Abstract
Two gray γ-irradiation is a widely employed basic module for total body irradiation (TBI) in allogeneic hematopoietic cell transplantation (HCT). The effects of γ-irradiation on hematopoietic and immune cells have been well investigated, but its effects on the bone marrow microenvironment (BMM) are unknown. Given the crucial contribution of mesenchymal/stromal stem cells (MSCs) in the BMM to hematopoiesis and osteogenesis, we investigated whether γ-irradiation affects the hallmark characteristics of human bone marrow-derived MSCs (BM-MSCs). Expansion of 2 Gy γ-irradiated BM-MSCs was delayed but eventually recovered. Colony formation and osteogenic, adipogenic, and chondrogenic differentiation capabilities of these cells were extensively suppressed. Irradiation of BM-MSCs did not affect the expansion of CD34 + hematopoietic stem and progenitor cells or production of CD11b + mature myeloid cells in co-cultures. However, it reduced production of CD19 + B-cells, as well as expression of CXCL12 and interleukin-7, which are essential for B-cell lymphopoiesis, in 2 Gy γ-irradiated BM-MSCs. Collectively, colony formation, osteogenic differentiation, and B-cell lymphopoiesis-supportive capabilities of γ-irradiated BM-MSCs were reduced. These effects may predispose survivors receiving HCT with TBI to defective bone formation and a perturbed humoral immune response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total body irradiation (TBI) has been applied as a conditioning regimen in allogeneic hematopoietic cell transplantation (HCT). Early research of bone marrow transplantation demonstrated that splitting TBI into multiple smaller doses reduces toxicity and improves outcomes in comparison with delivering a single large dose [1, 2]. In standard myeloablative conditioning, six fractions of 2 Gy/fraction are usually applied in the cyclophosphamide-TBI 12 Gy regimen [3]. One or two fractions of 2 Gy/fraction are applied in the fludarabine-TBI 2 or 4 Gy reduced-intensity conditioning regimen [4]. Accordingly, 2 Gy γ-irradiation is a widely employed basic module for TBI in the current clinical setting.
HCT has greatly contributed to the growing number of patients who have been cured of their original diseases. However, complications such as immune suppression, osteoporosis, and lung injury are important clinical issues in survivors who have undergone HCT [5]. Several factors, including the type of graft source, the presence of chronic graft-versus-host disease, and the use of TBI, are associated with these complications [6]. Whereas it is well-recognized that γ-irradiation in TBI allows donor hematopoietic cells to engraft in bone marrow by eliminating malignant cells and affecting immune cells, the effects of γ-irradiation on the bone marrow microenvironment (BMM) have been poorly investigated. In the BMM, mesenchymal stromal/stem cells (MSCs) contribute not only to regulation of hematopoiesis and the immune response but also to bone homeostasis [7]. Previous studies demonstrated the effects of γ-irradiation on bone marrow-derived MSCs (BM-MSCs) [8, 9]. These studies examined doses as low as 0.1 Gy emitted from natural sources such as minerals in the ground, or doses > 30 Gy emitted from man-made sources such as x-rays, which are used for medical examination and depletion of nucleated cells from blood products before transfusion [10]. Such low-/high-dose irradiation is not employed for medical treatment. The differentiation- and hematopoiesis-supportive characteristics of human BM-MSCs immediately after γ-irradiation at the reduced dose of 2.5 Gy were recently demonstrated [11]. However, the characteristics of these cells in the acute phase might not necessarily reflect their phenotypes at steady state in the late phase. This study explored the impact of widely employed 2 Gy γ-irradiation on the expansion, differentiation, colony formation, and hematopoiesis-related characteristics of human BM-MSCs in the post-acute phase by comparing these cells with human BM-MSCs exposed to 4 or 12 Gy γ-irradiation.
Materials and methods
Antibodies
A phycoerythrin-conjugated mouse antibody against human CD34 and a fluorescein isothiocyanate-conjugated mouse antibody against CD45 were purchased from BD Pharmingen (San Diego, CA). Phycoerythrin-conjugated mouse antibodies against human CD19 and CD11b were purchased from eBioscience/Thermo Fisher Scientific (San Diego, CA). A mouse antibody against runt-related transcription factor 2 (Runx2), and rabbit antibodies against alkaline phosphatase (ALP) and fatty acid-binding protein 4 (FABP4), were purchased from Abcam (Cambridge, UK). Rabbit antibodies against osteocalcin (OCN) and peroxisome proliferator-activated receptor γ (PPARγ) were purchased from Millipore/Merck Millipore (Burlington, MA) and Cell Signaling Technology (Danvers, MA), respectively. A rabbit antibody against β-actin and a mouse antibody against GAPDH were purchased from Sigma-Aldrich (St. Louis, MO) and Chemicon/Merck Millipore (Burlington, MA), respectively.
Isolation of human BM-MSCs
Bone marrow of multiple healthy adults aged 21–44 years (#4362, #4615, and #4641) was purchased from AllCells (Emeryville, CA). Human BM-MSCs were isolated from bone marrow according to our previously published methods [12, 13]. In brief, a single-cell suspension of 1 × 106 bone marrow mononuclear cells was seeded into a 15 cm culture dish. Adherent cells were cultured in advanced-minimal essential medium (Invitrogen/Thermo Fisher Scientific, Waltham, MA) supplemented with 5% fetal bovine serum (Invitrogen/Thermo Fisher Scientific), 100 µM ascorbic acid (Wako Pure Chemicals Industries, Osaka, Japan), 2 mM l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Gibco/Thermo Fisher Scientific, Waltham, MA). Primary cultures were passaged to disperse colony-forming cells (passage 1). Cells at passage 3 were used as BM-MSCs in this study. Before experiments, flow cytometric analysis was performed to confirm that these cells fulfilled the criteria for human MSCs [14].
Expansion and differentiation assays of γ-irradiated BM-MSCs
For the expansion assay, BM-MSCs (0.5 × 105) were prepared in a 10 cm cell culture dish. On the next day, these cells were exposed to 0, 2, 4, or 12 Gy γ-irradiation (Cesium-137) at a rate of 0.8 Gy/min using a Gammacell Irradiator (Best Theratronics, Ontario, Canada; day 0). The media were changed twice per week. The viable cell number was determined on days 7, 14, 21, and 28 by trypan blue dye staining until cells were almost confluent. The in vitro adipogenic, chondrogenic, and osteogenic differentiation assays were performed according to our published procedures [12, 15]. Expression of adipogenesis-associated molecules and lipid deposition in γ-irradiated BM-MSCs were evaluated after 2 weeks of culture by immunoblotting analysis and Oil Red O staining, respectively. Cartilage matrix deposition was evaluated after 4 weeks of culture by Alcian blue staining. Expression of osteogenesis-associated molecules and mineralization in γ-irradiated BM-MSCs were evaluated after 2 weeks of culture by immunoblotting analysis and Alizarin Red S staining, respectively.
Co-culture of human CD34+ cells with γ-irradiated human BM-MSCs
Human CD34+ cells were isolated from umbilical cord blood using anti-CD34 immunomagnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the enriched CD34+ cell population was confirmed by flow cytometric analysis using a phycoerythrin-conjugated antibody against human CD34. For the CD34+ cell expansion assay, human BM-MSCs were seeded into a 24-well culture plate at a density of 2 × 104 cells/well. On the next day, the cells were γ-irradiated at 0, 2, 4, or 12 Gy. After a further 2 days, CD34+ cells (1.5 × 103 cells/well) were applied onto the γ-irradiated BM-MSCs. After 10 days of co-culture, the numbers of CD34+ and CD11b+ cells among expanded hematopoietic cells were examined by flow cytometric analysis as previously described [15, 16]. For the B-cell production assay, CD34+ cells were co-cultured with γ-irradiated BM-MSCs in the presence of 10 ng/mL stem cell factor (SCF) (Wako Pure Chemicals Industries) and 5 ng/mL fms-related tyrosine kinase 3 (Wako Pure Chemicals Industries) as previously described [17]. Half the media was changed twice per week. After 28 days of co-culture, the number of CD19+ cells among the expanded hematopoietic cells was examined by flow cytometric analysis. The study protocol was approved by the ethics committee of Kyoto University.
Colony-forming unit fibroblast assay
A total of 1 × 106 bone marrow mononuclear cells was seeded into a 10 cm culture dish and cultured for 10–14 days. Then, adherent cells were washed twice with phosphate-buffered saline, fixed with 2% paraformaldehyde, and stained with 0.1% toluidine blue O. Aggregates containing > 50 cells were counted as a colony.
Immunoblot analysis
Cell lysates were boiled in sodium dodecyl sulfate sample buffer, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. Primary antibodies against adipogenic markers (PPARγ and FABP4), osteogenic markers (Runx2, ALP, and OCN), and loading controls (β-actin and GAPDH) were used. Immunoreactive proteins were detected using horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (GE Healthcare, Little Chalfont, UK) and visualized using enhanced chemiluminescence or enhanced chemiluminescence prime kits (GE Healthcare).
Quantitative real-time PCR
Total RNA was extracted from γ-irradiated BM-MSCs using a QIAamp RNA Blood Mini Kit (Qiagen Japan, Tokyo, Japan). cDNA was prepared using a PrimeScript RT reagent kit (Perfect Real-Time; Takara Bio Inc., Shiga, Japan). Real-time PCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems, Carlsbad, CA) and a Universal ProbeLibrary (Roche Applied Science, Upper Bavaria, Germany). Gene expression levels were normalized to the mRNA level of GAPDH. All samples were analyzed in duplicate. The following primer sets and universal probes were used: CXCL12, ccaaactgtgcccttcagat (5′-3′ forward primer), tggctgttgtgcttacttgttt (5′-3′ reverse primer), and Universal Probe #80; interleukin (IL)-7, aatggtcagcatcgatcaatta (5′-3′ forward primer), aattcattattcaggcaattgctac (5′-3′ reverse primer), and Universal Probe #56; stem cell factor, gcgctgcctttccttatg (5′-3′ forward primer), ccttcagttttgacgagagga (5′-3′ reverse primer), and Universal Probe #68; and GAPDH, agccacatcgctcagacac (5′-3′ forward primer), gcccaatacgaccaaatcc (5′-3′ reverse primer), and Universal Probe #60.
Statistical analysis
The unpaired Student’s t-test was used for analysis unless otherwise indicated. Data in bar graphs indicate the mean ± standard deviation (SD). Statistical significance is expressed as follows: *, p < 0.05; **, p < 0.01; n.s., not significant.
Results
Expansion of human BM-MSCs is delayed but eventually recovers after exposure to 2 Gy γ-irradiation
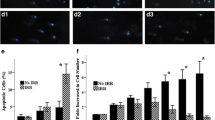
Human bone marrow mononuclear cells exposed to 2 Gy γ-irradiation formed fewer colonies than non-irradiated cells in the colony-forming unit fibroblast assay (mean value, 16.3 versus 6.6). γ-Irradiation at 4 Gy or higher suppressed colony formation to an even greater extent (Fig. 1a). We next examined whether 2 Gy γ-irradiation affected the expansion of human BM-MSCs. Non-irradiated (i.e., 0 Gy) BM-MSCs expanded and reached confluency within 2 weeks (Fig. 1b, black line). Meanwhile, 2 Gy γ-irradiation suppressed the expansion of BM-MSCs at around day 7, but expansion recovered thereafter, and cells reached confluency at day 28 (Fig. 1b, blue line). Additionally, 4 Gy γ-irradiation suppressed the expansion of BM-MSCs, and 12 Gy γ-irradiation prevented cell expansion during all 28 days of culture (Fig. 1b, orange and red lines). These results indicated that γ-irradiation suppressed the expansion of human BM-MSCs in a dose-dependent manner, but expansion recovered after 2 Gy γ-irradiation.
Colony formation and expansion of γ-irradiated human BM-MSCs. a Colony-forming unit fibroblast assay after γ-irradiation. Representative images are shown. Blue arrows indicate colonies of BM-MSCs. b BM-MSCs were prepared in a 10-cm cell culture dish. On the next day, these cells were exposed to 2 Gy γ-irradiation. The viable cell number was counted 7, 14, 21, and 28 days after γ-irradiation by trypan blue dye staining (blue line). The black, orange, and red lines indicate the viable number of BM-MSCs exposed to 0 (non-irradiated), 4, and 12 Gy γ-irradiation, respectively. The data are shown as mean ± SD of three lots of BM-MSCs
Two gray γ-irradiation impairs the osteogenic, adipogenic, and chondrogenic differentiation capabilities of human BM-MSCs
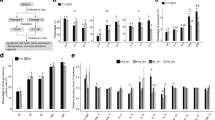
We next examined whether γ-irradiation affected the multi-differentiation capability of human BM-MSCs. These cells were γ-irradiated at 2, 4, or 12 Gy, cultured for 28 days, and then differentiated into adipogenic, chondrogenic, or osteogenic cells in vitro. Upon culture under adipo-inductive conditions, the number of lipid-laden cells assessed by Oil Red O staining, and expression of PPARγ and FABP4 assessed by immunoblot analysis, were lower among γ-irradiated BM-MSCs than among non-irradiated BM-MSCs (Fig. 2a, b). Alcian blue staining demonstrated that γ-irradiated BM-MSCs exhibited decreased cartilage matrix deposition under chondro-inductive conditions (Fig. 2c). Thus, γ-irradiation at 2 Gy or higher impaired the adipogenic and chondrogenic differentiation capabilities of BM-MSCs.
Multi-differentiation capabilities of γ-irradiated human BM-MSCs. a, b In vitro adipogenic differentiation of BM-MSCs exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. a Oil Red O staining of γ-irradiated BM-MSCs cultured in adipogenesis-inducing conditions. Representative images are shown. Yellow arrows indicate lipid deposits in cells. Scale bars = 200 μm. b Immunoblot analysis examining the expression of PPARγ and FABP4 in γ-irradiated BM-MSCs cultured in adipogenesis-inducing conditions. β-actin was used as a loading control. c In vitro chondrogenic differentiation of BM-MSCs exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. Alcian blue staining with Nuclear fast red counterstaining of γ-irradiated BM-MSCs cultured in chondrogenesis-inducing conditions. Representative images are shown. Black dotted circles indicate the areas of cartilage matrix deposition that were stained blue. Scale bars = 50 μm. d, e In vitro osteogenic differentiation of BM-MSCs exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. d Alizarin Red S staining of γ-irradiated BM-MSCs cultured in osteogenesis-inducing conditions. Representative images are shown. Calcium deposits, indicative of mineralization, were stained red. Scale bars = 200 μm. e Immunoblot analysis examining the expression of Runx2, ALP, and OCN in γ-irradiated BM-MSCs cultured in osteogenesis-inducing conditions. GAPDH was used as a loading control
With regard to the osteogenic differentiation capability, Alizarin Red S staining demonstrated that mineralization of 2 Gy γ-irradiated BM-MSCs was lower than that of non-irradiated BM-MSCs (Fig. 2d, left panels). Mineralization of BM-MSCs exposed to γ-irradiation at 4 Gy or higher seemed to be enhanced, with deposition of aggregate-like large minerals (Fig. 2d, right panels). This staining pattern was particularly apparent in cultures of 12 Gy γ-irradiated BM-MSCs. To further examine osteogenesis of γ-irradiated BM-MSCs, osteogenesis-related molecules were detected by immunoblot analysis. Expression of GAPDH, a loading control, was comparable between non-irradiated (0 Gy), 2 Gy γ-irradiated, and 4 Gy γ-irradiated BM-MSCs (Fig. 2e). The expression level of Runx2 relative to that of GAPDH was 1.0, 0.85, and 0.65 in non-irradiated (0 Gy), 2 Gy γ-irradiated, and 4 Gy γ-irradiated BM-MSCs, respectively. Similarly, the expression level of ALP relative to that of GAPDH was 1.0, 0.36, and 0.30, respectively. The expression levels of Runx2 and ALP and the loading control GAPDH were low in 12 Gy γ-irradiated BM-MSCs and, therefore, it was difficult to quantitatively compare them between 12 Gy γ-irradiated BM-MSCs and non-irradiated (0 Gy), 2 Gy γ-irradiated, and 4 Gy γ-irradiated BM-MSCs (Fig. 2e). Expression of OCN was high in 12 Gy γ-irradiated BM-MSCs (Fig. 2e). These results indicated that γ-irradiation at 2 Gy or higher altered the osteogenic differentiation capability of human BM-MSCs.
CD34+ cell-derived B-cell production is reduced in co-cultures with γ-irradiated BM-MSCs
To examine whether γ-irradiation affects the capability of human BM-MSCs to support the expansion of human hematopoietic stem and progenitor cells (HSPCs), we co-cultured CD34+ cells with BM-MSCs that had been exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. The number of CD34+ cells was comparable between the co-cultures (Fig. 3a). Similarly, the number of CD11b+ cells did not significantly differ between the co-cultures (Fig. 3b). We further examined the effects of γ-irradiation on the capability of BM-MSCs to support B-cell production. The number of CD19+ cells was significantly lower in co-cultures with BM-MSCs that had been γ-irradiated at 2 Gy or higher than in co-cultures with non-irradiated BM-MSCs (Fig. 3c, d). Collectively, expansion of CD34+ HSPCs and production of CD11b+ mature myeloid cells were unaffected by γ-irradiation of BM-MSCs even at 12 Gy, whereas production of CD19+ B-cells from CD34+ cells was reduced by γ-irradiation of BM-MSCs even at 2 Gy.
Effects of γ-irradiation on the production of hematopoietic cells and expression of hematopoiesis-associated soluble factors in human BM-MSCs. a, b Purified human CD34+ cells were co-cultured with BM-MSCs that had been exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. The numbers of CD34+ and CD11b+ cells in the expansion cultures were assessed by flow cytometric analysis. The data are shown as mean ± SD of three lots of BM-MSCs. c, d Production of B-cells from CD34+ HSPCs. Purified human CD34+ cells were co-cultured with BM-MSCs that had been exposed to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation in B-cell-inducing conditions. The number of CD19+ cells in the differentiation cultures was assessed by flow cytometric analysis. **, p < 0.05. Representative counter plots are shown in d. e–i Quantitative real-time PCR of expression of the hematopoiesis-associated soluble factors CXCL12, IL-7, and SCF in BM-MSCs at 7 days (e–g), 2 weeks (left panels of h, i), or 3 weeks (right panels of h, i) after exposure to 0 (non-irradiated), 2, 4, or 12 Gy γ-irradiation. j–l Expression of CXCL12, IL-7, and SCF in BM-MSCs from healthy donors (C) and three cases receiving TBI. The data are shown as mean ± SD of three lots of BM-MSCs. **, p < 0.01; *, p < 0.05; n.s., not significant
Hematopoiesis-related cytokine expression in γ-irradiated BM-MSCs
γ-Irradiation of BM-MSCs perturbed production of CD19+ cells from CD34+ cells in co-cultures; therefore, we investigated whether it affected the expression of B-cell hematopoiesis-associated cytokines/chemokines in BM-MSCs. mRNA expression of CXCL12 and IL-7 was significantly lower in BM-MSCs at 7 days after γ-irradiation, irrespective of the dose than in non-irradiated BM-MSCs (Fig. 3e, f). Expression of CXCL12 and IL-7 was also downregulated in BM-MSCs at 2 and 3 weeks after γ-irradiation (Fig. 3h, i). SCF expression in BM-MSCs at 7 days after γ-irradiation did not significantly differ between the γ-irradiation conditions (Fig. 3g). We further examined the expression of these molecules in BM-MSCs derived from three cases receiving 2 Gy-based TBI in allogeneic HCT. Consistent with the investigation of in vitro γ-irradiated human BM-MSCs, expression of CXCL12 was significantly reduced in two of these three cases, and expression of IL-7 was significantly reduced in all three cases (Fig. 3j, k). Expression of SCF was unaffected in all cases (Fig. 3l). This implied that the impaired B-cell production was associated with the reduced expression of CXCL12 and IL-7 in BM-MSCs.
Discussion
The primary aim of this study was to examine the impact of 2 Gy γ-irradiation employed for TBI in HCT on the hallmark characteristics of human BM-MSCs. The analyses focused on the expansion, multi-differentiation, colony formation, and hematopoiesis-supportive capabilities of cultured γ-irradiated human BM-MSCs. We showed that expansion of BM-MSCs was delayed but eventually recovered after exposure to 2 Gy γ-irradiation, while the osteogenic, adipogenic, and chondrogenic differentiation capabilities of these cells were extensively suppressed. In addition, 2 Gy γ-irradiation suppressed colony formation. Whereas the exposure of BM-MSCs to 2 Gy γ-irradiation did not affect the expansion of CD34+ HSPCs or production of CD11b+ mature myeloid cells in co-cultures, it did reduce the production of CD19+ B-cells. These results suggest that γ-irradiation affected some characteristics of human BM-MSCs, while others were unaffected. Ionizing radiation damages DNA directly and indirectly. DNA is damaged following a single exposure to radiation of even 0.1 Gy [18,19,20], and 2 Gy γ-irradiation induces about 3,000 DNA breaks per cell [21]. The DNA damage response begins within several minutes after the occurrence of DNA damage [22]. Approximately 70,000 lesions, which can arise due to oxidative damage during metabolism or base hydrolysis, are estimated to occur in each human cell per day [23]. DNA damage induced by 2 Gy γ-irradiation may be partially repaired in human BM-MSCs. Variation in susceptibility to γ-irradiation might account for the differences in its impact on the hallmark characteristics of BM-MSCs. Our results are consistent with a previous study by Preciado et al. with regard to adipogenic differentiation and Runx2 expression, but inconsistent with regard to viability, mineralization, and CXCL12 expression [11]. We speculate that these inconsistencies are due to differences in the assay systems and/or cells used. Preciado et al. used BM-MSCs immediately after γ-irradiation, while we used BM-MSCs at 7–28 days after γ-irradiation. We further described the effects of reduced-dose γ-irradiation on the hematopoiesis-supportive characteristics of BM-MSCs, and reported the previously undescribed findings that production of CD34+ HSPCs and CD11b+ mature myeloid cells was unaffected and production of CD19+ B-cells from CD34+ cells was reduced in co-cultures.
The rationale for this study is that patients receiving TBI in HCT develop multiple complications including bone defects (osteoporosis) and suppression of the humoral immune response, and that BM-MSCs contribute to the regulation of bone generation and hematopoiesis [5, 7]. As expected, both the osteogenic differentiation capability of 2 Gy γ-irradiated BM-MSCs and the production of CD19+ B-cells from CD34+ HSPCs in co-cultures with 2 Gy γ-irradiated BM-MSCs were reduced. Importantly, this reduced B-cell production occurred concomitantly with decreased expression of CXCL12 and IL-7, which are essential for B-cell lymphopoiesis, in 2 Gy γ-irradiated BM-MSCs [24, 25]. The reductions in B-cell production and CXCL12/IL-7 expression were not exacerbated when BM-MSCs were γ-irradiated at 4 Gy or higher, suggesting that the B-cell-supportive characteristics of human BM-MSCs are highly susceptible to γ-irradiation. The osteogenic differentiation capability of 12 Gy γ-irradiated BM-MSCs was particularly enhanced; Alizarin Red S staining showed strong mineralization and immunoblot analysis showed strong OCN expression in these cells, respectively. OCN is a marker of mature osteoblasts [26]. BM-MSCs exposed to high-dose γ-irradiation may have been skewed toward osteogenic differentiation with loss of their expansion and colony-forming capabilities. Runx2 promotes early osteogenic differentiation and inhibits late osteogenic differentiation, while ALP is an early osteoblast differentiation marker [27, 28]. Differentiated osteogenic cells eventually undergo apoptosis, and γ-irradiated BM-MSCs of insufficient quality does not support normal bone homeostasis.
In contrast to the reduction in CD19+ B-cells, the numbers of CD34+ and CD11b+ cells were not decreased in co-cultures with 2 Gy γ-irradiated BM-MSCs. The numbers of these cells in co-cultures with BM-MSCs γ-irradiated at 4 Gy or higher were comparable to those in co-cultures with non-irradiated BM-MSCs. These results do not contradict the clinical observation that neutrophil engraftment occurs after infusion of donor HSPCs in both reduced-intensity conditioning and myeloablative conditioning with 12 Gy TBI [29, 30]. The findings may be explained by the maintenance of SCF expression in γ-irradiated BM-MSCs, and the myeloid bias resulting from reduced responsiveness to IL-7 [31]. Another possibility is that expansion of CD34+ HSPCs is not strictly dependent on BM-MSCs, or that various factors such as fms-related tyrosine kinase 3 ligand expressed in BM-MSCs cooperatively support the generation of these cells. It is important to overcome impaired T-cell function in patients receiving HCT [32]. MSCs exist in the human thymus as well as in BM; however, their contribution to early T-cell development is limited compared with that of thymic epithelial cells [32,33,34,35]. In contrast to the well-known regulatory role of BM-MSCs in peripheral T-cell differentiation when exogenously infused, the effect of reduced-dose γ-irradiation on T-cell production from CD34+ HSPCs may be limited.
In conclusion, 2 Gy γ-irradiation affected a broad spectrum of characteristics of human BM-MSCs. The reduced colony formation, osteogenic differentiation, and B-cell lymphopoiesis-supportive capabilities of these cells may predispose survivors receiving HCT with TBI to impaired bone formation and a perturbed humoral immune response. Further studies are needed to elucidate the contribution of human BM-MSCs to the pathophysiology of multiple complications in such patients.
References
Thomas ED, Clift RA, Hersman J, Sanders JE, Stewart P, Buckner CD, et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8:817–21. https://doi.org/10.1016/0360-3016(82)90083-9.
Deeg HJ, Sullivan KM, Buckner CD, Storb R, Appelbaum FR, Clift RA, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow Transplant. 1986;1:151–7.
Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–33.
Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–8. https://doi.org/10.1200/jco.2012.45.0247.
Majhail NS, Rizzo JD. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48:1145–51. https://doi.org/10.1038/bmt.2012.258.
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–92. https://doi.org/10.1182/blood-2007-03-082933.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. https://doi.org/10.1038/nature09262.
Fekete N, Erle A, Amann EM, Fürst D, Rojewski MT, Langonné A, et al. Effect of high-dose irradiation on human bone-marrow-derived mesenchymal stromal cells. Tissue Eng Part C Methods. 2015;21:112–22. https://doi.org/10.1089/ten.tec.2013.0766.
Fujishiro A, Miura Y, Iwasa M, Fujii S, Sugino N, Andoh A, et al. Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells. Inflamm Regen. 2017;37:19. https://doi.org/10.1186/s41232-017-0049-2.
Pelszynski MM, Moroff G, Luban NL, Taylor BJ, Quinones RR. Effect of gamma irradiation of red blood cell units on T-cell inactivation as assessed by limiting dilution analysis: implications for preventing transfusion-associated graft-versus-host disease. Blood. 1994;83:1683–9.
Preciado S, Muntión S, Rico A, Pérez-Romasanta LA, Ramos TL, Ortega R, et al. Mesenchymal stromal cell irradiation interferes with the adipogenic/osteogenic differentiation balance and improves their hematopoietic-supporting ability. Biol Blood Marrow Transplant. 2018;24:443–51. https://doi.org/10.1016/j.bbmt.2017.11.007.
Iwasa M, Miura Y, Fujishiro A, Fujii S, Sugino N, Yoshioka S, et al. Bortezomib interferes with adhesion of B cell precursor acute lymphoblastic leukemia cells through SPARC up-regulation in human bone marrow mesenchymal stromal/stem cells. Int J Hematol. 2017;105:587–97. https://doi.org/10.1007/s12185-016-2169-x.
Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, et al. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells. 2018;36:434–45. https://doi.org/10.1002/stem.2759.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. https://doi.org/10.1080/14653240600855905.
Sugino N, Miura Y, Yao H, Iwasa M, Fujishiro A, Fujii S, et al. Early osteoinductive human bone marrow mesenchymal stromal/stem cells support an enhanced hematopoietic cell expansion with altered chemotaxis- and adhesion-related gene expression profiles. Biochem Biophys Res Commun. 2016;469:823–9. https://doi.org/10.1016/j.bbrc.2015.12.061.
Yao H, Miura Y, Yoshioka S, Miura M, Hayashi Y, Tamura A, et al. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32:2245–55. https://doi.org/10.1002/stem.1701.
Ichii M, Oritani K, Yokota T, Schultz DC, Holter JL, Kanakura Y, et al. Stromal cell-free conditions favorable for human B lymphopoiesis in culture. J Immunol Methods. 2010;359:47–55. https://doi.org/10.1016/j.jim.2010.06.002.
Alessio N, Del Gaudio S, Capasso S, Di Bernardo G, Cappabianca S, Cipollaro M, et al. Low-dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget. 2015;6:8155–66. https://doi.org/10.18632/oncotarget.2692.
Osipov AN, Pustovalova M, Grekhova A, Eremin P, Vorobyova N, Pulin A, et al. Low doses of X-rays induce prolonged and ATM-independent persistence of γH2AX foci in human gingival mesenchymal stem cells. Oncotarget. 2015;6:27275–87. https://doi.org/10.18632/oncotarget.4739.
Laurent A, Blasi F. Differential DNA damage signaling and apoptotic threshold correlate with mouse epiblast-specific hypersensitivity to radiation. Development. 2015;142:3675–85. https://doi.org/10.1242/dev.125708.
Tubbs A, Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–56. https://doi.org/10.1016/j.cell.2017.01.002.
Lomax M, Folkes L, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013;25:578–85. https://doi.org/10.1016/j.cell.2017.01.002.
Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA. 2003;100:12871–6. https://doi.org/10.1073/pnas.2135498100.
Dias S, Silva H Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–9. https://doi.org/10.1084/jem.20042393.
Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–34. https://doi.org/10.1016/s1074-7613(01)00185-6.
Zhou H, Choong P, McCarthy R, Chou S, Martin T, Ng KW. In situ hybridization to show sequential expression of osteoblast gene markers during bone formation in vivo. J Bone Miner Res. 1994;9:1489–99. https://doi.org/10.1002/jbmr.5650090922.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. https://doi.org/10.1016/s0092-8674(00)80258-5.
Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–8. https://doi.org/10.1097/bco.0b013e3282630851.
Cutler C, Ballen K. Reduced-intensity conditioning and umbilical cord blood transplantation in adults. Bone Marrow Transplant. 2009;44:667–71. https://doi.org/10.1038/bmt.2009.283.
Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–30. https://doi.org/10.1182/blood-2006-04-020172.
Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–8. https://doi.org/10.1182/blood-2003-10-3448.
Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2020. https://doi.org/10.1038/s41577-020-00457-z.
Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. https://doi.org/10.1089/scd.2007.0024.
Patenaude J, Perreault C. Thymic mesenchymal cells have a distinct transcriptomic profile. J Immunol. 2016;196:4760–70. https://doi.org/10.4049/jimmunol.1502499.
Suniara RK, Jenkinson EJ, Owen JJ. An essential role for thymic mesenchyme in early T cell development. J Exp Med. 2000;191:1051–6. https://doi.org/10.1084/jem.191.6.1051.
Acknowledgements
We thank Ms. Yoko Nakagawa for excellent technical assistance.
Funding
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#18K08323 to Y.M., #19K17856 to S.F.) and by the Japanese Society of Hematology Research Grant (Y.M. and S.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Ichinohe has received research funding from FUJIFILM Wako Chemicals, Repertoire Genesis Inc., and Takara Bio Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iwasa, M., Fujii, S., Fujishiro, A. et al. Impact of 2 Gy γ-irradiation on the hallmark characteristics of human bone marrow-derived MSCs. Int J Hematol 113, 703–711 (2021). https://doi.org/10.1007/s12185-020-03072-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03072-9