Abstract
Vitamin K2 in the form of menatetrenone has clinical benefits for osteoporosis and cytopenia. Given the dominant role of mesenchymal-osteolineage cells in the regulation of hematopoiesis, we investigated whether menatetrenone alters the hematopoiesis-supportive capability of human bone marrow mesenchymal stromal/stem cells (BM-MSCs). Menatetrenone up-regulated fibronectin protein expression in BM-MSCs without affecting their proliferation and differentiation capabilities. In addition, menatetrenone treatment of BM-MSCs enhanced generation of the CD34+ cell population in co-cultures through acceleration of the cell cycle. This effect was associated with cell–cell interactions mediated by VLA-4 and fibronectin. This proposal was supported by cytokine array and quantitative real-time PCR analyses, in which there were no significant differences between the expression levels of hematopoiesis-associated soluble factors in naïve and menatetrenone-treated BM-MSCs. Profiling of hematopoietic cells in co-cultures with menatetrenone-treated BM-MSCs demonstrated that they included significantly more CD34+CD38+ hematopoietic progenitor cells and cells skewed toward myeloid and megakaryocytic lineages than those in co-cultures with untreated BM-MSCs. Notably, myelodysplastic syndrome-derived cells were induced to undergo apoptosis when co-cultured with BM-MSCs, and this effect was enhanced by menatetrenone. Overall, our findings indicate that pharmacological treatment with menatetrenone bestows a unique hematopoiesis-supportive capability on BM-MSCs, which may contribute to the clinical improvement of cytopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin K is required for post-transcriptional γ-carboxylation of vitamin K-dependent proteins [1]. This process involves proteins such as osteocalcin, which is important for bone maturation and quality [2]. Vitamin K exists naturally in two bioactive forms: vitamin K1 (phylloquinone) and vitamin K2 (menaquinone-4). Unlike vitamin K1, vitamin K2 plays a highly effective role in bone metabolism, and the synthetic vitamin K2 analog menatetrenone is used clinically for the treatment of osteoporosis [3]. In addition, in combination with vitamin D3 (VD3), menatetrenone improves cytopenia in patients with myelodysplastic syndromes (MDS) [4,5,6,7]; however, the mechanisms by which menatetrenone facilitates hematopoiesis have not been fully characterized [8, 9]. A better understanding of the effect of menatetrenone on hematopoiesis will contribute to the development of effective drug therapies in patients with cytopenia.
Adult hematopoiesis is regulated not only by intrinsic signals, but also by extrinsic signals from the hematopoiesis-supportive bone marrow microenvironment (BMM). The major cellular constituents of the BMM are non-hematopoietic mesenchymal stromal/stem cells (MSCs) and their osteogenic progenies [10, 11]. Mature osteoblasts regulate hematopoietic stem cells (HSCs) through an angiopoietin-1/Tie2 interaction [12]. Osteocytes control lymphopoiesis and support myeloid expansion through the production of granulocyte colony-stimulatory factor [13, 14]. Osteogenesis-associated soluble factors, such as parathyroid hormone and calcium ions, contribute to hematopoiesis [15, 16]. In our previous studies, we reported that, unlike naïve bone marrow MSCs (BM-MSCs), osteoinductive and parathyroid hormone-treated BM-MSCs support the enhanced expansion of hematopoietic stem and progenitor cells (HSPCs) [17, 18], which indicates a distinct hematopoiesis-supportive capability of osteogenic-induced BM-MSCs. In this study, we examined whether menatetrenone alters the hematopoiesis-supportive capability of human BM-MSCs.
Materials and methods
Reagents
Menatetrenone, VD3, stem cell factor (SCF), interleukin-3 (IL-3), and fms-related tyrosine kinase 3 (FLT3) ligand were purchased from Wako Pure Chemical Industries (Osaka, Japan). Thrombopoietin and erythropoietin were provided by Kyowa Hakko Kirin (Tokyo, Japan). The antibodies used in this study are listed in Supplementary Table 1.
Isolation and expansion of human BM-MSCs
Normal human BM samples were purchased from AllCells (Alameda, CA). To confirm consistency among donors, multiple batches of BM-MSCs were isolated as described previously [17,18,19]. Single-cell suspensions of 1 × 107 BM mononuclear cells were seeded into T-75 culture flasks, and adherent cells were cultured in advanced-minimal essential medium (Invitrogen/Thermo Fisher Scientific, Waltham, MA) supplemented with 5% fetal bovine serum (FBS, Invitrogen/Thermo Fisher Scientific), 100 μM ascorbic acid (Wako Pure Chemicals Industries), and 2 mM l-glutamine (Gibco/Thermo Fisher Scientific). Primary cultures were passaged to disperse the colony-forming cells (passage 1). Cells at passage 3 were used as BM-MSCs in this study. Prior to experiments, the surface antigen profiles of CD14, CD19, CD34, CD45, CD73, CD90, and CD105 were examined by flow cytometric analysis to confirm that the cells expressed mesenchymal stem cell-associated markers, but did not express hematopoietic cell markers, as proposed by the International Society for Cellular Therapy [20, 21] (Supplementary Figure). For the expansion assay, 2 × 105 BM-MSCs were seeded into a 6 cm dish. Twenty-four hours later, BM-MSCs were treated with 1 or 10 µM menatetrenone for a further 24 h. Subsequently, BM-MSCs were collected with 0.05% trypsin/ethylenediaminetetraacetic acid, washed with Dulbecco’s phosphate-buffered saline (D-PBS, Wako Pure Chemical Industries) three times, and re-seeded into new 6 cm dishes at a density of 2 × 105 cells per dish. The number of cells was counted by staining with 0.5% trypan blue on days 4, 6, and 8 after menatetrenone treatment.
Co-culture of human CD34+ cells and human BM-MSCs
Human CD34+ HSPCs were isolated from BM mononuclear cells using anti-CD34 immunomagnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the isolated CD34+ cell population was confirmed by flow cytometric analysis using an anti-human CD34 antibody. BM-MSCs were seeded into 24-well culture plates at a density of 5 × 104 cells per well and treated with 1 or 10 µM menatetrenone. Subsequently, 0.6 × 103 CD34+ cells were co-cultured with the BM-MSCs in StemSpan Serum-Free Expansion Medium (STEMCELL Technologies, Vancouver, Canada) supplemented with 100 ng/mL SCF, 100 ng/mL FLT3 ligand, 50 ng/mL thrombopoietin, and 20 ng/mL IL-3. After 10 days of co-culture, the hematopoietic cells were collected by vigorous pipetting, and the cell number and surface marker expression profiles were examined by flow cytometric analysis.
Erythroid induction of human CD34+ cells
Human CD34+ HSPCs were cultured for 14 days in Iscove’s modified Dulbecco’s medium (Invitrogen/Thermo Fisher Scientific) supplemented with 900 ng/mL iron sulfate, 90 ng/mL iron nitrate, 10 µl/mL insulin (all from Sigma-Aldrich, St. Louis, MO), 1% bovine serum albumin (Wako Pure Chemical Industries), 10% heat-inactivated FBS (HyClone, Little Chalfont, UK), 10 ng/mL IL-3, 200 ng/mL SCF, 3 U/mL erythropoietin, and 1.2 µg/mL transferrin (Calbiochem, Darmstadt, Germany) [22,23,24]. Erythroid differentiation was confirmed by flow cytometric analysis in which erythroblasts were determined as CD45+CD71+CD235a−/CD45+CD71+CD235a+/CD45+CD71−CD235a+ cells. In some experiments, HSPCs were erythroid-induced in co-cultures with BM-MSCs that were treated with or without menatetrenone. We also performed co-culture assays of HSPCs and VD3-treated BM-MSCs using the same procedure as that used for HSPCs and menatetrenone-treated BM-MSCs.
Blocking experiments for co-cultures of human CD34+ cells and human BM-MSCs
Cell culture inserts with a pore size of 0.4 µm (Corning, One Riverfront Plaza, NY) were applied to abrogate direct interactions between human BM-MSCs and human CD34+ HSPCs. To investigate the involvement of integrin α4 in cell–cell interactions, HSPCs were pre-incubated at 37 °C for 30 min with 5 nM of BIO1211 (Bio-Techne, Minneapolis, MN). Subsequently, the media were removed, and the cells were washed twice and co-cultured with BM-MSCs.
In vitro differentiation of human BM-MSCs
To induce osteogenic differentiation of human BM-MSCs that were treated with menatetrenone for 24 h, osteogenesis-inducing cocktails comprising 100 μM ascorbic acid, 1.8 mM potassium dihydrogen phosphate (Sigma-Aldrich), and 100 nM dexamethasone (Sigma-Aldrich) were added to the culture media. Mineralization was evaluated by 1% Alizarin Red S staining after 6 weeks of osteogenesis-inducing culture. To induce adipogenic differentiation of menatetrenone-treated BM-MSCs, 0.5 mM isobutyl-methyl xanthine, 60 μM indomethacin, 0.5 μM hydrocortisone, and 10 μg/mL insulin (all from Sigma-Aldrich) were added to the culture media. Oil Red O staining was used to assess lipid-laden fat cells after 3 weeks of adipogenesis-inducing culture. The Alizarin Red S-stained area and the number of Oil Red O+ cells were quantified as described previously [17]. Images were acquired using an Axiovert 40C (Carl Zeiss, Oberkochen, Germany).
Quantitative real-time PCR (qRT-PCR)
BM-MSCs (2 × 105) were seeded into a 6 cm dish. Twenty-four hours later, the BM-MSCs were treated with menatetrenone for a further 24 h. Cell lysates were then prepared from the menatetrenone-treated BM-MSCs, and total RNA was extracted using the QIAamp RNA Blood Mini Kit (Qiagen, Hilden, Germany) and then reverse transcribed. The 10 μL PCR mixture contained TaqMan Fast Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA), cDNA, primer pairs, and a TaqMan probe (Universal Probe Library). The cDNA was amplified using the Step One Plus Real-Time PCR system (Applied Biosystems) using the following parameters: 95 °C for 20 s, followed by 40 cycles of 95 °C for 1 s and 60 °C for 20 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize any loading differences. The primer sets and universal probes used are listed in Supplementary Table 2.
Cell cycle assay
The cell cycle assay of CD34+ cells was performed 8 days after starting co-cultures with menatetrenone-treated BM-MSCs by using Click-iT Plus EdU Flow Cytometry Assay Kits (Thermo Fisher Scientific) and FxCycle Violet Stain (Invitrogen/Thermo Fisher Scientific). 5-Ethynyl-2′-deoxyuridine (EdU) was added to the culture media, and the culture media was mixed by pipetting gently. The cells were incubated with EdU at 37 °C for 1 h and then evaluated by flow cytometric analysis.
Immunoblot analysis
BM-MSCs (2 × 105) were seeded into a 10 cm dish 24 h before menatetrenone treatment and then treated with menatetrenone for a further 24 h. Subsequently, the BM-MSCs were washed with D-PBS three times and cultured with advanced-minimal essential medium-based culture medium. Three days after menatetrenone treatment, cell lysates were prepared from BM-MSCs with radioimmunoprecipitation assay buffer (Wako Pure Chemical Industries). Total proteins extracted from the cell lysates were boiled in sodium dodecyl sulfate sample buffer (Invitrogen/Thermo Fisher Scientific), separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Invitrogen/Thermo Fisher Scientific), and transferred to polyvinylidene fluoride membranes (EDM Millipore). Immunoreacted proteins were detected using horseradish peroxidase-conjugated sheep anti-mouse (for fibronectin) or donkey anti-rabbit (for actin) IgG, and visualized using enhanced chemiluminescence kits (GE Healthcare, Little Chalfont, UK). Immunoblotting detection was performed using the ChemiDoc XRS + system (Bio-Rad, Hercules, CA).
Cytokine array
Human BM-MSCs were seeded into a 6-well culture plate at a density of 2 × 105 cells per well and treated with menatetrenone for 24 h. These cells were washed with D-PBS three times and cultured in 1 mL of menatetrenone-free culture medium for 2 days. Subsequently, the lower part of the culture supernatant (800 μL) was collected, centrifuged, and analyzed using the Proteome Profiler Array Human Cytokine Array Kit (R&D Systems, Minneapolis, MN). Detection was performed using the ChemiDoc XRS + system.
MDS-L cell culture
MDS-L cells, a cell line derived from a patient with MDS [25, 26], were cultured in RPMI 1640 (Wako Pure Chemical Industries) supplemented with 10% heat-inactivated FBS, 20 µM 2-mercaptoethanol, 50 U/mL penicillin, 10 mM 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (all from Sigma-Aldrich), and 50 ng/mL IL-3. For the treatment experiments, menatetrenone was added to the culture medium at a final concentration of 0, 1, or 10 µM. Apoptosis of MDS-L cells was assessed by flow cytometric analysis 10 days after menatetrenone treatment.
Statistical analysis
Unpaired Student’s t tests were used for analyses. Data in bar graphs indicate the mean ± standard deviation (SD). Statistical significance is expressed as follows: *P < 0.05; **P < 0.01; ***P < 0.001; n.s. not significant.
Results
Menatetrenone treatment of human BM-MSCs enhances the hematopoietic progenitor cell population through cell cycle acceleration
CD34+ HSPCs were co-cultured with BM-MSCs that were pre-treated with menatetrenone at a concentration of 1–50 µM (Fig. 1a). Compared with that of cells co-cultured with untreated BM-MSCs, the number of CD45+ cells in co-cultures with menatetrenone-treated BM-MSCs increased in a dose-dependent manner (1–10 µM); however, cell numbers declined at 50 µM (Fig. 1b). We therefore treated BM-MSCs with menatetrenone at 1 and 10 µM for the following assays.
Menatetrenone treatment of human BM-MSCs increases the number of hematopoietic cells in co-culture. a Schematic showing the experiments. b Flow cytometry analyses showing the numbers of CD45+ cells in co-cultures with BM-MSCs treated with 0, 1, 5, 10, or 50 µM menatetrenone (n = 5 per group). Data are expressed as the mean ± SD. ***P < 0.001
The number of CD34+ cells was significantly higher in co-cultures with menatetrenone-treated BM-MSCs than in those with untreated BM-MSCs (Fig. 2a). Cell cycle assays of the co-cultures indicated that the frequency of CD34+ cells in the S/G2/M phases was significantly increased by menatetrenone treatment, whereas the frequency of CD34+ cells in the G0/G1 phases was significantly decreased (Fig. 2d). The number of CD34+CD38+ cells, a population of hematopoietic progenitor cells (HPCs), was higher in co-cultures with menatetrenone-treated BM-MSCs than in those with untreated BM-MSCs, whereas the number of CD34+CD38− cells, a population of HSCs, was comparable between the co-cultures with treated and untreated BM-MSCs (Fig. 2a). The percentage of CD34+CD38+ cells in co-cultures with menatetrenone-treated BM-MSCs was higher than the percentage of those in co-cultures with untreated BM-MSCs, whereas the percentage of CD34+CD38− cells in co-cultures with menatetrenone-treated BM-MSCs was lower than the percentage of those in cultures with untreated BM-MSCs (Fig. 2b, c). These results suggest that menatetrenone treatment of BM-MSCs enhances the HPC population, and that this process is associated with accelerating the cell cycle of CD34+ cells and skewing CD34+CD38− cells toward a more differentiated state.
Menatetrenone treatment of human BM-MSCs increases the HPC population in co-cultures. Flow cytometry analyses showing the number (a) and the percentage (b) of CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells in co-cultures with BM-MSCs treated with 0, 1, or 10 µM menatetrenone (n = 5 per group). c Representative dot plots of CD34 versus CD38 expression. The numbers in each box indicate the percentages of cells. d Percentages of cells in the G0/G1 and S/G2/M phases in co-cultures with BM-MSCs treated with 0 µM (black bar), 1 µM (deep blue bar), or 10 µM (light blue bar) menatetrenone (n = 4 per group). a, b, d Data are shown as the mean ± SD. *P < 0.05. **P < 0.01. n.s. not significant
Menatetrenone-treated human BM-MSCs support the maturation of CD34+ cells toward multiple cell lineages
Next, we examined the profiles of hematopoietic cells in the co-cultures of CD34+ HSPCs and menatetrenone-treated human BM-MSCs using flow cytometry. The frequencies of CD34−CD33+, CD34−CD13+, and CD34−CD41a+ cells were significantly higher in co-cultures with BM-MSCs that were treated with 10 µM menatetrenone than in those with BM-MSCs that were untreated (Fig. 3a, b). In erythroid-inductive co-cultures of CD34+ HSPCs with menatetrenone-treated BM-MSCs, the generation of erythroblasts was enhanced compared with co-cultures with untreated BM-MSCs, although the difference was not statistically significant (Fig. 3c). These results indicate that menatetrenone treatment of BM-MSCs supports the enhancement of HSPC maturation toward multiple cell lineages, especially at a concentration of 10 µM. In erythroid-inductive cultures of HSPCs with BM-MSCs, pretreatment of the BM-MSCs with VD3 enhanced the generation of erythroblasts significantly in a dose-dependent manner (Fig. 3d, e). These results indicate that the BM-MSC-mediated maturation of HSPCs toward erythroid cells is enhanced by VD3 rather than menatetrenone.
Menatetrenone treatment of human BM-MSCs supports the maturation of CD34+ cells in co-cultures toward multiple cell lineages. a, b Flow cytometric analyses showing myeloid and megakaryocytic cells in co-cultures with BM-MSCs treated with 0, 1, or 10 µM menatetrenone. a Frequencies of CD34−CD33+ cells, CD34−CD13+ cells, and CD34−CD41a+ cells in the CD45+ cell population; n = 5 per group. b Representative dot plots of CD34 versus CD33, CD34 versus CD13, and CD34 versus CD41a. The numbers in each box indicate the percentages of cells. c Flow cytometric analyses showing the numbers of erythroblasts in co-cultures with BM-MSCs treated with 0, 1, or 10 µM menatetrenone; n = 5 per group. d Flow cytometric analyses showing the numbers of erythroblasts in co-cultures with BM-MSCs treated with 0, 0.1, 1, or 10 nM VD3; n = 5 per group. e Representative cytospin images of May–Giemsa staining of cells co-cultured with VD3-treated BM-MSCs. The yellow arrows indicate erythroblasts. a, c, d Data are represented as the mean ± SD. *P < 0.05. **P < 0.01
Menatetrenone does not affect the differentiation and proliferation capabilities of human BM-MSCs
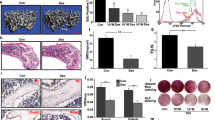
When BM-MSCs were treated with menatetrenone for 24 h and then cultured in an osteoinductive medium for 6 weeks, their mineralization was comparable to that of untreated BM-MSCs, as assessed by Alizarin Red S staining (Fig. 4a, b). This result was supported by the results of a qRT-PCR analysis showing that the mRNA expression levels of various osteogenesis-associated genes, including runt-related transcription factor 2, osterix, alkaline phosphatase, osteopontin, and osteocalcin, were not up-regulated in menatetrenone-treated BM-MSCs (Fig. 4c). When menatetrenone-treated BM-MSCs were cultured in adipogenesis-inducing medium for 3 weeks, their fat deposition was similar to that of untreated BM-MSCs, as assessed by Oil Red O staining (Fig. 4d, e). Furthermore, the proliferation of menatetrenone-treated BM-MSCs was comparable to that of untreated BM-MSCs (Fig. 4f). Overall, these results indicate that the multi-differentiation and proliferation capabilities of BM-MSCs are not affected by treatment with menatetrenone for 24 h.
Menatetrenone does not affect the differentiation or proliferation capabilities of BM-MSCs. a, b Osteogenic differentiation of BM-MSCs treated with or without menatetrenone, as assessed by Alizarin Red S staining (a). Red nodules indicate mineralization (b). Representative images are shown. c Expression levels of osteogenesis-associated genes in BM-MSCs treated with or without menatetrenone, as assessed by qRT-PCR. RUNX2 runt-related transcription factor 2, OSX osterix, COL collagen, ALP alkaline phosphatase, OPN osteopontin, OCN osteocalcin. d, e Adipogenic differentiation of BM-MSCs treated with or without menatetrenone, as assessed by Oil Red O staining (d). Red-staining indicates fat-laden cells (e). Representative images are shown. f The proliferation of BM-MSCs treated with or without menatetrenone. a, c, d, f Data are represented as the mean ± SD; n = 5 per group; n.s. not significant
The increase in the CD34+ cell population mediated by menatetrenone-treated human BM-MSCs depends on direct cell–cell interactions
Next, we examined the molecules that are involved in enhanced hematopoietic expansion in co-cultures with menatetrenone-treated BM-MSCs. Array analyses of hematopoiesis-associated soluble factors (cytokines/chemokines) showed that their expression profiles did not differ markedly between menatetrenone-treated and untreated BM-MSCs (Fig. 5a). In addition, qRT-PCR analyses showed that the mRNA expression levels of representative hematopoiesis-associated soluble factors did not differ significantly between menatetrenone-treated and untreated BM-MSCs (Fig. 5b).
Direct cell–cell interactions, but not soluble factors, mediate enhanced HPC expansion in co-cultures with menatetrenone-treated human BM-MSCs. a Cytokine arrays of supernatants from cultures of BM-MSCs treated with 0, 1, or 10 µM menatetrenone. CCL2 C–C motif chemokine ligand 2, CXCL12 C-X-C motif chemokine ligand 12, MIF macrophage migration inhibitory factor, SERPINE1 serpin family E member 1, IL-6 interleukin-6. b The mRNA expression levels of multiple hematopoiesis-associated genes in BM-MSCs treated with 0 µM (black bars), 1 µM (gray bars), or 10 µM (white bars) menatetrenone, as assessed by qRT-PCR; n = 5 per group. Ang-1 angiopoietin-1, FLT3L fms-related tyrosine kinase 3 ligand, G-CSF granulocyte colony-stimulating factor, IL-6 interleukin-6, IL-11 interleukin-11, Jag-1 jagged-1, LIF leukemia inhibitory factor, M-CSF macrophage colony-stimulating factor, SCF stem cell factor. c Co-culture of CD34+ HSPCs with BM-MSCs treated with 0 (black bars), 1 (blue bars), or 10 µM (gray bars) menatetrenone in the presence (+, red squares) or absence (−) of cell culture inserts (n = 4 per group). Flow cytometry analyses showing the numbers of CD45+ cells, CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells. d Co-culture of CD34+ HSPCs with BM-MSCs treated with 0, 1, or 10 µM menatetrenone in the presence (red bars) or absence (black bars) of cell culture inserts (n = 4 per group). Flow cytometry plots showing the percentages of CD34−CD33+ cells, CD34−CD13+ cells, and CD34−CD41a+ cells. b–d Data are presented as the mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001; n.s. not significant
We then performed a co-culture experiment of CD34+ HSPCs and menatetrenone-treated BM-MSCs using cell culture inserts. The numbers of CD45+ cells, CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells in co-cultures with inserts were significantly lower than those in co-cultures without inserts (Fig. 5c). Importantly, increased generation of CD45+ and CD34+ cells in co-cultures with menatetrenone-treated BM-MSCs did not occur in the presence of the cell culture inserts (Fig. 5c, red squares). These results indicate that direct cell–cell interactions support the dose-dependent generation of CD34+ cells in co-cultures with menatetrenone-treated BM-MSCs.
The percentages of CD34−CD33+ and CD34−CD41a+ cells in co-cultures containing the cell culture inserts were significantly higher than those in co-cultures lacking the cell culture inserts (Fig. 5d). In addition, in the presence of the inserts, menatetrenone treatment of BM-MSCs did not affect the frequencies of these mature cells in co-cultures significantly (Fig. 5d, red bars).
Fibronectin contributes to the enhancement of HPC expansion by menatetrenone-treated human BM-MSCs
Extracellular matrices and adhesion molecules in the BMM play functional roles in homeostatic and regenerative hematopoiesis. Among the various kinds of extracellular matrix produced by BM-MSCs, fibronectin is a crucial molecule that supports HPCs [27]. An immunoblot analysis revealed that fibronectin expression was up-regulated by menatetrenone treatment of BM-MSCs in a dose-dependent manner (Fig. 6a). Integrin α4β1 (very late antigen-4, VLA-4) is a fibronectin receptor expressed on the surface of HSPCs [28]. Therefore, we performed a blocking experiment using a selective inhibitor of integrin α4, BIO1211. Increased expansion of CD45+ cells, CD34+ cells, and CD34+CD38+ cells in co-cultures with menatetrenone-treated BM-MSCs was abolished in BIO12-treated HSPCs (Fig. 6b). These results indicate that fibronectin-mediated interactions between HSPCs and menatetrenone-treated BM-MSCs contribute to the generation of HPCs.
Fibronectin enhances HPC expansion mediated by menatetrenone-treated human BM-MSCs. a Immunoblot analysis showing the expression of fibronectin in BM-MSCs treated with 0, 1, or 10 µM menatetrenone. Actin was used as a loading control. b Flow cytometry plots showing the numbers of CD45+ cells, CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells in co-cultures of CD34+ HSPCs treated with a selective inhibitor of integrin α4 and BM-MSCs treated with 0 µM (black bars), 1 µM (blue bars), or 10 µM (gray bars) menatetrenone (n = 3 per group). b Data are presented as the mean ± SD
Direct treatment of CD34+ cells with menatetrenone does not promote enhancement of the CD34+ cell population
Next, CD34+ HSPCs were directly exposed to menatetrenone and then cultured alone (Fig. 7a). Treatment with 1 and 5 µM menatetrenone reduced the numbers of CD45+ cells, CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells in cultures (Fig. 7b). Treatment with 10 and 50 µM menatetrenone reduced the numbers of these cells to undetectable levels. These results suggest that menatetrenone has opposing effects on BM-MSCs and HSPCs with regard to expansion of CD34+ hematopoietic cells.
The CD34+ cell population is not enhanced by direct treatment of CD34+ cells with menatetrenone. a Schema of the experiments. b Flow cytometric analyses showing the numbers of CD45+ cells, CD34+ cells, CD34+CD38− cells, and CD34+CD38+ cells in single cultures of CD34+ HSPCs treated with 0 µM (black bars), 1 µM (dark gray bars), or 10 µM (light gray bars) menatetrenone (n = 5 per group). Data are presented as the mean ± SD. **P < 0.01
Effects of menatetrenone on human MDS-derived cells
Menatetrenone is clinically considered for the treatment of cytopenia in MDS patients; therefore, we examined the effects of menatetrenone on MDS-derived cells. Treatment of MDS-L cells, a unique cell line derived from an MDS patient, with 1 or 10 µM menatetrenone did not increase the percentage of Annexin V+ propidium iodide (PI)− cells (early apoptotic cells; Fig. 8a, left panel, black bars). By contrast, the frequency of Annexin V +PI+ cells (late apoptotic cells) was increased at 10 μM but not at 1 μM menatetrenone (Fig. 8a, right panel, black bars). Notably, when MDS-L cells were cultured in the presence of BM-MSCs, the frequency of Annexin V +PI− cells was decreased and the frequency of Annexin V +PI+ cells was increased (Fig. 8a, white bars versus black bars, and b). These results indicate that BM-MSCs induce a shift of MDS-L cells from an early apoptotic state to a late apoptotic state. We further co-cultured MDS-L cells with BM-MSCs in the presence of cell culture inserts. The percentage of Annexin V +PI− cells with cell culture inserts was higher than that without cell culture inserts, and the percentage of Annexin V +PI+ cells with cell culture inserts tended to be lower than that without cell culture inserts (Fig. 8c). These results suggest that the shift of MDS-L cells from an early apoptotic state to a late apoptotic state requires cell–cell interactions between MDS-L cells and BM-MSCs. When normal CD34+ HSPCs were cultured in the presence of BM-MSCs, the frequencies of Annexin V +PI− and Annexin V +PI+ cells were not affected by menatetrenone treatment (Fig. 8d).
Apoptosis of MDS-L cells treated with menatetrenone. a, b Flow cytometric analyses showing the apoptosis of MDS-L cells co-cultured with or without BM-MSCs, and treated with or without menatetrenone. a Percentages of Annexin V +PI− and Annexin V +PI+ cells in MDS-L cells co-cultured with (MSC + , white bars) or without (MSC −, black bars) BM-MSCs treated with 0, 1, or 10 µM menatetrenone (n = 5 per group). *P < 0.05; **P < 0.01; ***P < 0.001. b Representative dot plots of Annexin V versus propidium iodide (PI) expression. The numbers in each box indicate the percentages of cells. c Flow cytometric analyses showing the percentages of Annexin V +PI− and Annexin V +PI+ cells in MDS-L cells co-cultured with BM-MSCs in presence (white bars) or absence (gray bars) of cell culture inserts (n = 4 per group). *P < 0.05; **P < 0.01; ***P < 0.001. d Flow cytometry plots showing apoptosis of CD34+ HSPCs after treatment with 0 µM (black bars), 1 µM (gray bars), or 10 µM (white bars) menatetrenone (n = 5 per group)
Discussion
Cytopenia is a common manifestation in acquired bone marrow failure syndromes. Immunosuppressive agents [29], cytokine/erythropoietin stimulating agents [30], and lenalidomide [31] are effective in some patients with low-risk MDS, but supportive transfusion of blood products is still a major therapy [32]. Because MDS is primarily a disease of people aged 70 years or older, available therapies are limited [33, 34]. In combination with VD3 intake, daily oral intake of menatetrenone at osteoporosis-treatment doses improves cytopenia in some patients with MDS, without any severe adverse effects [4,5,6]. Therefore, we examined the pharmacological effects of menatetrenone on human BM-MSCs, of which hematopoiesis-supportive characteristics have recently been reported. As expected, the generation of CD45+ cells and the CD34+CD38+ HPC population were enhanced by menatetrenone treatment of BM-MSCs, and maturation of CD34+ HSPCs toward myeloid and megakaryocytic cells was also enhanced. These results provide mechanistic information regarding the improvement of cytopenia by menatetrenone. In addition, we also found that VD3 promoted erythropoiesis, indicating that the combined use of menatetrenone and VD3 could improve anemia.
The pharmaceutical product information and an interview form from Glakay® (a brand name of menatetrenone) demonstrate that the average concentration of menatetrenone in the human body is about 0.9 µM [35]. Another piece of information was provided by a previous study, which indicates that the half maximal inhibitory concentration (IC50) of menatetrenone is 5 µM [9]. Therefore, we believe that such concentrations will be the appropriate therapeutic and circulating levels in patients. In addition, the concentration of a single oral dose of menatetrenone peaks at around 4–6 h after administration and decreases to a low level at around 12–24 h [35]. We speculate that the effect of menatetrenone pretreatment on MSCs is temporary.
VLA-4 expressed by HSPCs binds to fibronectin, highlighting the significance of interactions between HSPCs and stromal components to the adult hematopoietic system in the BMM [28]. Here, fibronectin expression in BM-MSCs was increased by menatetrenone treatment, and the enhanced generation of CD34+ cells by menatetrenone was abolished by a selective inhibitor of integrin α4. These findings suggest that a VLA-4/fibronectin-mediated interaction between HSPCs and BM-MSCs contributes to the generation of CD34+ cells. We also found that menatetrenone treatment of BM-MSCs accelerated the cell cycle of CD34+ cells. Thus, our study reveals a novel menatetrenone-mediated pharmacological effect related to the established molecular mechanism of hematopoietic cell generation.
In our current study, BM-MSCs promoted the generation of normal CD34+ cells but the apoptosis of MDS-L cells. The potential role of MSCs in the suppression or proliferation of cancer cells has been extensively explored in solid tumors, including breast cancers, lung cancers, pancreatic cancers, melanoma, and prostate cancers, by co-culture experiments using normal MSCs. Some studies showed inhibitory effects of MSCs on tumor cells, while others demonstrated proliferative effects, regardless of the type of cancer and source of MSCs [36,37,38,39,40,41,42]. Furthermore, studies reported suppression of both proliferation and apoptosis by MSCs [43, 44]. A dual role of MSCs was also demonstrated in hematological malignant cells of B cell acute lymphoblastic leukemia (B-ALL), T-cell acute lymphoblastic leukemia, acute myeloid leukemia, and chronic myeloid leukemia [45]. The reasons for the discrepancies between the effects of MSCs on tumor cells are still unknown, but might be related to the type of cells used (cell lines or primary cells) or the specific culture conditions of leukemia cells and MSCs. We showed previously that, when adhered to BM-MSCs, human B-ALL cells acquire chemoresistance and pro-survival characteristics, alongside enhanced Akt/Bcl-2 pathway activity and increased G0 cell cycle populations [46]. In addition, murine BM-MSCs support the generation of physiological precursor B-cells and the proliferation of leukemic precursor B-cells, possibly through the production of C-X-C chemokine receptor type 4 [47]. Our previous investigations revealed proliferative effects of BM-MSCs on B-ALL cells. In the current study, MDS-L cells were induced to be in a late apoptotic state in the presence of normal BM-MSCs, and this effect was enhanced by treatment of the MSCs with menatetrenone. Accordingly, the effect of menatetrenone-treated MSCs on MDS-L cells is opposite to that on normal CD34+ cells. Moreover, cell–cell adhesion between MDS-L cells and menatetrenone-treated BM-MSCs plays a role in the shift of MDS-L cells from an early apoptotic state to a late apoptotic state. We were unable to identify previous investigations related to our observations in public databases, probably due to difficulties associated with obtaining primary MDS cells and established MDS cell lines. Several studies demonstrated that BM-MSCs isolated from low-risk MDS patients differ from normal BM-MSCs in that they are defective in cell adhesion [48,49,50,51] and hematopoiesis-supportive function [51,52,53,54]. Further investigations using BM-MSCs isolated from low-risk MDS patients are warranted to examine the pro-apoptotic effect of MSCs on MDS cells. A recent report suggested that warfarin, an antagonist of vitamin K, compromises hematopoiesis and HSC function via impairment of the hematopoiesis-supportive function of macrophages and MSCs in the BMM, in association with impairment of the integrin β3/periostin axis [55]. Of note, the authors of the study suggested that warfarin is associated with a possible risk of MDS.
In conclusion, the results presented here indicate that menatetrenone enhances the HPC population and generation of myeloid and megakaryocytic cells via effects on human BM-MSCs in vitro. Direct cell–cell interactions, mediated in part through fibronectin/VLA-4 rather than hematopoiesis-related soluble factors, are implicated in the process. In contrast to normal CD34+ cells, MDS-L cells undergo apoptosis in the presence of menatetrenone-treated BM-MSCs (Fig. 9). These data connect the pharmacological activation of BM-MSCs with the previously recognized clinical improvement of cytopenia by menatetrenone, and may contribute to the enhancement of drug therapy in patients with cytopenia.
Suggested model for the improvement of cytopenia by menatetrenone treatment. Menatetrenone-treated human BM-MSCs support generation of the HPC population and the maturation of HSPCs toward multiple cell lineages in BMM. Up-regulated fibronectin mediates cell–cell interactions between HSPCs and menatetrenone-treated BM-MSCs, thereby contributing to the generation of HPCs. BM-MSCs induce a shift of menatetrenone-induced apoptosis of MDS cells from an early apoptotic state to a late apoptotic state
Change history
15 September 2020
In the original publication of the article, the Figs.��4 C, F and 5 B, C were published with unexpected appearance of dots.
References
Suttie JW. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–77. https://doi.org/10.1146/annurev.bi.54.070185.002331.
Shitaki S, Tsugawa N, Okano T. Recent advances in vitamin K-dependent Gla-containing proteins and vitamin K nutrition. Osteoporos Sarcopenia. 2015;1:22–38. https://doi.org/10.1016/j.afos.2015.07.009.
Su S, He N, Men P, Song C, Zhai S. The efficacy and safety of menatetrenone in the management of osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2019;30:1175–86. https://doi.org/10.1007/s00198-019-04853-7.
Takami A, Nakao S, Ontachi Y, Yamauchi H, Matsuda T. Successful therapy of myelodysplastic syndrome with menatetrenone, a vitamin K2 analog. Int J Hematol. 1999;69:24–6.
Miyazawa K, Nishimaki J, Ohyashiki K, Enomoto S, Kuriya S, Fukuda R, et al. Vitamin K2 therapy for myelodysplastic syndromes (MDS) and post-MDS acute myeloid leukemia: information through a questionnaire survey of multi-center pilot studies in Japan. Leukemia. 2000;14:1156–7. https://doi.org/10.1038/sj.leu.2401790.
Takami A, Asakura H, Nakao S. Menatetrenone, a vitamin K2 analog, ameliorates cytopenia in patients with refractory anemia of myelodysplastic syndrome. Ann Hematol. 2002;81:16–9. https://doi.org/10.1007/s00277-001-0391-x.
Akiyama N, Miyazawa K, Kanda Y, Tohyama K, Omine M, Mitani K, et al. Multicenter phase II trial of vitamin K(2) monotherapy and vitamin K(2) plus 1alpha-hydroxyvitamin D(3) combination therapy for low-risk myelodysplastic syndromes. Leuk Res. 2010;34:1151–7. https://doi.org/10.1016/j.leukres.2010.04.006.
Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, et al. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia. 1997;11:779–87. https://doi.org/10.1038/sj.leu.2400667.
Nishimaki J, Miyazawa K, Yaguchi M, Katagiri T, Kawanishi Y, Toyama K, et al. Vitamin K2 induces apoptosis of a novel cell lines established from a patient with myelodysplastic syndrome in blastic transformation. Leukemia. 1999;13:1399–405.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. https://doi.org/10.1038/nature12984.
Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity. 2018;48:632–48. https://doi.org/10.1016/j.immuni.2018.03.024.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. https://doi.org/10.1016/j.cell.2004.07.004.
Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H, et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18:749–58. https://doi.org/10.1016/j.cmet.2013.09.014.
Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu X, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121:930–9. https://doi.org/10.1182/blood-2012-06-437160.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. https://doi.org/10.1038/nature02040.
Paredes-Gamero EJ, Barbosa CM, Ferreira AT. Calcium signaling as a regulator of hematopoiesis. Front Biosci (Elite Ed). 2012;4:1375–84.
Yao H, Miura Y, Yoshioka S, Miura M, Hayashi Y, Tamura A, et al. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32:2245–55. https://doi.org/10.1002/stem.1701.
Sugino N, Miura Y, Yao H, Iwasa M, Fujishiro A, Fujii S, et al. Early osteoinductive human bone marrow mesenchymal stromal/stem cells support an enhanced hematopoietic cell expansion with altered chemotaxis- and adhesion-related gene expression profiles. Biochem Biophys Res Commun. 2016;469:823–9. https://doi.org/10.1016/j.bbrc.2015.12.061.
Fujishiro A, Miura Y, Iwasa M, Fujii S, Sugino N, Andoh A, et al. Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells. Inflamm Regen. 2017;37:19. https://doi.org/10.1186/s41232-017-0049-2.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. https://doi.org/10.1080/14653240600855905.
Miura Y. Human bone marrow mesenchymal stromal/stem cells: current clinical applications and potential for hematology. Int J Hematol. 2016;103:122–8. https://doi.org/10.1007/s12185-015-1920-z.
Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. https://doi.org/10.1038/nbt1047.
Boehm D, Murphy WG, Al-Rubeai M. The potential of human peripheral blood derived CD34 + cells for ex vivo red blood cell production. J Biotechnol. 2009;144:127–34. https://doi.org/10.1016/j.jbiotec.2009.08.017.
Li B, Ding L, Yang C, Kang B, Liu L, Story MD, et al. Characterization of transcription factor networks involved in umbilical cord blood CD34 + stem cells-derived erythropoiesis. PLoS One. 2014;9:e107133. https://doi.org/10.1371/journal.pone.0107133.
Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24:748–55. https://doi.org/10.1038/leu.2009.296.
Drexler HG, Dirks WG, Macleod RA. Many are called MDS cell lines: one is chosen. Leuk Res. 2009;33:1011–6. https://doi.org/10.1016/j.leukres.2009.03.005.
Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adhes Migr. 2014;8:563–77. https://doi.org/10.4161/19336918.2014.968501.
Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–41.
Okamoto T, Okada M, Yamada S, Takatsuka H, Wada H, Tamura A, et al. Good response to cyclosporine therapy in patients with myelodysplastic syndromes having the HLA-DRB1*1501 allele. Leukemia. 2000;14:344–6. https://doi.org/10.1038/sj.leu.2401665.
Hellstrom-Lindberg E, Ahlgren T, Beguin Y, Carlsson M, Carneskog J, Dahl IM, et al. Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: results from a randomized phase II study and long-term follow-up of 71 patients. Blood. 1998;92:68–75.
Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. https://doi.org/10.1182/blood-2007-01-068833.
Nilsson-Ehle H, Birgegard G, Samuelsson J, Antunovic P, Astermark J, Garelius H, et al. Quality of life, physical function and MRI T2* in elderly low-risk MDS patients treated to a haemoglobin level of ≥ 120 g/L with darbepoetin alfa ± filgrastim or erythrocyte transfusions. Eur J Haematol. 2011;87:244–52. https://doi.org/10.1111/j.1600-0609.2011.01654.x.
Tefferi A, Vardiman JM. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. https://doi.org/10.1056/NEJMra0902908.
Tefferi A. Myelodysplastic syndromes–many new drugs, little therapeutic progress. Mayo Clin Proc. 2010;85:1042–5.
Interview form of Glakay®. (https://medical.eisai.jp/content/000000488.pdf). Accessed 5 May 2020.
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–9. https://doi.org/10.1002/stem.559.
Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. https://doi.org/10.1016/j.canlet.2008.04.032.
Li L, Tian H, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226:1860–7. https://doi.org/10.1002/jcp.22511.
Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, et al. Mesenchymal stromal cells alone or expressing interferon-β suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–25. https://doi.org/10.3109/14653241003631815.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. https://doi.org/10.1038/nature06188.
Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–73. https://doi.org/10.1089/scd.2007.0181.
Lin G, Yang R, Banie L, Wang G, Ning H, Li L, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066–73. https://doi.org/10.1002/pros.21140.
Yulyana Y, Ho IA, Sia KC, Newman JP, Toh XY, Endaya BB, et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015;23:746–56. https://doi.org/10.1038/mt.2015.13.
Wu YL, Li HY, Zhao XP, Jiao JY, Tang DX, Yan LJ, et al. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017;108:897–909. https://doi.org/10.1111/cas.13202.
Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH, Yoo KH. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia. 2019;33:597–611. https://doi.org/10.1038/s41375-018-0373-9.
Iwasa M, Miura Y, Fujishiro A, Fujii S, Sugino N, Yoshioka S, et al. Bortezomib interferes with adhesion of B cell precursor acute lymphoblastic leukemia cells through SPARC up-regulation in human bone marrow mesenchymal stromal/stem cells. Int J Hematol. 2017;105:587–97. https://doi.org/10.1007/s12185-016-2169-x.
Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, Tamura A, et al. CCAAT/enhancer-binding protein β expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem Cells. 2014;32:730–40. https://doi.org/10.1002/stem.1555.
Aanei CM, Eloae FZ, Flandrin-Gresta P, Tavernier E, Carasevici E, Guyotat D, et al. Focal adhesion protein abnormalities in myelodysplastic mesenchymal stromal cells. Exp Cell Res. 2011;317:2616–29. https://doi.org/10.1016/j.yexcr.2011.08.007.
Roversi FM, Lopes MR, Machado-Neto JA, Longhini AL, Duarte Ada S, Baratti MO, et al. Serine protease inhibitor kunitz-type 2 is downregulated in myelodysplastic syndromes and modulates cell-cell adhesion. Stem Cells Dev. 2014;23:1109–20. https://doi.org/10.1089/scd.2013.0441.
Wu Y, Aanei CM, Kesr S, Picot T, Guyotat D, Campos Catafal L. Impaired expression of focal adhesion kinase in mesenchymal stromal cells from low-risk myelodysplastic syndrome patients. Front Oncol. 2017;7:164. https://doi.org/10.3389/fonc.2017.00164.
Corradi G, Baldazzi C, Očadlíková D, Marconi G, Parisi S, Testoni N, et al. Mesenchymal stromal cells from myelodysplastic and acute myeloid leukemia patients display in vitro reduced proliferative potential and similar capacity to support leukemia cell survival. Stem Cell Res Ther. 2018;9:271. https://doi.org/10.1186/s13287-018-1013-z.
Matthes TW, Meyer G, Samii K, Beris P. Increased apoptosis in acquired sideroblastic anaemia. Br J Haematol. 2000;111:843–52.
Zhao ZG, Xu W, Yu HP, Fang BL, Wu SH, Li F, et al. Functional characteristics of mesenchymal stem cells derived from bone marrow of patients with myelodysplastic syndromes. Cancer Lett. 2012;317:136–43. https://doi.org/10.1016/j.canlet.2011.08.030.
Geyh S, Oz S, Cadeddu RP, Fröbel J, Brückner B, Kündgen A, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013;27:1841–51. https://doi.org/10.1038/leu.2013.193.
Verma D, Kumar R, Pereira RS, Karantanou C, Zanetti C, Minciacchi VR, et al. Vitamin K antagonism impairs the bone marrow microenvironment and hematopoiesis. Blood. 2019;134:227–38. https://doi.org/10.1182/blood.2018874214.
Acknowledgements
We thank Ms. Yoko Nakagawa (Kyoto University) for her excellent technical assistance.
Funding
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#18K08323 to Y.M., #19K17856 to S.F.), by a Japanese Society of Hematology Research Grant (to Y.M. and S.F.), and by the Takeda Science Foundation (S.F.).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: AF, MI, SF, TM, AA, KT, AT-K, and YM. Acquisition of data: AF, MI, SF, and YM. Analysis and interpretation of data: AF, MI, SF, TM, AA, KT, AT-K, and YM. Drafting of the article: AF, MI, SF, TM, AA, KT, AT-K, and YM. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics
All materials were obtained from commercially available sources.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Fujishiro, A., Iwasa, M., Fujii, S. et al. Menatetrenone facilitates hematopoietic cell generation in a manner that is dependent on human bone marrow mesenchymal stromal/stem cells. Int J Hematol 112, 316–330 (2020). https://doi.org/10.1007/s12185-020-02916-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02916-8