Abstract
Poor graft function (PGF) is a fatal complication following hematopoietic stem cell transplantation and is influenced by multiple factors, such as donor-specific anti-HLA antibodies, a poor infused CD34+ cell count, and the donor source. Alloantibodies against human platelet antigen 15 (HPA-15) recognize platelet membrane glycoprotein CD109, which is expressed not only on platelets, but also on megakaryocytes and specific hematopoietic stem cells. HPA-15 antibodies are known to induce platelet transfusion refractoriness and neonatal alloimmune thrombocytopenia, but their effects on graft function following hematopoietic stem cell transplantation remain unknown. We encountered a case of HPA-15 mismatched cord blood transplantation with a high HPA-15b antibody titer. Prolonged PGF and megakaryocyte aplasia with sustained high-titer HPA-15b antibodies were attenuated by rituximab therapy, and rapid recovery of hematopoiesis was achieved. HPA-15-compatible platelet transfusions were highly effective for platelet recovery. Methylcellulose assays and megakaryocyte cultures revealed that patient serum inhibited in vitro hematopoietic development from patient bone marrow cells. These results suggest that HPA-15 antibodies might be a cause of PGF and that reducing the HPA-15 antibody titer might improve graft function in HPA-15 mismatched transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor graft function (PGF) is a fatal complication following hematopoietic stem cell transplantation. Recent studies have defined PGF as at least bilinear severe cytopenia (persistent thrombocytopenia ≤ 20 × 109/L, hemoglobin ≤ 70 g/L, and/or neutropenia ≤ 0.5 × 109/L) or the requirement of transfusion support after engraftment with hypocellular bone marrow and full donor chimerism, without severe graft-versus-host disease (GVHD) or disease relapse [1]. Several pre-transplant conditions are associated with PGF, including donor-specific anti-HLA antibodies, infused CD34+ cell counts, and HLA disparity between the donor and recipient. Post-transplant complications, such as cytomegalovirus (CMV) infections, and myelosuppressive drugs, including ganciclovir (GCV), also damage graft function [1]. The prognosis of PGF is poor, with a mortality rate of 75%, and PGF is recognized as a severe complication following hematopoietic stem cell transplantation [2].
Alloantibodies against human platelet antigen (HPA) recognize platelet membrane glycoproteins and induce platelet transfusion refractoriness and neonatal alloimmune thrombocytopenia [3]. The HPA-15 alloantigen is localized on CD109, a glycosylphosphatidylinositol-linked glycoprotein, and HPA-15 alleles differ by a single nucleotide polymorphism, resulting in a Tyr/Ser substitution at amino acid 682 [4]. CD109 is expressed not only on platelets, but also on megakaryocytes and specific hematopoietic stem cells [5]. However, the clinical significance of HPA-15 antibodies for graft function following hematopoietic stem cell transplantation is unknown.
We herein report a relationship between a reduction in HPA-15b antibodies in a recipient of HPA-15 mismatched cord blood transplantation (CBT) and the recovery of PGF.
We also observed the inhibitory effects of patient serum on megakaryocyte expansion and erythroid colony formation.
Materials and methods
Study approval
The present study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of Rinku General Medical Center, the Japanese Red Cross Kinki Block Blood Center, and Osaka University Graduate School of Medicine.
Methylcellulose colony assay
Bone marrow samples were obtained from patient on day 733 post-transplant in clinical examination with informed consent. Patient’s serum containing high titer HPA-15b antibodies were collected before transplant and on day 110 when no cytotoxic drugs were used. Bone marrow mononuclear cells (BMNCs) were isolated by density gradient centrifugation. A total of 2 × 105 cells were incubated with 50 μL of pre-transplant patient or control serum at 37 °C for 1 h, suspended in Iscove’s modified Dulbecco’s medium-based methylcellulose medium supplemented with recombinant human (rh) stem cell factor (SCF), rh interleukin-3 (IL-3), rh IL-6, rh erythropoietin, and rh granulocyte–macrophage colony-stimulating factor (Methocult GF3434 Classic; StemCell Technologies, Vancouver, BC, Canada), and then cultured at 37 °C in a 5% CO2 incubator. After 12 days, colonies were enumerated and classified as granulocyte colony-forming units (CFU-G), macrophage colony-forming units (CFU-M), granulocyte–macrophage colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E), or mixed erythroid-myeloid colony-forming units (CFU-Mix) based on their shape and color under an inverted microscope.
Megakaryocyte culture
To evaluate the development of megakaryocytes, 1 × 106 BMNCs were incubated with 100 μL of pre-transplant patient or control serum at 37 °C for 30 min, and then cultured in a 24-well plate (2 × 105/well) with StemSpan™ SPFM (StemCell Technologies, Vancouver, BC) supplemented with rh SCF, rh IL-3, rh IL-9, rh thrombopoietin, and 20 μg/mL human low-density lipoproteins. Fresh media containing 50 μL of healthy control or patient serum were supplied to each well on days 4, 8, and 11. After 14 days, cells were collected and subjected to flow cytometric analyses.
Flow cytometry
Cultured cells were collected and incubated with a purified mouse IgG2a, κ Isotype Control (BD Pharmingen, San Diego, CA, USA) and subsequently stained with FITC anti-human CD45 (BioLegend, San Diego, CA, USA), PE anti-human CD42b (BD Pharmingen, San Diego, CA, USA), and APC anti-human CD41 (BD Pharmingen, San Diego, CA, USA). Flow cytometric analyses were performed with FACS CANTOII (BD Bioscience, San Jose, CA, USA).
Statistical analysis
All statistical analyses were performed using EZR software [6].
Case presentation
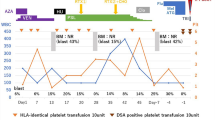
A 42-year-old nonparous woman with myelodysplastic/myeloproliferative disease, unclassifiable (MDS/MPN-U) was referred to our hospital for the introduction of CBT. She had a history of splenectomy at 8 years of age with a diagnosis of spherocytosis. After surgery, she developed transit anemia with opportunistic infections and received red blood cell (RBC) transfusions. Three years before being referred to our hospital, anemia with thrombocytosis progressed, and she required RBC transfusions every 2–3 weeks. A bone marrow (BM) examination at the local hospital revealed an increase in blasts to 5% with dysplasia of myeloid and megakaryocyte lineages, and she was diagnosed with MDS/MPN-U. Since there was no available HLA-identical donor in her family or from the Japan Marrow Donor Program, CBT was planned from a serologically 6/8 antigen matched male donor. She received fludarabine 25 mg/m2 i.v. on days − 7 to − 2, busulfan 3.2 mg/kg i.v. on days − 3 and − 2, and total body irradiation of 4 Gy in two fractions on days − 2 and − 1 as a conditioning regimen followed by CBT (nucleated cell count = 2.9 × 107 cells/kg and CD34+ cell count = 0.64 × 105 cells/kg). Prophylaxis for GVHD was cyclosporine-A and short-term methotrexate. The patient’s clinical course after CBT is shown in Fig. 1. She developed post-transfusion refractoriness for platelets in the early phase after CBT. A serological examination revealed that the patient possessed a high titer of HPA-15b antibodies without other HPA or HLA antibodies. A genotyping test showed that the recipient was HPA-15a/a and the donor was HPA-15b/b, indicating that she underwent HPA-15-mismatched CBT. Based on these results, platelet transfusions were selected from HPA-15 compatible donors as much as possible. The engraftment of neutrophils (> 0.5 × 109/L) was noted on day 21; however, the recovery of RBC and platelets was delayed, and the patient was in transfusion dependency. Grade I acute GVHD of the skin was observed but regressed without any specific treatment. The engraftment of donor cells was confirmed by the sex chromosomes of BM samples on day 103 (46 XX: 99.2%) and by a polymerase chain reaction (PCR) analysis of the short tandem repeats of peripheral T cells on day 132 (complete chimerism of the donor type), and she was diagnosed with PGF. The BM aspirate on day 103 revealed normal myeloid and erythroid lineages with severe megakaryocyte hypoplasia (Fig. 2). Her clinical course was complicated by prolonged CMV antigenemia (CMV Ag) and viral cystitis requiring intermittent treatment with foscarnet and GCV. The administration of GCV for CMV Ag on day 132 caused pancytopenia following the rapid decrease in neutrophils. A BM examination revealed severe aplasia (data not shown). She developed melena and shock on day 142, and fiberoptic colonoscopy revealed severe mucosal edema and redness at the terminal ileum, which was suggestive of post-transplant lymphoproliferative disorder (PT-LPD). The weekly administration of rituximab was initiated with the intent to treat suspected PT-LPD and reduce the HPA-15b antibody titer; however, histological findings showed the accumulation of inflammatory lymphocytes, and immunohistochemical staining and PCR with peripheral blood were negative for EB virus. Therefore, EB-LPD was unlikely. Thereafter, the number of neutrophils increased by more than 1.0 × 109/L, the number of reticulocytes was also elevated, and the patient became independent of RBC transfusions 7 weeks after the administration of rituximab. Although the HPA-15b antibody titer remained high with a transient decrease for 4 months after transplantation without the administration of rituximab, the titer was permanently decreased by its administration and became negative from 9 months after transplantation. Thereafter, thrombopoiesis promptly recovered in association with the disappearance of the HPA-15b antibody, and the number of platelets increased to 150 × 109/L within 1 month. A BM examination on day 363 showed normocellularity, and a large number of mature megakaryocytes were noted (Fig. 2).
Clinical course of the patient and transition of the HPA-15 antibody index. The sample was considered to be positive for HPA-15 alloantibodies when the signal-to-noise (S/N) ratio was > 2.0. The black triangle denotes random or HPA-15-incompatible platelet transfusions, while the white triangle indicates HPA-15-compatible platelet transfusions
Histology and immunohistochemical analysis of bone marrow. (magnification × 100). a, b Hematoxylin–eosin staining (H&E stain) showed slightly hypoplastic bone marrow with myeloid and erythroid lineages, and immunohistochemical (IHC) staining showed CD42b-positive megakaryocytes were not observed on day 103. c, d Normocellular bone marrow with H&E staining, and yellow arrows indicate increased CD42b-positive megakaryocytes with IHC staining on day 363
To evaluate the clinical impact of HPA-15b antibodies, we assessed the efficacy of HPA-15-compatible platelet transfusions. The patient received 76 platelet transfusions in the 9 months after transplantation. Among these transfusions, the 24-h corrected count increment (CCI-24 h) was calculated in eight HPA-15-compatible, 17 random, and one HPA-15-incompatible transfusions, and the results obtained are summarized in Table 1. CCI-24 h was significantly higher in HPA-15-compatible platelet transfusions than in HPA-15-incompatible or random transfusions; however, no significant differences were observed in platelet transfusion refractoriness (CCI-24 h less than 4500/μL).
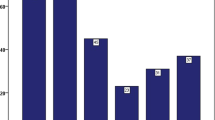
To investigate the effects of HPA-15 antibodies on hematopoiesis, we performed a colony formation assay with or without patient serum containing HPA-15 antibodies using post-transplant bone marrow samples (Fig. 3A). The addition of patient serum inhibited myeloid and erythroid colony formation, particularly the growth of BFU-E. In addition, the growth of megakaryocytes was significantly impaired in the presence of patient serum (Fig. 3B, C).
Inhibitory effects of patient serum on megakaryocyte expansion and colony formation. A BMNCs were subjected to methylcellulose colony formation assays to evaluate the growth of CFU-G/M/GM, BFU-E, and CFU-Mix (n = 3 in each). B BMNCs were subjected to a megakaryocyte differentiation assay. Representative flow cytometry plots are shown. Each number indicates the percentage of the CD41+CD42b+ mature megakaryocyte population within the CD45+ fraction. C The percentage of the CD41+CD42b+ mature megakaryocyte population are shown (n = 4 in each). Statistical analyses were conducted using a standard Student’s t test. Data are shown as the mean ± SEM. Significant differences are represented by asterisks (P < 0.05)
Discussion
HPA-15 antibodies target CD109, which is reportedly expressed not only on platelets, but also on megakaryocytes and specific hematopoietic stem and progenitor cells [5]. This expression profile has led to the hypothesis that alloantibodies against HPA-15 might dysregulate hematopoietic recovery after HPA-15 mismatched hematopoietic stem cell transplantation. In the present case, prolonged thrombocytopenia was sustained for more than 8 months after CBT, but rapidly recovered with the growth of megakaryogenesis along with a decrease in the HPA-15 titer thereafter. In the in vitro study using bone marrow samples, we also observed the strong inhibitory effects of patient serum on megakaryocyte expansion and erythroid colony formation. Although it is important to consider the damaging effects of viral infections and myelosuppressive drugs on graft function, HPA-15 antibodies might also be a possible cause of PGF.
We assessed the efficacy of HPA-15-compatible platelet transfusions to evaluate the clinical significance of HPA-15b antibodies. HPA-15b incompatibility had a significant impact on CCI-24 h, which indicates that HPA-15 antibodies exert inhibitory effects in vivo. However, we did not observe an increase in PRT in HPA-15-incompatible or random transfusions. Platelets have been shown to express low levels of CD109 [4], which might contribute to the moderate impact of HPA-15 antibodies on platelet recovery. However, this is not consistent with our previous findings showing the minor impact of HPA-15 alloantibodies on platelet transfusions [7]. Anatomic asplenia due to surgical splenectomy might have influenced the efficacy of platelet transfusions in the present case.
To the best of our knowledge, this is the first case report to show a change in the HPA-15 antibody titer following hematopoietic stem cell transplantation. Difficulties are generally associated with the detection of HPA-15 antibodies, with only a few laboratories successfully achieving this [8]. Using the CL-MR-MAIPA method [9], we accurately detected the HPA-15 antibody titer for a long time. It is important to note that the HPA-15 antibody titer transiently decreased soon after CBT. HPA-15 antibodies were previously shown to be reduced by HPA-incompatible platelet transfusions [7], suggesting that antibody absorption with HPA-15 random transfusion might influence the decrease in HPA-15 antibodies in the early phase after transplantation.
HPA-15 genotyping in the Japanese population revealed gene frequencies of 0.551 and 0.449 for HPA-15a and -15b, respectively, and the percentage of HPA-15 antibodies in heavily transfused patients was reported to be 2.3% (0.66% for HPA-15a and 1.64% for HPA-15b) [10]. Since the incidence of PGF is 5.18% [2], HPA-15 alloantibodies might contribute to PGF, particularly in HPA-15b cases.
The limitations of the present study are that it is a case report and lacks assessments of the direct effects of HPA-15 antibodies for hematopoietic stem and progenitor cells.
In conclusion, we herein report a case of HPA-15 mismatched CBT that suggests a relationship between the emergence of HPA-15 antibodies and poor graft function. Further evaluations with a large number of patients and a functional analysis of HPA-15 antibodies for hematopoietic stem and progenitor cells are needed.
References
Chen J, Wang H, Zhou J, Feng S. Advances in the understanding of poor graft function following allogeneic hematopoietic stem-cell transplantation. Ther Adv Hematol. 2020;11:1–13.
Zhao Y, Gao F, Shi J, Luo Y, Tan Y, Lai X, et al. Incidence, risk factors, and outcomes of primary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1898–907.
Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia: perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus. 2015;13(3):380–90.
Schuh AC, Watkins NA, Nguyen Q, Harmer NJ, Lin M, Prosper JY, et al. A tyrosine703serine polymorphism of CD109 defines the Gov platelet alloantigens. Blood. 2002;99:1692–8.
Murray LJ, Bruno E, Uchida N, Hoffman R, Nayar R, Yeo EL, et al. CD109 is expressed on a subpopulation of CD34+ cells enriched in hematopoietic stem and progenitor cells. Exp Hematol. 1999;27:1282–94.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Lewin A, Al Khan S, Beaudin L, Meilleur L, Clarke G, Richard L. Report on the 19th International Society of Blood Transfusion Platelet Immunology Workshop 2018. Vox Sang. 2020;115:767–82.
Hayashi T, Amakishi E, Matsuyama N, Yasui K, Furuta RA, Hori Y, et al. Detection of antibodies against human platelet antigens 15a and 15b by using a cell line panel. Br J Haematol. 2010;151:402–4.
Inoue H, Sakamoto R, Nishimiya H, Sakamoto H, Terasu S, Aminaka R, et al. Minor impact of patient alloantibodies against human platelet antigen (HPA)-15 in the effectiveness of platelet transfusion: a pilot study. Transfusion. 2021;61:738–43.
Matsuhashi M, Tsuno NH, Sone S, Mishima Y, Nagura Y, Watanabe-Okochi N, et al. The role of alloantibodies against human platelet antigen-15 in multiply platelet transfused patients. Transfusion. 2014;54:1093–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yasumi, M., Yokota, T., Endo, T. et al. Relationship between donor-specific HPA-15 antibodies and poor graft function in HPA-15 mismatched cord blood transplantation. Int J Hematol 115, 753–758 (2022). https://doi.org/10.1007/s12185-022-03286-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03286-z