Abstract
We analyzed the incidence of bone marrow fibrosis in 91 newly diagnosed Japanese multiple myeloma (MM) patients and evaluated the impact of fibrosis on clinical characteristics and therapeutic outcomes. Thirty-four (37%) patients had greater than grade 1 bone marrow fibrosis. The presence of bone marrow fibrosis did not affect laboratory data, the percentage of plasma cells in bone marrow or cytogenetic findings. It also had no significant effect on response to initial treatment, engraftment after autologous hematopoietic stem cell transplantation or overall survival. Interestingly, the incidence of extramedullary disease at diagnosis was significantly higher in patients with bone marrow fibrosis (p = 0.006). Analysis of biological characteristics of MM cells revealed that expression of CD49e, an alpha5/beta1 integrin, was downregulated in MM cells derived from patients with bone marrow fibrosis (p = 0.026). When seven of the original 34 patients were re-evaluated for fibrosis grading after treatment, five (71%) showed a reduction in fibrosis. Our present findings suggest that the presence of bone marrow fibrosis may predict development of extramedullary disease in MM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone marrow microenvironment plays an important role in the pathophysiology of multiple myeloma (MM) by supporting the growth and survival of MM cells, suppressing immune cell function and inducing clonal evolution of MM cells [1,2,3]. From a clinical point of view, abnormalities in the bone marrow environment seen in MM are one of the major obstacles to curing the disease [1].
The bone marrow microenvironment is a complex system composed of cellular components, growth factors, cytokines and extracellular matrix (ECM). Increased production and deposition of ECM, known as tissue fibrosis, is often found in a variety of solid cancers and is associated with an aggressive phenotype of the disease and refractoriness to treatment [4]. An increase in reticulin or collagen fibers in bone marrow is observed in many types of hematological malignancies, i.e., myeloproliferative neoplasms, myelodysplastic syndrome or acute myeloid leukemia [5, 6]. In addition to myeloid malignancies, myelofibrosis is sometimes found in lymphoid malignancies, including multiple myeloma [5, 6].
The role of bone marrow fibrosis in MM has been analyzed by both biological studies and clinical observations. Fibroblast-like cells isolated from primary bone marrow samples of MM patients expressed higher levels of numerous types of ECM remodeling proteins, i.e., laminin, matrix metalloproteinases or prolyl-4-hydroxylase, lysyl-hydroxylase, than those derived from normal individuals or monoclonal gammopathy of undetermined significance (MGUS) patients [7]. Interestingly, these proteins and enzymes are involved in the progression of bone marrow fibrosis in myeloproliferative neoplasms [8,9,10]. Another study revealed an increase in serum amino terminal propeptide procollagen III (PIIINP), a putative biomarker for the synthesis of reticulin fibers, in MM patients [11]. The serum levels of PIIINP were correlated with the disease status of MM [11]. Other studies have revealed the presence of an activated form of fibroblasts defined as “cancer-associated fibroblasts (CAFs)” in the bone marrow of advanced-stage MM [7, 12]. It is well accepted that CAFs modify ECM homeostasis by producing, remodeling and depositing several types of ECM components [13]. In turn, altered ECM supports the survival and acquisition of drug resistance in cancer cells, leading to poor prognosis in these patients [13, 14]. In agreement with these biological observations, previous case series studies have indicated that a significant proportion of MM patients had bone marrow fibrosis and that MM patients harboring bone marrow fibrosis had poorer survival [15,16,17]. However, these reports had a limitation in that most of the patients in these studies were treated with an old-fashioned type of treatment composed of chemotherapeutic agents and did not receive proteasome inhibitors (PIs) or immunomodulatory drugs (IMiDs), which have become standard treatment regimens after the 2010s. Very recently, Paul et al. evaluated the impact of bone marrow fibrosis on the treatment outcome of newly diagnosed MM patients treated with modern era regimens [18]. They demonstrated that the presence of bone marrow fibrosis at diagnosis has a negative impact on treatment outcome even in MM patients treated with novel agents [18]. Although the study included approximately 400 MM patients, the distribution of ethnic background of the patients was biased; 63.1% were Caucasian, and 34.5% were African American [18]. Therefore, the data for patients with Asian ethnic backgrounds are still missing.
In this study, we investigated the incidence of bone marrow fibrosis in Japanese MM patients and attempted to determine the impact of bone marrow fibrosis on the clinical characteristics and treatment outcomes of these patients. This is the first study to evaluate the clinical significance of the presence of bone marrow fibrosis in MM patients with an Asian background.
Materials and methods
Ethical conduct of the study
This was a single-center, retrospective cohort study approved by the Institutional Review Board (IRB) at the University of Yamanashi and was conducted in accordance with the Declaration of Helsinki.
Patients and definitions
We enrolled 91 patients with MM diagnosed at the Department of Hematology and Oncology, University of Yamanashi, between January 2012 and March 2020. The diagnosis of MM was made according to the International Myeloma Working Group (IMWG) diagnostic criteria [19, 20]. Bone marrow trephine biopsy was performed at the time of diagnosis. Patient data were collected from electronic medical records that included laboratory data at the time of diagnosis, treatment regimen, treatment response and survival data. Extramedullary disease (EMD) was defined according to previous papers, and we considered only bone-independent extramedullary disease and primary plasma cell leukemia (PCL) as EMD according to recent recommendations [21, 22]. For the definition of plasma cell leukemia, we used the recently proposed revised diagnostic criteria [22, 23]. According to these criteria, patients with more than 5% circulating plasma cells are defined as PCL. Genetic abnormalities were analyzed using both conventional G-banding and fluorescence in situ hybridization (FISH). High-risk cytogenetic abnormalities were defined according to the updated definition of the IMWG [24]. A surface marker expression study using flow cytometry was performed at SRL Corporation (Tokyo, Japan) within the insurance coverage. Plasma cells were identified depending on the expression of CD38 and side scatter analysis, and the expression of CD19, CD56, mature plasma cell-1 (MPC-1), CD45 and CD49e was analyzed in this population of cells.
The response to treatment was evaluated using the IMWG treatment response criteria [25]. Overall survival (OS) was defined as the duration from the date of diagnosis of MM to the date of last follow-up when the patient was known to be alive or the date of death. Progression-free survival (PFS) was defined as the duration from the start of treatment to first progression or death, whichever occurred earlier.
Pathological analysis
For the evaluation of fibrosis, bone marrow biopsy samples were analyzed by reticulin staining and Masson’s trichrome staining. The degree of myelofibrosis was scored according to the World Health Organization (WHO) criteria [26]. To confirm the presence of MM cells, an immunohistochemical study with an anti-CD138 antibody was also performed.
Statistical analysis
OS and PFS were analyzed by the Kaplan–Meier method, and statistical significance was evaluated using the log-rank test. The Mann–Whitney U test was used for analysis of continuous variables between the patients with fibrosis and the patients without fibrosis. For the analysis of categorical variables, Fisher`s exact test was used. All statistical analyses were performed using GraphPad Prism version 9.20 software (GraphPad Software, San Diego, CA, USA).
Results
Characteristics of patients
Bone marrow fibrosis was detected in 34 cases (37%) at the diagnosis of MM; 31 patients showed grade 1 fibrosis, 2 patients showed grade 2 fibrosis, and 1 patient had grade 3 fibrosis. The representative pathological findings of the patient with grade 3 levels of bone marrow fibrosis are presented in Fig. 1. Several studies have indicated pre-existing or concomitant myeloproliferative neoplasms (MPNs), especially primary myelofibrosis, with MM [27, 28]. In contrast, none of the patients in this study had a previous history of MPNs and showed elevation of red blood cells or platelets at MM diagnosis. Furthermore, we analyzed MPN driver gene mutations in 9 of the patients with available bone marrow cell DNA samples. All patients who had grade 2 or higher levels of myelofibrosis were included in this analysis. We confirmed that none of the MPN driver mutation, including JAK2V617F, CALR exon9 mutation and MPL W515 mutation, were detected in these patients (data not shown).
We divided the patients into two groups according to the presence or absence of fibrosis: the “with fibrosis group”, which showed any grade of bone marrow fibrosis, and the “without fibrosis group”. The clinical characteristics of each group of patients are presented in Table 1. There were no significant differences between the “with fibrosis” and “without fibrosis” groups with respect to sex, age, type of M protein or affected light chain type. The distribution of the International Staging System (ISS) stages showed no statistical significance. The presence of chromosomal abnormalities in conventional analysis and FISH-defined high-risk cytogenetic abnormalities were not significantly different between the two groups. In contrast, we found that the patients with bone marrow fibrosis tended to have extramedullary disease at initial presentation compared to the “without fibrosis group”, and this difference was confirmed to be significantly different (p = 0.006). Five cases with EMM are summarized in Table 2. Among them, one case showed MF-3 levels of fibrosis, whereas 4 cases had MF-1 level fibrosis. Three patients had an increase in plasma cells of more than 5% in peripheral blood, 2 patients had invasion of MM cells into the pleura, and one patient had invasion of MM cells into the lymph node and pancreas. Two case were confirmed to have t(11;14) with FISH.
We also investigated the differences in laboratory data between the two groups (Fig. 2). The proportion of plasma cells in bone marrow was not significantly different. In addition, we did not find any differences in hemoglobin, lactate dehydrogenase (LDH), estimated glomerular filtration rate, serum calcium levels, serum albumin or serum beta2-microglobulin levels. The numbers of megakaryocytes in the bone marrow were also not different between the two groups of patients.
Expression of cell surface antigens on myeloma cells
Next, we compared the expression levels of cell surface antigens on myeloma cells between the two groups. As shown in Fig. 3, the ratio of CD56-positive cells was not different between the two groups. We did not find any differences in MPC-1-positive cells or in CD45-positive cells. However, CD49e, also known as very late antigen 5 (VLA-5), and the positive cell ratio were significantly decreased in patients with bone marrow fibrosis (p = 0.026).
Reponses to treatment and survival
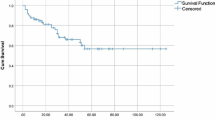
The patients were treated with a variety of regimens. We divided these regimens into four groups depending on the drugs used in the regimens: proteasome inhibitor (PI)-dependent regimen, immunomodulatory agent (IMiD)-dependent regimen, PI plus IMiD regimen and other types. Thirty of the 57 (52%) patients without fibrosis were treated with PI-dependent regimens, 5 (9%) were treated with IMiD-based regimens, and 10 patients (18%) were treated with a combination of PI and IMiDs, most of which were bortezomib plus lenalidomide (Table 3). In the patients with bone marrow fibrosis, 18 of 34 (51%) were treated with a PI-dependent regimen, 5 (14%) were treated with an IMiD-based regimen, and 6 (17%) were treated with PI plus IMiD regimens (Table 3). There were no differences in the treatment patterns in the groups with and without fibrosis. The response to initial treatment is also presented in Table 3. The rates of very good partial response (VGPR) or better were 28.1% and 44.1% for patients without fibrosis and with fibrosis, respectively. There was no significant association between the presence or absence of bone marrow fibrosis and the therapeutic response (p = 0.17). The OS and PFS of each group were also analyzed. As illustrated in Fig. 4, we did not find significant differences in OS and PFS between the two groups.
Impact of bone marrow fibrosis on the outcome of autologous hematopoietic stem cell transplantation
In this study, 8 patients without bone marrow fibrosis and 7 patients with fibrosis underwent autologous hematopoietic stem cell transplantation (ASHCT). We evaluated the effects of bone marrow fibrosis on the collection of CD34 cells and engraftment after transplantation. For induction therapy before peripheral blood stem cell collection, 3 patients received bortezomib plus dexamethasone; 4 patients were treated with a combination of bortezomib, cyclophosphamide and dexamethasone; and the remaining 8 patients were treated with bortezomib, lenalidomide and dexamethasone. Peripheral blood stem cells were collected following high-dose cyclophosphamide treatment (13 patients) or G-CSF alone (2 patients). We did not find significant differences between the groups in the collected number of CD34-positive cells (patients without fibrosis: 9.26 ± 3.76 × 106/kg vs. patients with fibrosis: 4.74 ± 1.43 × 106/kg, p = 0.5). The interval from transplant to granulocyte recovery was also not different in patients without fibrosis (11.7 ± 0.5 days) and patients with fibrosis (mean 11.3 ± 0.3 days). The presence of bone marrow fibrosis did not affect OS after ASHCT (data not shown).
Changes in fibrosis grading after treatment
Among the 34 patients who initially had MF-1 or greater fibrosis, 7 patients were re-evaluated for bone marrow fibrosis grade to examine treatment response (Table 4). Four patients (#2, 3, 4 and 7) were examined for bone marrow findings at the timepoint after ASHCT, and 2 patients (#5 and #6) were analyzed before ASHCT. Five of 7 patients showed regression of bone marrow fibrosis levels, whereas the bone marrow fibrosis levels remained unchanged even after the treatment in 2 patients. The changes in the pathological findings of case #6 are shown in Fig. 5.
Regression of bone marrow fibrosis after treatment for MM. A–C At the diagnosis of MM, the patient showed increased bone marrow cells with marked invasion of anti-CD138-positive MM cells. Increases in reticulin fibers were also found. D–F After the completion of 4 courses of therapy composed of bortezomib, lenalidomide and dexamethasone, a second bone marrow biopsy was performed. At this time, CD138-positive cells disappeared with regression of bone marrow fibrosis
Discussion
This is the first study to evaluate the impact of bone marrow fibrosis in MM in an era of new age treatment and in Asian ethnic background patients. We found that approximately one-third of newly diagnosed Japanese multiple myeloma patients had myelofibrosis. The prevalence of fibrosis is almost the same as that of previous studies. Although we did not find any correlations among the presence of bone marrow fibrosis and clinical characteristics, laboratory data, or therapeutic response, we found a statistically significant association between the presence of fibrosis and concomitant extramedullary disease. We also noticed that myeloma cells from patients with fibrosis had lower expression levels of CD49e than that of cells from patients without fibrosis.
The clinical significance and impact on the therapeutic response of bone marrow fibrosis in MM patients have been studied by several investigators. Abidgaard N and colleagues reported that an increase in reticulin was observed in 9 of 25 (36%) MM patients who underwent bone marrow biopsies [11]. They also found a positive correlation between the degree of reticulin fibrosis and the burden of myeloma cells in biopsy specimens [11]. Dolgikh et al. reported that the area of fibrosis was correlated with anemia and blood transfusion dependence in MM patients [15]. Another study from India indicated that 9 of 44 (20.5%) newly diagnosed MM patients showed more than grade 1 fibrosis when evaluated using European Consensus Methods [16]. They reported that an increase in bone marrow fibrosis was correlated with a poorly differentiated plasma cell phenotype [16]. The survival of patients with bone marrow fibrosis was shorter than that of patients without fibrosis, but the differences were not statistically significant [16]. Babarovic et al. also reported that the presence of fibrosis at diagnosis did not impact the therapeutic response [17]. In contrast, they found that patients with stable or increased bone marrow fibrosis after treatment had significantly worse survival. In the above studies, most of the cases were treated with conventional chemotherapies composed of vincristine, doxorubicin and dexamethasone. Therefore, it is still unclear whether the presence of bone marrow fibrosis affects the treatment outcome in the modern treatment era for MM. Very recently, Paul B and colleagues tried to answer this question. In the study, a total of 393 MM patients were analyzed [18]. The presence of bone marrow fibrosis was associated with shorter OS and progression-free survival. However, after adjusting for age, ISS, and cytogenetic risk, these differences were not statistically significant [18]. In our study, most of the patients were treated with PI, IMiDs or a combination of agents. In accordance with the reports by Paul [18], we did not find any impact of bone marrow fibrosis on the treatment response or survival.
The effects of bone marrow fibrosis on the outcome of patients who underwent ASHCT were reported by Suyani et al. [29]. In the study, 50 patients, including 16 MM patients who did not have fibrosis and 19 patients (16 cases were MM) with bone marrow fibrosis, received ASHCT. They found that the presence of bone marrow fibrosis did not affect engraftment after transplantation or OS. Although the number was limited, we drew the same conclusion that the presence of bone marrow fibrosis did not affect the mobilization of hematopoietic stem cells, engraftment after transplantation or survival.
It is unclear whether the bone marrow fibrosis observed in MM patients is reversible with treatment. Babarovic et al. reported that among the 22 subjects who had bone marrow fibrosis at diagnosis, regression of fibrosis grade was observed in 8 patients, whereas 9 patients showed progression of fibrosis, and these patients did not respond to therapy [17]. In our study, we found that 5 of 7 patients who underwent a second bone marrow biopsy showed regression of bone marrow fibrosis, and it should be noted that a patient with regression of fibrosis achieved stringent complete response (sCR) at the second examination. Altogether, it is speculated that bone marrow fibrosis shown in MM could be resolved after treatment for MM.
The most important and novel finding of our present study is that the presence of bone marrow fibrosis was correlated with EMM at diagnosis. We statistically confirmed the higher incidence of extramedullary disease in MM patients with bone marrow fibrosis. In solid cancer, progression of the fibrotic tumor microenvironment is one of the important steps for metastasis of cancer cells [4]. Together with this widely accepted notion, our present observation suggests that the development of bone marrow fibrosis is also important for extramedullary dissemination of MM cells.
Although we do not have a precise biological explanation for the association between the progression of EMM and bone marrow fibrosis at present, we focused our attention on CD49e. CD49 is also known as very late antigen 5 (VLA5), a member of the integrin family of adhesion molecules composed of a5 integrin and b5 integrin. In the present study, we observed that the CD49e-positive MM cell population was decreased in patients with bone marrow fibrosis. In accord with our observation, previous studies have demonstrated that MM cells derived from EMM patients or PCL showed reduced expression of cell surface molecules, including CD49e [30, 31]. Based on this notion, it would be hypothesized that downregulation of adhesion molecules, including CD49e, may enhance the escape of MM cells from the bone marrow microenvironment, leading to the development of EMM. VLA5 is involved in a variety of types of pathological fibrosis. Hairy leukemia cells express abundant VLA5, which interacts with fibronectin, one of the major components of the ECM, and enhances assembly of the protein [32]. Matsuura and colleagues demonstrated that JAK2V617F-positive megakaryocytes expressed higher levels of cell surface VLA5. High levels of VLA5 enhanced the interaction of megakaryocytes with fibronectin, resulting in the expansion of these cells [33]. VLA5 also mediates an interaction between cancer cells and CAFs in gastric cancer [34]. Although the above reports suggested that VLA5 positively regulates fibrosis, another study showed that downregulation of VLA5 enhanced the differentiation of pulmonary fibroblasts to myofibroblasts, an activated form of fibroblasts, leading to enhanced production of ECM in idiopathic pulmonary fibrosis [35]. These findings suggested that VLA5 might have different roles in the regulation of fibrosis depending on the type of cell or tissue. Further studies will be required to understand the biological role of CD49e in the accumulation of reticulin and collagen fibers in bone marrow in MM.
Our study has several limitations. First, the number of patients included in this study was relatively small. Second, some of the important data, including cytogenetic studies, were missing for many patients. In addition, the treatment regimens were heterogeneous in each patient. Therefore, it is difficult to draw concrete conclusions regarding the role of the presence of bone marrow fibrosis in clinical findings and the treatment outcome of MM. However, our present observation that the presence of bone marrow fibrosis may be associated with the development of EMM, including PCL, provides evidence that pretreatment evaluation of bone marrow fibrosis is important for predicting the development of EMM in MM patients. Further studies with large-scale cohorts should be performed to confirm this notion.
References
Lomas OC, Tahri S, Ghobrial IM. The microenvironment in myeloma. Curr Opin Oncol. 2020;32(2):170–75.
Furukawa Y, Kikuchi J. Molecular basis of clonal evolution in multiple myeloma. Int J Hematol. 2020;111(4):496–511. https://doi.org/10.1007/s12185-020-02829-6.
de Jong MME, Kellermayer Z, Papazian N, Tahri S, Hofste op Bruinink D, Hoogenboezem R, et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat Immunol. 2021;22(6):769–80. https://doi.org/10.1038/s41590-021-00931-3.
Boulter L, Bullock E, Mabruk Z, Brunton VG. The fibrotic and immune microenvironments as targetable drivers of metastasis. Br J Cancer. 2021;124(1):27–36. https://doi.org/10.1038/s41416-020-01172-1.
Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–62. https://doi.org/10.1111/j.1365-2141.2007.06807.x.
Abdallah Abou Z, Mohamed ES, Nicole C, Douglas T, Srdan V, Ruben M, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660–71. https://doi.org/10.3324/haematol.2015.141283.
Frassanito MA, Rao L, Moschetta M, Ria R, Di Marzo L, De Luisi A, et al. Bone marrow fibroblasts parallel multiple myeloma progression in patients and mice: in vitro and in vivo studies. Leukemia. 2014;28(4):904–16. https://doi.org/10.1038/leu.2013.254.
Wang JC, Wong C, Kao WW. Immunoreactive prolyl hydroxylase in patients with primary and secondary myelofibrosis. Br J Haematol. 1987;65(2):171–4. https://doi.org/10.1111/j.1365-2141.1987.tb02260.x.
Abbonante V, Chitalia V, Rosti V, Leiva O, Matsuura S, Balduini A, et al. Upregulation of lysyl oxidase and adhesion to collagen of human megakaryocytes and platelets in primary myelofibrosis. Blood. 2017;130(6):829–31. https://doi.org/10.1182/blood-2017-04-777417.
Leiva O, Ng SK, Matsuura S, Chitalia V, Lucero H, Findlay A, et al. Novel lysyl oxidase inhibitors attenuate hallmarks of primary myelofibrosis in mice. Int J Hematol. 2019;110(6):699–708. https://doi.org/10.1007/s12185-019-02751-6.
Abildgaard N, Bendix-Hansen K, Kristensen JE, Vejlgaard T, Risteli L, Nielsen JL, et al. Bone marrow fibrosis and disease activity in multiple myeloma monitored by the aminoterminal propeptide of procollagen III in serum. Br J Haematol. 1997;99(3):641–8. https://doi.org/10.1046/j.1365-2141.1997.4503260.x.
Ciavarella S, Laurenzana A, De Summa S, Pilato B, Chillà A, Lacalamita R, et al. u-PAR expression in cancer associated fibroblast: new acquisitions in multiple myeloma progression. BMC Cancer. 2017;17(1):215. https://doi.org/10.1186/s12885-017-3183-y.
Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2): 188356. https://doi.org/10.1016/j.bbcan.2020.188356.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98. https://doi.org/10.1038/nrc.2016.73.
Dolgikh TY, Yuliya AD, Domnikova NP, Maltseva NA, Kachesov IV. Clinical-laboratory significance of myelofibrosis in patients with multiple myeloma. Int Biomed. 2014;4(2):72–5.
Subramanian R, Basu D, Dutta TK. Significance of bone marrow fibrosis in multiple myeloma. Pathology. 2007;39(5):512–5. https://doi.org/10.1080/00313020701570038.
Babarović E, Valković T, Štifter S, Budisavljević I, Seili-Bekafigo I, Duletić-Načinović A, et al. Assessment of bone marrow fibrosis and angiogenesis in monitoring patients with multiple myeloma. Am J Clin Pathol. 2012;137(6):870–8. https://doi.org/10.1309/AJCPT5Y2JRIUUCUB.
Paul B, Zhao Y, Loitsch G, Feinberg D, Mathews P, Barak I, et al. The impact of bone marrow fibrosis and JAK2 expression on clinical outcomes in patients with newly diagnosed multiple myeloma treated with immunomodulatory agents and/or proteasome inhibitors. Cancer Med. 2020;9(16):5869–80. https://doi.org/10.1002/cam4.3265.
The International Myeloma Working G. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–57. https://doi.org/10.1046/j.1365-2141.2003.04355.x.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. https://doi.org/10.1016/S1470-2045(14)70442-5.
Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1–20. https://doi.org/10.1038/s41375-019-0660-0.
Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021;11(9):161. https://doi.org/10.1038/s41408-021-00527-y.
Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ, et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8(12):116. https://doi.org/10.1038/s41408-018-0140-1.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–9. https://doi.org/10.1200/jco.2015.61.2267.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–5. https://doi.org/10.1182/blood-2010-10-299487.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
Malhotra J, Kremyanskaya M, Schorr E, Hoffman R, Mascarenhas J. Coexistence of myeloproliferative neoplasm and plasma-cell dyscrasia. Clin Lymphoma Myeloma Leukemia. 2014;14(1):31–6. https://doi.org/10.1016/j.clml.2013.09.015.
Langseth ØO, Myklebust TÅ, Johannesen TB, Hjertner Ø, Waage A. Patterns of previous and secondary malignancies in patients with multiple myeloma. Eur J Haematol. 2021;106(4):529–36. https://doi.org/10.1111/ejh.13581.
Suyanı E, Akı SZ, Yegin ZA, Ozkurt ZN, Altındal S, Akyürek N, et al. The impact of bone marrow fibrosis on the outcome of hematopoietic stem cell transplantation. Transpl Proc. 2010;42(7):2713–9. https://doi.org/10.1016/j.transproceed.2010.05.150.
Pellat-Deceunynck C, Barillé S, Puthier D, Rapp MJ, Harousseau JL, Bataille R, et al. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 1995;55(16):3647–53.
Klimienė I, Radzevičius M, Matuzevičienė R, Sinkevič-Belliot K, Kučinskienė ZA, Pečeliūnas V. Adhesion molecule immunophenotype of bone marrow multiple myeloma plasma cells impacts the presence of malignant circulating plasma cells in peripheral blood. Int J Lab Hematol. 2021;43(3):403–8. https://doi.org/10.1111/ijlh.13387.
Burthem J, Cawley JC. The bone marrow fibrosis of hairy-cell leukemia is caused by the synthesis and assembly of a fibronectin matrix by the hairy cells. Blood. 1994;83(2):497–504.
Matsuura S, Thompson CR, Ng SK, Ward CM, Karagianni A, Mazzeo C, et al. Adhesion to fibronectin via α5β1 integrin supports expansion of the megakaryocyte lineage in primary myelofibrosis. Blood. 2020;135(25):2286–91. https://doi.org/10.1182/blood.2019004230.
Miyamoto S, Nagano Y, Miyazaki M, Nagamura Y, Sasaki K, Kawamura T, et al. Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Lett. 2021. https://doi.org/10.1016/j.canlet.2021.11.008.
Shochet GE, Brook E, Bardenstein-Wald B, Grobe H, Edelstein E, Israeli-Shani L, et al. Integrin alpha-5 silencing leads to myofibroblastic differentiation in IPF-derived human lung fibroblasts. Ther Adv Chronic Dis. 2020;11:2040622320936023. https://doi.org/10.1177/2040622320936023.
Acknowledgements
This study was supported by a research grant from the Japanese Society of Hematology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Koshiishi, M., Kawashima, I., Hyuga, H. et al. Presence of bone marrow fibrosis in multiple myeloma may predict extramedullary disease. Int J Hematol 116, 544–552 (2022). https://doi.org/10.1007/s12185-022-03373-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03373-1