Abstract
A 43-year-old woman was referred to our department for hematopoietic stem cell transplantation for acute myeloid leukemia, as she failed to achieve remission following induction therapy. Umbilical cord blood transplantation was initially planned; however, multiple anti-human leukocyte antigen (HLA) antibodies with a mean fluorescence intensity of over 10,000 were detected, and optimal umbilical cord blood could not be obtained. The plan was then switched to peripheral blood stem cell transplantation (PBSCT) from the patient’s son, who had a 5/8 HLA haploidentical match. However, the patient had donor-specific antibodies against the donor’s HLA-B 0702 and HLA-C 0702. To address this issue, after rituximab therapy, the patient was given platelet transfusions from B0702- and C0702-positive donors on day − 1 and day 0, and immunoglobulin on day 0, followed by PBSCT. Donor-specific antibodies decreased by over 90%, and engraftment was confirmed on day 13. Since then, the patient has remained relapse-free and healthy. This case suggests that appropriate management of donor-specific antibodies can enable safe transplantation, even in donors who test positive for these antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Donor selection for allogeneic hematopoietic stem cell transplantation (HSCT) is based on human leukocyte antigen (HLA) matching between donors and patients. Recently, in cases where an HLA-matched donor is unavailable, HLA-mismatched donors, including haploidentical-related donors or umbilical cord blood, are increasingly being chosen. Anti-HLA antibodies, targeting class I/II HLA antigens are formed after exposure to different HLA antigens through blood transfusion or childbirth. The presence of donor-specific anti-HLA antibodies (DSA) has been associated with reduced engraftment rates, attributed to antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity [1]. The impact of DSA on HSCT outcomes has been documented across diverse transplantation settings, including umbilical cord blood transplantation [2], unrelated bone-marrow transplantation [3], and HLA–haploidentical transplantation [4]. If a patient has a broad type of anti-HLA antibody and a DSA-negative donor cannot be identified, a DSA-positive donor must be selected. Strategies for desensitization therapy aimed at reducing DSA antibody titers, such as rituximab, plasma exchange, intravenous high-dose immunoglobulin therapy (IVIg), bortezomib, daratumumab, and DSA-specific platelet transfusion have been reported; however, their therapeutic management has not been established [5]. We report the case of a patient with high-titer DSA who was treated with a combination therapy, resulting in an effective reduction of DSA titers and successful engraftment.
Case presentation
A 43-year-old female presented with complaints of skin rash, abdominal discomfort, and decreased appetite, which were initially observed by a previous physician. Blood tests revealed peripheral blood leukocytosis, anemia, and thrombocytopenia. Bone-marrow examination revealed 95% blast cells, leading to a diagnosis of acute myeloid leukemia (AML). Induction therapy with daunorubicin plus cytarabine and venetoclax plus azacitidine was administered, but resulted in treatment failure with no achievement of remission. Subsequently, the patient was referred to our department for HSCT. Upon assessment in our department, bone-marrow examination revealed 36% blast cells. In addition, flow cytometry showed positivity for myeloid markers, namely, CD13, CD33, CD117, and MPO, along with B-cell lineage markers CD19 and cyCD79a. Based on these findings and revised scoring system for the diagnosis of acute mixed phenotype leukemia from European Group for the Immunologic Classification of Leukemia, the patient was rediagnosed with acute mixed-lineage leukemia (mixed-phenotype acute leukemia, MPAL).
Early HSCT was required because of uncontrolled leukemia. For donor selection, umbilical cord blood, a relatively accessible donor source, was initially planned. Her HLA typing was as follows: HLA-A 01:01/24:02, HLA-B 37:01, HLA-C 06:02/14:02, HLA-DRB1 10:01. When searching for a suitable donor, the patient was found to have 1 type of anti-HLA antibody against HLA-A, 36 types against HLA-B, and 1 type against HLA-Cw. No anti-HLA antibodies were detected against HLA-DR, DPB1, and DQ. These results made it difficult to identify the optimal umbilical cord blood match. Consequently, we concluded that an ideal umbilical cord blood unit was not available.
Subsequently, her 25-year-old son emerged as a donor candidate. His HLA typing was as follows: HLA-A 01:01/24:02, HLA-B 07:02/37:01, HLA-C 06:02/07:02, HLA-DRB1 01:01/10:01. DSA was performed by multiplex assay with mean fluorescence intensity (MFI) of 2385 and 5061 for HLA-B 07:02 and HLA-C 07:02, respectively. However, finding a more suitable donor was challenging because of these results. Therefore, the decision was made to proceed with a peripheral blood stem cell transplantation (PBSCT) using the patient’s son as the donor after reducing the MFI levels.
The patient received venetoclax (50 mg/body (day 1), 100 mg/body (day 2), 200 mg/body (day 3-) plus azacitidine (75 mg/m2, days 1–5) therapy for the uncontrolled leukemia; however, owing to an increase in blast cells, the treatment was deemed ineffective. Given the positivity of lymphoid markers on flow cytometry, the introduction of steroids and rituximab (375 mg/m2, day 20) resulted in a decrease in the number of blasts. As a chemotherapy approach for the lymphoid tumor, R-CHOP therapy (including rituximab, cyclophosphamide, adryamicin, vincristine, and prednisolone) and clofarabine (30 mg/m2, days 44–47) were administered, but disease remission was not achieved. In the non-remission state, a conditioning regimen was conducted with fludarabine (30 mg/m2, day − 5 to day − 1), melphalan (40 mg/m2, day − 2, day − 1), and total body irradiation (2 Gy × 2, day 0). Subsequently, allogeneic PBSCT was performed using the haploidentical DSA-positive donor (see Fig. 1).
Clinical course before transplantation. WBC, white blood cell; Plt, platelet; AZA, azacitidine; VEN, venetoclax; HU. Hydroxyurea; PSL, prednisolone; RTX, rituximab; Clo, clofarabine; Flu, fludarabine; Mel, melphalan; ATG, anti-thymocyte globulin; PBSCT, peripheral blood stem cell transplantation; IVIg, intravenous immunoglobulin; TBI, total body irradiation; BM, bone marrow; NR, non-remission
Allogeneic PBSCT, utilizing 7.7 × 106/kg of CD34 cells, was conducted; 20 units of DSA-positive platelets were administered the day before transplantation to reduce DSA titers, followed by an infusion of 25,000 mg of immunoglobulin and 15 units of DSA-positive platelets on the day of transplantation. On the second day after the transplantation, the MFI of antibodies against HLA-C and HLA-B decreased by 92.1% and 96.8%, respectively (see Fig. 2).
To prevent GVHD, we administered of antithymocyte globulin (1.25 mg/kg, day − 2 to day − 1) along with tacrolimus and short-term methotrexate (day 1: 10 mg/m2; day 3, 6, and 11: 7 mg/m2). Lenograstim (5 mg/kg) was administered from day 5 to day 14. Neutrophil engraftment was confirmed on day 13 and platelet engraftment was confirmed on day 16. On day 28, a bone-marrow examination revealed complete donor chimerism by short tandem repeat PCR, confirming the achievement of remission. On day 22, acute GVHD of the skin developed, and by day 24, it worsened to Stage 3, prompting the introduction of methylprednisolone at a dose of 2 mg/kg/day. However, the symptoms progressed to Stage 4 (Grade IV), and in addition, gut Stage 1 was observed. Consequently, on day 28, mesenchymal stem cells were administered.
Following treatment initiation, both skin and digestive symptoms gradually improved. Although the patient’s physical condition and activities of daily living were significantly impaired because of GVHD and prolonged hospitalization, diligent rehabilitation led to an improvement in ambulation with the use of a cane. After the overall condition stabilized, maintenance therapy with azacitidine was initiated, and the patient was discharged on day 93. Presently, the patient continues outpatient maintenance therapy and maintains remission of the underlying condition.
Discussion
In the case of the patient possessing a broad spectrum of anti-HLA antibodies and high-titer DSA, multiple desensitization therapies were administered prior to PBSCT from a related donor. A high antibody titer immediately decreased after PBSCT, and donor engraftment was promptly confirmed. After HSCT, the patient developed severe GVHD, which was controlled using steroid plus mesenchymal stem cell therapy. The uncontrolled leukemia was in remission. The patient recovered well and remained relapse-free for 10 months after HSCT.
DSA can induce engraftment failure via complement- and antibody-dependent stem cell injury. Ciurea et al. [6] and Yoshihara et al. [3] demonstrated a higher incidence of engraftment failure in patients with a DSA MFI exceeding 5000, suggesting the necessity of reducing antibody titers before allogeneic bone-marrow transplantation in patients with antibody titers above 5000 to prevent engraftment failure.
Several studies have reported on desensitization therapy for DSA and its significance in engraftment. Reports have attempted to reduce MFI through the use of bortezomib and daratumumab in desensitization therapy [7,8,9]. However, there is few reports on performing allogeneic transplantation after the reduction of MFI, leaving the impact of these interventions on allogeneic transplantation unclear. In terms of indications for allogeneic transplantation, studies have reported on the impact of rituximab administration, plasma exchange (PE), IVIg, DSA-positive platelets, or their combination on reducing MFI and influencing engraftment. Chang et al. reported a decrease in the incidence of engraftment failure in haploidentical transplantation with a single dose of rituximab leading to MFI reduction [10]. However, its effectiveness was deemed insufficient with MFI above 5000, so additional strategies may be necessary for such patients. Maneesh et al. reported a case of successful PBSCT with significant MFI reduction achieved through 23 PEs [11]. Reverberi et al. suggested that a single PE could remove 60–70% of intravascular IgG [12], while Britany et al. stated that 4–5 PEs are necessary for DSA removal [5], suggesting that PE is a viable strategy in facilities where it can be safely implemented. Jinye et al. reported favorable engraftment and survival rates in 19 cases of patients with DSA MFI exceeding 5000 who underwent haploidentical transplantation after reduction of MFI with IVIg [13]. Bernd et al. demonstrated significant MFI reduction and successful engraftment with seven DSA-positive platelet transfusions [14].
Furthermore, efforts are also underway to reduce antibody titers more effectively by combining several techniques. Ciurea et al. reported that when DSA had a high titer before transplantation, rituximab administration and PE were performed twice each, followed by haploidentical transplantation, resulting in successful engraftment [15]. Accordingly, Gergis et al. also reported that a combination of bortezomib, rituximab, IVIg, and PE effectively reduced the DSA titer [16]. Choe et al. reported a similar approach to desensitization therapy in patients with DSA with an MFI of 2000 or higher [17]. However, Brittany et al. pointed out that they were all single-center, open-label studies with a small number of cases [5]. Various reports have been made on desensitization therapies, but multiple factors, such as conditioning regimens, CD34-positive cell counts, and the underlying condition, influence engraftment. Therefore, despite reviewing these reports, there is no established treatment protocol. The current situation involves attempting diverse desensitization therapies, as seen in this case, acknowledging the complexity of factors influencing engraftment.
Here, DSA with MFI values of 2385 and 5061 were confirmed at the time of transfer to our center. A high rejection rate was estimated, and a strategy to reduce the high titer of antibodies was required. Given the bias of leukemia markers towards lymphoid tumors, we opted for R-CHOP therapy as a bridging therapy before transplantation, with the aim of achieving anti-tumor and desensitization effects. The MFI 3 days before transplantation was lower than that at the time of transfer, but the effect was limited. Considering the existing reports, the effectiveness of rituximab appears to be limited, and it is believed to have minimal contribution to the reduction of antibody titers. PEs could not be performed because of persistent thrombocytopenia.
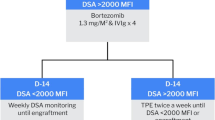
Transfusions of IVIg and DSA-positive platelets were administered immediately prior to allogeneic PBSCT to desensitize DSA titer. Particularly, after DSA-positive platelet transfusion, a significant reduction in MFI was observed, suggesting its effectiveness in desensitization (Fig. 2). In addition, we chose peripheral blood stem cells as the donor source to avoid the limitation of cell numbers associated with cord blood, which resulted in sufficient CD34 cell numbers and is believed to have had a favorable impact on engraftment. The detailed management of high-titer antibodies is unknown, it is possible that with an MFI around 5000, and if a sufficient number of CD34-positive cells can be ensured, engraftment might be achievable with DSA-positive platelets and IVIg alone (Fig. 3).
The treatment management for patients with high-titer DSA positivity has not yet been firmly established. It has been demonstrated that through strategies such as pre-transplantation chemotherapy, IVIg administration, DSA-positive platelet transfusions, and careful donor selection, it is possible to overcome this challenge. However, many uncertainties remain regarding when to intervene and the extent to which antibody titers can be predicted to decrease as a result of these interventions. Further accumulation of cases is required for a more comprehensive understanding.
Data availability
All data generated in this study are included in this manuscript. Further inquiries can be directed to the corresponding author.
References
Hanajiri R, Murata M, Sugimoto K, Murase M, Sakemura R, Goto T, et al. Integration of humoral and cellular HLA-specific immune responses in cord blood allograft rejection. Bone Marrow Transpl. 2015;50(9):1187–94.
Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46.
Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transpl. 2012;47(4):508–15.
Spellman S, Bray R, Bronson SR, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–8.
Brittany F, Huang Y, Peedin A, Gergis U. The impact of HLA donor-specific antibodies on engraftment and the evolving desensitization strategies. Bone Marrow Transpl. 2022;57(4):526–31.
Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2015;21:1392–8.
Everly MJ, Everly JJ, Susskind B, Arend LJ, Alloway RR, Chaudhury PR, et al. Proteasome inhibition reduces donor-specific antibody levels. Transpl Proc. 2009;41(1):105–7.
Miki H, Ozaki S, Tanaka O, Lee E, Takimoto T, Watanabe H, et al. Marked improvement of platelet transfusion refractoriness after bortezomib therapy in multiple myeloma. Int J Hematol. 2009;89:223–6.
Ibrahim U, Keyzner A. Daratumumab for donor-specific anti-HLA antibody desensitization in a case of HLA-mismatched allogeneic stem cell transplantation. Hematol Transfus Cell Ther. 2023;45(4):510–2.
Chang YJ, Xu LP, Wang YU, Zhang XH, Chen H, Chen YH, et al. Rituximab for desensitization during HLA-mismatched stem cell transplantation in patients with a positive donor-specific anti-HLA antibody. Bone Marrow Transpl. 2020;55(7):1326–36.
Misra MK, Xin JJ, Brown NK, Weidner JG, Upchurch RL, Bishop MR, et al. Effective desensitization for a strong donor-specific HLA antibody in a case of HLA-mismatched allogeneic hematopoietic cell transplantation. HLA Immune Response Genet. 2019;94(3):307–11.
Reverberi R, Reverberi L. Removal kinetics of therapeutic apheresis. Blood Transfus. 2007;5(3):164–74.
Zhu J, Wang Q, Liu Y, Dong Y, Liang Z, Yin Y, et al. High-Dose immunoglobulin Intervention as an effective and simple strategy for donor specific anti-HLA antibody desensitization in haploidentical transplant. Int Immunopharmacol. 2023;120:110299.
Spriewald BM, Bach C, Zingsem J, Strobel J, Winkler J, Mackensen A, et al. Depletion of donor-specific anti-HLA A2 alloantibodies in a hematopoietic cell transplant recipient using directed mismatched platelet transfusions. Bone Marrow Transpl. 2018;53(6):791–4.
Ciurea SO, Lima MD, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88(8):1019–24.
Gergis U, Mayer S, Gordon B, Mark T, Pearse R, Shore T, et al. A strategy to reduce donor-specific HLA Abs before allogeneic transplantation. Bone Marrow Transpl. 2014;49:722–4.
Choe H, Gergis U, Phillips A, Hsu JM, Sharma V, Shore TB, et al. Bortezomib and immune globulin desensitization for donor-specific HLA antibodies in haplo-cord stem cell transplantation. Transpl Cell Ther. 2017;23(3):S265.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HN reports honoraria from Novartis and Daiichi-Sankyo, outside the submitted work. TT reports honoraria from Pfizer, Otsuka, MSD, Abbvie, Chugai, Asahi Kasei-Pharma, and Astellas, outside the submitted work. HN reports grant/research support from Daiichi-Sankyo, Cellgene, Chugai, Nihon-Shinyaku, outside the submitted work. HN reports scholarship from Astellas, Asahi Kasei-Pharma, Chugai, Takeda, Pfizer, and Eisai, outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
New findings: In hematopoietic stem cell transplantation, when the recipient has anti-HLA antibodies, especially donor-specific antibodies (DSA), desensitization therapy is required to reduce their titers, but this strategy has not yet been established. We have experience with a recipient with high DSA titers who was successfully treated with rituximab, immunoglobulin, and DSA-positive platelets to reduce titers and achieve successful engraftment. This suggests a strategy to effectively reduce DSA titers.
About this article
Cite this article
Katsuki, K., Tachibana, T., Izumi, A. et al. Acute mixed-lineage leukemia treated with desensitization therapy prior to HLA–haploidentical transplantation with high donor-specific antibodies. Int J Hematol 120, 256–261 (2024). https://doi.org/10.1007/s12185-024-03775-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-024-03775-3