exogenous conserved components of pathogens or endogenous “danger signals” and further trigger intracellular signals (Akira et al. 2001; Tang et al. 2007; Zhang et al. 2013). In brain, TLRs are primarily present in immune cells like microglia and astrocytes (Jack et al. 2005). Astrocytes carry a series of TLRs, including TLR2, TLR3, TLR4, and TLR9 (Bowman et al. 2003; Carpentier et al. 2005). Increasing evidence indicates that TLRs play important roles in the pathologies of PD. For example, Noelker et al. showed that mice with TLR4 deficiency were less vulnerable to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication (Noelker et al. 2013). Additionally, Ma et al. showed that activation of astrocytes via TLRs exhibited neurotoxic effect to neurons in vitro (Ma et al. 2013). Therefore, we speculated that astrocytic TLRs might be involved in the neuroinflammatory conditions in PD, and proper regulation of astroglial-related inflammation may offer a potential approach for PD therapy.
Curcumin, a bioactive component primary extracted from Curcuma longa rhizome, is shown to exert multiple pharmacological properties, such as anti-inflammatory (Jurenka 2009), anti-oxidant (Ak and Gulcin 2008), and anti-tumor actions (Lin et al. 2007). Recently, numerous studies have shown the therapeutic potential of curcumin in neurodegenerative and neuroinflammatory diseases (Lee et al. 2013; Mansouri et al. 2012; Ray and Lahiri 2009). For example, Zhu et al. (2014) showed that curcumin attenuated acute inflammation in an experimental traumatic brain injury model. Kaur et al. (2015) demonstrated that curcumin attenuated cognitive deficits in an experimental chronic epilepsy model via inhibition of glial activation and inflammatory mediator production. Seyedzadeh et al. (2014) further revealed that curcumin exerted anti-inflammatory actions on reactive astrocytes in vitro, suggesting the potential regulatory role of curcumin in astroglial-related inflammation. Based on these findings, we now investigate the effects of curcumin on 1-methyl-4-phenylpyridinium ion (MPP+)-stimulated primary mesencephalic astrocytes and depict the role of TLR4 in the inflammatory process.
Materials and methods
Cell culture
Primary astrocytes were isolated from the brain tissues of 1–3-day-old rat pups as previously described (Ma et al. 2013). Briefly, the mesencephalic tissues were minced and digested in 0.1 % trypsin for 30 min at 37 °C. After being washed with PBS, the cells were resupended in Dulbecco modified eagle medium (DMEM, Gibco, Rockville, MD, USA) containing 10 % fetal bovine serum (FBS, Hyclone, Logan, UT, USA). The cells were passaged at 6 days, and astrocytes were purified from the primary mixed cultures by three to four repetitions of trypsinization and replating. The purity of astrocytes was evaluated by immunoflurescence staining with anti-glial fibrillary acidic protein (GFAP) antibody, a specific marker of astrocytes.
Cell viability assay
The effect of curcumin on cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT, Sigma Co., St. Louis, MO, USA). Briefly, cells (2 × 103/well) were seeded in 96-well plates and incubated in a humidified atmosphere containing 5 % CO2. The cells were then treated with different concentrations of curcumin (0, 1, 2, 4, 8, or 16 μM) for 48 h, followed by incubation in MTT (0.2 mg/ml) for an additional 4 h. After that, the medium was removed, and the crystal dye was dissolved in 100 μl of DMSO for each well. The absorbance was measured at 490 nm with a microplate reader (ELx800, BioTek, Winooski, VT, USA).
Real-Time PCR
Total RNA from primary astrocytes was extracted using an RNA extraction kit (Tiangen Co., Beijing, China), then converted into complementary DNA (cDNA) using Super MMLV Reverse Transcriptase (BioTeke, Beijing, China) according to the manufacturer’s instructions. The expression of TLR4, MyD88, TRIF/TRAM, NF-κB, and IRF3 was determined using Exicycler™ 96 real-time (RT)-PCR machine (Bioneer, Daejeon, Korea). The sequences of all RT-PCR primers are as follows: NF-κB, 5ʹ-CGCATTCTGACCTTGCCTATC-3ʹ (forward), 5ʹ-AGTCCAGTCTCCGAGTGAAGC-3ʹ (reverse); IRF3, 5ʹ-TGGCTGACTTTGGCATCT-3ʹ (forward), 5ʹ-AATCGCAACACTTCTTTCC-3ʹ (reverse); MyD88, 5ʹ-TCCCACTCGCAGTTTGTT-3ʹ (forward), 5ʹ-TGCCTCCCAGTTCCTTTG-3ʹ (reverse); TRAM, 5ʹ-GGACAGACCCGAGGCAAAG-3ʹ (forward), 5ʹ-CCAGGCGGACCCATTTAC-3ʹ (reverse); TLR4, 5ʹ-GGGGGGTATTTGACACACTCTA-3ʹ (forward), 5ʹ-TCCTTTGGATGTCTCTATGCGA-3ʹ (reverse); β-actin, 5ʹ-GGAGATTACTGCCCTGGCTCCTAGC-3ʹ (forward), 5ʹ-GGCCGGACTCATCGTACTCCTGCTT-3ʹ (reverse).
Measurement of cytokines
Cells were pretreated with curcumin for 30 min, followed by MPP+ stimulation (800 μM) for 48 h. The concentrations of TNF-α, IL-6, and IL-10 in the supernatants were determined using enzyme-linked immunosorbent assay (ELISA) according to the products’ manufacturers (Boster Bio-Engineering Co., Ltd, Wuhan, China).
Measurement of oxygen species
Reactive oxygen species (ROS) levels were determined as previously described (Morimoto et al. 2013). ROS formation was detected by monitoring a conversion of 2', 7'-Dichlorodihydrofluorescein diacetate (DCFH-DA) to DCF according to the manufacturer’s instruction (Invitrogen, Carlsbad, CA, USA). Briefly, single-cell suspensions were incubated with 10 μM DCF H2-DA for 20 min at 37 °C. After washing three times using PBS, the cells were analyzed in a BD Accuri C6 flow cytometer (Becton, Dickinson, & Co. (BD), Franklin Lakes, NJ, USA).
Measurement of glutathione
Cells lysates were prepared by freezing and thawing method. GSH level from the supernatants was estimated using a glutathione (GSH) assay kit (Nanjing Jiancheng Institute, Nanjing, China) according to the manufacturer’s instructions. GSH levels were expressed as micromoles of GSH formed per milligram of protein.
Western blotting
Cell samples were lysed using NP-40 lysis reagent (Beyotime Technology, Haimen, China), and the protein concentration was measured using a bicinchoninic acid method (Beyotime). Each amount of protein samples were separated by SDS-PAGE, then transferred onto the polyvinylidene difluoride membrane (PVDF, GE Healthcare UK, Buckinghamshire, UK). After being blocked with 5 % skim milk, the membranes were incubated in appropriate primary antibodies: anti-TLR4 (1:100, sc-293072, Santa Cruz, CA, USA), anti-MyD88 (BA2321), anti-TRIF/TRAM (BA2933-2, 1:400), anti-NF-κB (BA0610, 1:400), and anti-IRF3(BA4351-2, 1:400) (all from Boster Bio-Engineering Co., Ltd). The membranes were subsequently incubated with secondary antibodies conjugated with horseradish peroxidase (goat anti-rabbit IgG horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, 1:5000, Beyotime) for 1 h. The target proteins were visualized with an enhanced ECL reagent, and the intensity of the bands was analyzed by using Gel-Pro Analyzer software 4.0 (Media Cybernetics Inc., Rockville, MD, USA).
Immunocytochemistry
Cells cultured on a glass coverslip were washed with PBS and fixed in 4 % paraformaldehyde, then blocked with normal goat serum. After that, the cells were incubated with diluted mouse monoclonal anti-GFAP antibody and anti-TLR4 antibody, respectively (1:50 diluted, Santa Cruz Biotechnology). Then, the cells were incubated with Cy3-labeled goat anti-mouse antibody (1:100, Beyotime) at room temperature for 1 h in the dark, followed by incubation with DAPI. Cell images were visualized with a fluorescent microscope (BX53; Olympus, Tokyo, Japan).
Statistical analysis
The statistical analysis was performed using GraphPad Prism Software version 5.0 (San Diego, CA, USA). Data are expressed as the mean ± SD. Comparisons were made between the groups using one-way ANOVA, followed by Bonferroni post hoc test. A P value less than 0.05 was considered to indicate a statistically significant difference.
Results
Effect of curcumin on cell viability
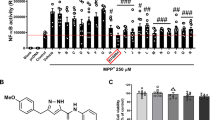
The potential cytotoxicity of curcumin on primary cultured astrocytes was evaluated using an MTT assay. As shown in Fig. 1, curcumin at low concentrations (1–8 μM) did not exhibit cytotoxicity to astrocytes. However, curcumin at high concentration (16 μM) caused a significant reduction in cell viability compared to the control untreated astrocytes (P < 0.01). Thus, we chose curcumin at a concentration of 8 μM for subsequent studies.
Effect of curcumin on cell viability in primary astrocytes. Cells were incubated with different concentrations of curcumin (0, 1, 2, 4, 8, or 16 μM) for 48 h. Cell viability was determined by MTT assay. Data are expressed as the mean ± standard deviation. **P < 0.01, compared with control untreated astrocytes
Curcumin inhibited the MPP+-induced activation of primary astrocytes
Previously, we have shown that curcumin inhibited astrocyte activation in the substantia nigra and striatum in MPTP-treated mice (Yu et al. 2010). Here, we further investigated the effect of curcumin on MPP+-induced astrocytes in vitro. Glial fibrillary acidic protein, the specific marker of astrocytes, was used to detect astrocyte activation. As shown in Fig. 2, MPP+ stimulation induced a reactive state in the astrocyte, as observed by increased GFAP expression and larger cell body of astrocytes determined by immunofluorescence. In contrast, curcumin pretreatment reversed these changes and mitigated the activation of astrocytes by MPP+. Curcumin per se had no obvious effects on the morphology of astrocytes.
Curcumin inhibited the production of pro-inflammatory cytokines in MPP+-stimulated primary astrocytes
Inflammatory cytokines are reported to be over-expressed following MPP+ insults. Thus, we investigated the effect of curcumin on inflammatory cytokine production in MPP+-stimulated astrocytes. Figure 3 shows the expression levels of TNF-α, IL-6, and IL-10 in the cell culture supernatants. MPP+ significantly increased the production of TNF-α and IL-6 compared to the control untreated astrocytes (P < 0.01, Fig. 3a, b). Pretreatment with curcumin reduced secretion of TNF-α and IL-6 (P < 0.05, P < 0.01 versus MPP+ group, respectively), while it increased the levels of IL-10 in MPP+-challenged astrocytes (P < 0.05, Fig. 3c). Curcumin per se did not affect the levels of TNF-α and IL-6.
Effect of curcumin on MPP+-induced cytokine production in primary astrocytes. Expression levels of a TNF-α, b IL-6, and c IL-10 in cell culture supernatants were measured. Curcumin downregulates TNF-a and IL-6 expression while it increased IL-10 production in MPP+-stimulated astrocytes. Data are expressed as the mean ± standard deviation. ## P < 0.01, compared with control untreated astrocytes; *P < 0.05, **P < 0.01, compared with MPP+-stimulated astrocytes
Curcumin attenuated MPP+-induced oxidative mediator in astrocytes
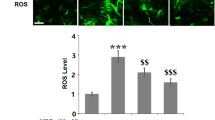
Reactive oxygen (ROS) plays a key role in inflammation and the pathogenesis of PD. Increased ROS production overwhelms the inherent antioxidant defense like GSH (Khan et al. 2014a). Therefore, we further assessed the effect of curcumin on oxidative mediator production in MPP+-stimulated astrocytes. As shown in Fig. 4, the levels of ROS significantly increased in MPP+-challenged astrocytes compared to the control untreated astrocytes (P < 0.01). Curcumin effectively suppressed ROS production induced by MPP+ (P < 0.01, Fig. 4a). In addition, curcumin notably increased the GSH levels that were repressed by MPP+ (P < 0.01, Fig. 4b).
Effect of curcumin on MPP+-induced oxidative stress in primary astrocytes. a Expression levels of ROS in astrocytes were determined by monitoring a conversion of DCF H-DA to DCF. b Expression levels of GSH in the medium of primary astrocytes. Data are expressed as the mean ± standard deviation. ## P < 0.01, compared with control untreated astrocytes; **P < 0.01, compared with MPP+-stimulated astrocytes
Curcumin inhibited NF-κB and IRF3 activation in MPP+-stimulated astrocytes
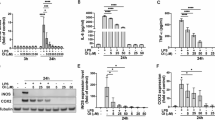
NF-κB and IRF3 play key roles in regulating inflammatory responses (Karin and Delhase 2000). To determine whether curcumin attenuates inflammation by affecting NF-κB and IRF3 pathways, we assess whether curcumin attenuates inflammation through the NF-κB and IRF3 pathways; the expression of NF-κB and IRF3 in astrocytes was detected by RT-PCR and Western blot analysis. As shown in Fig. 5, MPP+ challenge significantly increased the messenger RNA (mRNA) expression of NF-κB and IRF3 (P < 0.01, Fig. 5a, b) and the phosphorylation of NF-κB and IRF3 (Fig. 5e, f). Furthermore, the expression of TLR4 downstream signaling components including MyD88 and TRIF were similarly upregulated in MPP+-stimulated astrocytes (Fig. 5c–f). In contrast, curcumin reversed these changes induced by MPP+. Together, these results indicate that curcumin attenuates astrocytic inflammation which is partially mediated by inactivation of NF-κB and IRF3.
Curcumin inhibits MPP+-induced NF-κB and IRF3 activation in primary astrocytes. Real-time PCR was performed to detect the mRNA levels of a NF-κB, b IRF3, c MyD88, and d TIRF/TRAM in primary astrocytes. e Western blot analysis was performed to determine the protein levels of NF-κB, IRF3, MyD88, and TIRF/TRAM. f Relative protein levels were quantified by a gray analysis with normalization to β-actin. Data are presented as mean ± SD; ## P < 0.01, compared with control untreated astrocytes; *P < 0.05, **P < 0.01, compared with MPP+-stimulated astrocytes
Curcumin inhibited TLR4 expression in MPP+-stimulated astrocytes
Activated astrocytes via TLRs have shown neurotoxicity (Ma et al. 2013). Thus, we further determined the changes in astrocytic TLR expression after MPP+ challenge and the effect of curcumin on TLR expression. Results from RT-PCR and Western blot analysis showed that MPP+ exposure significantly increased the mRNA and protein levels of TLR4 in astrocytes compared to the control untreated cells. However, these elevations were repressed by curcumin pretreatment (Fig. 6a, b). Immunofluorescence results further confirmed that the MPP+-induced TLR4 immunoreactivity was diminished by curcumin (Fig. 6c). Together, these results indicate that the anti-inflammatory action of curcumin on MPP+-stimulated astrocytes is mediated by inhibition of the TLR4 signaling pathway.
Curcumin attenuates the expression and immunoreactivity of TLR4 in MPP+-induced astrocytes. a Real-time PCR analysis was performed to determine TLR4 mRNA level of TLR4 in primary astrocytes. b The protein levels of TLR4 were determined by Western blot analysis. Relative TLR4 protein level was quantified by a gray analysis with normalization to β-actin. c The immunofluorescence of TLR4 in astrocytes was determined. TLR4 was shown in red and DAPI was shown in blue. Magnification, ×400, scale bars = 20 μm. Data are presented as mean ± SD; ## P < 0.01, compared with control untreated astrocytes; *P < 0.05, compared with MPP+-stimulated astrocytes (color figure online)
Discussion
Repeated exposure to environmental toxins, such as MPTP, has been reported to cause PD symptoms (Schober 2004). MPP+ is the active metabolite of classical parkinsonian toxin MPTP and is commonly used to investigate the alteration of cell functions relevant to PD. The toxic mechanisms of MPP+ are demonstrated to inhibit complex I of the mitochondrial respiratory chain and induce ROS generation (Alvarez-Fischer et al. 2013; Bournival et al. 2012) as well as induce pro-inflammatory mediators of glial cells (Khan et al. 2014a; Zhou et al. 2016). We previously showed that curcumin prevented dopaminergic neuronal death and inhibited astrocyte activation in a mouse model of MPTP-induced PD (Yu et al. 2010). In the present study, we focused on the pro-inflammatory roles of astrocytes and we specifically addressed the effects of curcumin on MPP+-induced primary cultured astrocytes. Our results showed that curcumin prevented the inflammatory and oxidative mediators in MPP+-induced mesencephalic astrocytes. Furthermore, we demonstrated for the first time that these effects were partially mediated by inhibition of TLR4 and its downstream signaling pathway.
Neuroinflammation is a central event related to PD progression. A prominent feature of neuroinflammation is characterized by glial activation, concomitant with production of pro-inflammatory mediators including cytokines, chemokines, and ROS (Hirsch and Hunot 2009; van Loo et al. 2006). Previously, glial cell activation was confirmed in the postmortem brain of PD patients and MPTP-treated animals (Damier et al. 1993; De Miranda et al. 2015). In the present study, we found that MPP+-stimulated astrocytes exhibited increased GFAP expression and larger cell bodies compared to the control untreated astrocytes. In addition, MPP+ challenge led to production of TNF-α and IL-6 in astrocytes. However, curcumin pretreatment inhibited the MPP+-induced activation of astrocytes and decreased the expression levels of TNF-α and IL-6. Our results indicate that curcumin can diminish MPP+-induced inflammation and the activation of astrocytes, and our results were consistent with an earlier study showing that curcumin exerted an anti-inflammatory effect in lipopolysaccharide (LPS)-induced human astrocyte cell line (Seyedzadeh et al. 2014).
ROS production plays a key role in inflammatory process and the pathogenesis of PD. Previously, Wong et al. showed that the mesencephalic astrocytes were more prone to produce ROS than were other regional astrocytes after MPP+ stimulation (Wong et al. 1999). In the present study, we found that MPP+-treated astrocytes exhibited significant increase in ROS production. However, this change was reversed by curcumin pretreatment. Furthermore, curcumin restored the level of antioxidant GSH that is decreased by MPP+. Together, these results suggest that curcumin can mitigate oxidative stress in MPP+-induced mesencephalic astrocytes.
TLRs are a large class of immune molecules mediating innate immune responses through conserved signaling pathways (Akira et al. 2001; Beutler et al. 2003). There are two major downstream pathways in TLR signaling: the MyD88-dependent pathway and the MyD88-independent pathway (or TRIF pathway ) (Kumar et al. 2009). MyD88 is a key adaptor protein required by all TLRs except TLR3 (Akira et al. 2006). The MyD88-dependent pathway induces production of various pro-inflammatory mediators via NF-κB or MAPK activation (Kagan and Medzhitov 2006). In contrast, the MyD88-independent pathway is controlled by the signal transduction intermediates like TRIF, which trigger production of type I interferon via IRF3 activation (Akira and Sato 2003). Previous studies have shown that MPP+-induced cytotoxicity is related to NF-κB activation (Khan et al. 2014a; Liu et al. 2010) and ROS generation (Khan et al. 2014b). ROS is supposed to be one of the causes leading to TLR4 upregulation (Wei et al. 2015). In the present study, we showed that MPP+ stimulation increased the expression levels of NF-κB and MyD88 in astrocytes. Moreover, we also found that the expression levels of IRF3 and TRIF were upregulated in MPP+-stimulated astrocytes. Curcumin significantly inhibited the activation of NF-κB and IRF3 and decreased the levels of TLR4 and its downstream effectors including MyD88 and TIRF that were induced by MPP+. Together, these results suggest that the anti-inflammatory action of curcumin is mediated via an inhibition of TLR4 and its downstream signaling pathway. Previously, curcumin was shown to have a number of molecular targets involved in inflammation (Ghosh et al. 2015). Curcumin could inhibit LPS-induced NFκB activation through inhibiting IKKβ (Pan et al. 2000). Youn et al. (2006) further showed that curcumin attenuated inflammatory responses through inhibiting homodimerization of TLR4. Here, we showed that curcumin exerted anti-inflammatory action in MPP+-induced astrocytes through downregulating TLR4 and its downstream signaling. Our results were consistent with these findings.
In conclusion, our results demonstrated that curcumin decreased the production of pro-inflammatory and oxidative mediators (TNF-α, IL-6, and ROS) in MPP+-stimulated mesencephalic astrocytes. The protective mechanism was partially through inhibiting both MyD88-dependent and TRIF-dependent pathways in TLR4 signaling. Thus, TLR4-mediated astrocytic inflammation could be a potential target for PD therapy and curcumin administration could be a potential agent in the inflammatory pathology of PD.
References
Ak T, Gulcin I (2008) Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 174:27–37. doi:10.1016/j.cbi.2008.05.003
Akira S, Sato S (2003) Toll-like receptors and their signaling mechanisms. Scand J Infect Dis 35:555–562
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680. doi:10.1038/90609
Akira S, Uematsu S, Takeuchi O, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801. doi:10.1016/j.cell.2006.02.015
Alvarez-Fischer D et al (2013) Probenecid potentiates MPTP/MPP+ toxicity by interference with cellular energy metabolism. J Neurochem 127:782–792. doi:10.1111/jnc.12343
Beutler B, Hoebe K, Du X, Ulevitch RJ (2003) How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol 74:479–485. doi:10.1189/jlb.0203082
Bournival J, Plouffe M, Renaud J, Provencher C, Martinoli MG (2012) Quercetin and sesamin protect dopaminergic cells from MPP + -induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Med Cell Longev 2012:921–941. doi:10.1155/2012/921941
Bowman CC, Rasley A, Tranguch SL, Marriott I (2003) Cultured astrocytes express toll-like receptors for bacterial products. Glia 43:281–291. doi:10.1002/glia.10256
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49:360–374. doi:10.1002/glia.20117
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 52:1–6
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, Tjalkens RB (2015) Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci Off J Soc Toxicol 143:360–373. doi:10.1093/toxsci/kfu236
Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. doi:10.1016/j.it.2007.01.005
Ghosh S, Banerjee S, Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 83:111–124. doi:10.1016/j.fct.2015.05.022
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. doi:10.1016/S1474-4422(09)70062-6
Jack CS et al (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175:4320–4330
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternat Med Rev J Clin Ther 14:141–153
Kagan JC, Medzhitov R (2006) Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125:943–955. doi:10.1016/j.cell.2006.03.047
Karin M, Delhase M (2000) The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol 12:85–98. doi:10.1006/smim.2000.0210
Kaur H, Patro I, Tikoo K, Sandhir R (2015) Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int 89:40–50. doi:10.1016/j.neuint.2015.07.009
Khan MM, Kempuraj D, Zaheer S, Zaheer A (2014a) Glia maturation factor deficiency suppresses 1-methyl-4-phenylpyridinium-induced oxidative stress in astrocytes. J Mol Neurosci 53:590–599. doi:10.1007/s12031-013-0225-z
Khan MM, Zaheer S, Nehman J, Zaheer A (2014b) Suppression of glia maturation factor expression prevents 1-methyl-4-phenylpyridinium (MPP(+))-induced loss of mesencephalic dopaminergic neurons. Neuroscience 277:196–205. doi:10.1016/j.neuroscience.2014.07.003
Kumar H, Kawai T, Akira S (2009) Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388:621–625. doi:10.1016/j.bbrc.2009.08.062
Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11:338–378. doi:10.2174/1570159X11311040002
Lin YG et al (2007) Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res Off J Am Assoc Cancer Res 13:3423–3430. doi:10.1158/1078-0432.CCR-06-3072
Liu L, Xu H, Jiang H, Wang J, Song N, Xie J (2010) Ghrelin prevents 1-methyl-4-phenylpyridinium ion-induced cytotoxicity through antioxidation and NF-kappaB modulation in MES23.5 cells. Exp Neurol 222:25–29. doi:10.1016/j.expneurol.2009.11.009
Ma D et al (2013) The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol 254:10–18. doi:10.1016/j.jneuroim.2012.08.010
Mansouri Z, Sabetkasaei M, Moradi F, Masoudnia F, Ataie A (2012) Curcumin has neuroprotection effect on homocysteine rat model of Parkinson. J Mol Neurosci 47:234–242. doi:10.1007/s12031-012-9727-3
More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediat Inflamm 2013:952375. doi:10.1155/2013/952375
Morimoto H et al (2013) ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 12:774–786. doi:10.1016/j.stem.2013.04.001
Noelker C et al (2013) Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep 3:1393. doi:10.1038/srep01393
Pan MH, Lin-Shiau SY, Lin JK (2000) Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol 60:1665–1676
Parpura V et al (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27. doi:10.1111/j.1471-4159.2012.07664.x
Phani S, Loike JD, Przedborski S (2012) Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord 18(1):S207–209. doi:10.1016/S1353-8020(11)70064-5
Ray B, Lahiri DK (2009) Neuroinflammation in Alzheimer’s disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol 9:434–444. doi:10.1016/j.coph.2009.06.012
Rossi D (2015) Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130:86–120. doi:10.1016/j.pneurobio.2015.04.003
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224. doi:10.1007/s00441-004-0938-y
Seyedzadeh MH, Safari Z, Zare A, Gholizadeh Navashenaq J, Razavi SA, Kardar GA, Khorramizadeh MR (2014) Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int Immunopharmacol 22:230–235. doi:10.1016/j.intimp.2014.06.035
Tang SC et al (2007) Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A 104:13798–13803. doi:10.1073/pnas.0702553104
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37:510–518. doi:10.1016/j.nbd.2009.11.004
van Loo G et al (2006) Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol 7:954–961. doi:10.1038/ni1372
Wei M, Li Z, Xiao L, Yang Z (2015) Effects of ROS-relative NF-kappaB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol Immunol 68:261–271. doi:10.1016/j.molimm.2015.09.002
Wong SS, Li RH, Stadlin A (1999) Oxidative stress induced by MPTP and MPP(+): selective vulnerability of cultured mouse astrocytes. Brain Res 836:237–244
Youn HS, Saitoh SI, Miyake K, Hwang DH (2006) Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol 72:62–69. doi:10.1016/j.bcp.2006.03.022
Yu S et al (2010) Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res 13:55–64. doi:10.1089/rej.2009.0908
Zhang Q, Kang R, Zeh HJ 3rd, Lotze MT, Tang D (2013) DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy 9:451–458. doi:10.4161/auto.23691
Zhou P et al (2016) TLR4 signaling in MPP(+)-induced activation of BV-2 cells. Neural Plast 2016:5076740. doi:10.1155/2016/5076740
Zhu HT et al (2014) Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. J Neuroinflammation 11:59. doi:10.1186/1742-2094-11-59
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81203004) and the Postdoctoral Science Foundation of China (No. 2013M540234).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Yu, S., Wang, X., He, X. et al. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP+)-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress and Chaperones 21, 697–705 (2016). https://doi.org/10.1007/s12192-016-0695-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0695-3