Abstract
This multicenter phase II study (UMIN000008145) aims to investigate the efficacy and safety of six cycles of combination therapy (RBD) comprising rituximab, bendamustine, and dexamethasone (DEX) for relapsed or refractory (RR) indolent B-cell non-Hodgkin lymphoma (B-NHL) and mantle cell lymphoma (MCL). Although the initial study protocol comprised 20 mg/body DEX on days 1 and 2, and 10 mg/body on days 3–5 [high-dose (HD-) DEX group], the dose of DEX was later decreased to 8 mg/body on days 1 and 2 [low-dose (LD-) DEX group] due to frequent cytomegalovirus (CMV) antigenemia and recurrent retinitis. We enrolled 33 patients, and LD-DEX and HD-DEX were administered in 15 and 18 patients, respectively. The overall response and the 3-year progression-free survival rates were 88% and 75.5%, respectively. The leading adverse event was myelosuppression. Incidence of grade 3–4 leukocytopenia, neutropenia, and lymphocytopenia was 55%, 67%, and 91%, respectively. The most frequent nonhematological adverse events were CMV antigenemia and rash (33% and 30%, respectively). Incidence of CMV antigenemia over 10/100,000 white blood cells was significantly lower with LD-DEX than that with HD-DEX (P = 0.0127). In conclusion, RBD showed significant effectiveness for RR indolent B-NHL and MCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bendamustine structurally comprises a mechlorethamine group and a benzimidazole ring, which confer pharmacological properties of both alkylators and purine analogs, and has demonstrated clinical efficacy in patients with B-cell non-Hodgkin lymphoma (B-NHL). Single-agent bendamustine has demonstrated efficacy and tolerability in a phase II study in Japan, which enrolled 58 patients with relapsed or refractory (RR) indolent B-NHL and 11 with mantle cell lymphoma (MCL) [1]. Bendamustine exhibited an overall response rate (ORR) of 91% with a complete response rate (CRR) of 67% by the International Workshop Response Criteria (IWRC) [2] and an ORR of 93% with a CRR of 57% by the revised response criteria [3]. The estimated 1-year progression-free survival (PFS) rates were 70% and 90% among indolent B-NHL and MCL patients, respectively.
Previously, various chemotherapies combined with an anti-CD20 monoclonal antibody, rituximab, have exhibited efficacy advantage and safety in B-NHL. Reportedly, the combination of rituximab (375 mg/m2) and bendamustine (90 mg/m2, 2 consecutive days) (RB) is effective and well tolerated in the treatment of RR indolent B-NHL and MCL as phase II [4, 5] and phase III studies [6]. In Japan, Matsumoto et al. conducted a phase II study of RB in patients with RR indolent B-NHL and MCL [7].
Typically, bendamustine is well tolerated. The most clinically significant toxicity is myelosuppression [1]. Gastrointestinal (GI) events, including nausea and vomiting, and rash are also commonly reported as its nonhematological toxicities. For bendamustine-induced emesis, dexamethasone (DEX; 20 mg, 3 days followed by 10 mg for 2 days) was administered in phase I [8] and phase II studies [9] in patients with RR aggressive B-NHL in Japan. The 2010 clinical practice guideline for antiemesis by the Japanese Society of Clinical Oncology (JSCO) [10] recommends the use of 5-hydroxytryptamine and administration of DEX (8 mg) before bendamustine. In addition, the use of DEX may be promising in terms of antitumor effects and prophylaxis of rash.
Hence, we conducted a multicenter phase II study to ascertain the safety and efficacy of the combination chemotherapy composed of rituximab, bendamustine, and DEX (RBD) in patients with RR indolent B-NHL and MCL.
Patients and methods
Study design and endpoints
This multicenter, single-arm phase II study was conducted at the centers of the Kyoto Clinical Hematology Study Group (KOTOSG). While the primary endpoint was the ORR in all eligible patients, secondary endpoints were the CRR, PFS, safety, and the completion rates of the first three cycles and all six cycles of chemotherapy.
Safety and response assessments
We evaluated patients’ responses in accordance with the IWRC [2]. While the CRR was defined as the frequency of patients exhibiting complete response (CR) plus CR unconfirmed (CRu), the ORR was defined as the frequency of patients reporting CR, CRu, and partial response (PR). Estimates of PFS and OS distributions were evaluated using the Kaplan–Meier method, and times to distributions were compared using the log-rank test. We recorded adverse events in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) to evaluate safety.
Patient eligibility
Eligible patients had immunohistochemically and/or flowcytometrically CD20-positive indolent B-NHL. In this study, the histopathology of the lymphoma was consistent with follicular lymphoma (FL; grades 1, 2, and 3a), extranodal/nodal/splenic marginal zone lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, and MCL, according to the World Health Organization Classification of Neoplastic Diseases of the Lymphoid Tissues [11]. All patients were required to have RR disease after, at least, one prior therapy. Prior rituximab was permitted. When counting the number of previous chemotherapy regimens, maintenance therapy with rituximab was included in the previous chemotherapy. Additional inclusion criteria were between 20 and 79 years of age, at least, one measurable lesion > 1.5 cm in the largest diameter, Eastern Cooperative Oncology Group (ECOG) performance status < 2, an absolute neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 80 × 109/L, aspartate transaminase (AST)/alanine transaminase (ALT) < 2.5 times the institutional upper limit of normal, total bilirubin ≤ 2.0 mg/dL, creatinine ≤ 2.0 mg/dL, no clinically significant electrocardiogram abnormalities, and room-air blood oxygen saturation ≥ 95%.
The exclusion criteria comprised pregnant or nursing women, active secondary malignancies, seropositivity for hepatitis B virus surface antigen or hepatitis C virus antibody, human immunodeficiency virus infection, and the history of treatment with bendamustine or stem cell transplantation.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of each participating institution. The study was registered at UMIN000008145. We obtained written informed consent from each patient at the time of study entry.
Treatment
While 375 mg/m2 rituximab was administered intravenously on day 1, 90 mg/m2 bendamustine was administered intravenously on days 1 and 2. Twenty mg/body DEX were administered on days 1 and 2, and 10 mg/body on days 3–5 intravenously or orally as described in previous phase I [8] and phase II studies [9]. The treatment was provided every 28 days for up to six treatment cycles. Before the commencement of the second and subsequent cycles, the absolute neutrophil count ≥ 1000/μL, platelet counts ≥ 75,000/μL, and the absence of grade ≥ 3 toxicities were required. Treatment cycles could be delayed for a maximum of 3 weeks. If a longer delay was required, patients discontinued their participation. Dose reductions of bendamustine to 60 mg/m2 were executed at the discretion of the attending physician in patients who developed grade 4 thrombocytopenia, grade ≥ 3 neutropenia lasting for ≥ 1 week, or other grade 3 or 4 nonhematological toxicities. The recurrence of toxicity at 60 mg/m2 led to the treatment discontinuation. No dose escalation of bendamustine was allowed after a dose reduction. The dose reduction for rituximab was not allowed. Antiemetic prophylaxis with granisetron and opportunistic infection prophylaxis with trimethoprim–sulfamethoxazole and acyclovir were recommended. Monitoring of cytomegalovirus (CMV) antigenemia (C7-HRP) of each cycle was recommended. The trigger value to start preemptive therapy recommended in the protocol was CMV antigenemia > 10/100,000 white blood cells (WBCs). The use of granulocyte colony-stimulating factor for neutropenia was allowed. The prophylaxis for tumor lysis syndrome was recommended in patients with high tumor burden. Rituximab maintenance therapy following RBD was allowed.
Results
Patients’ characteristics
While 37 patients of seven institutions of KOTOSG applied to this study between July 2011 and December 2014, we enrolled 33 patients. Four patients were ineligible for inclusion, because 2 had been treated with bendamustine previously, one was diagnosed as FL grade 3b, and the other was > 80 years. Table 1 summarizes the major characteristics of 33 eligible patients at the time of enrollment.
Dose of DEX and bendamustine and the completion rate of the treatment
The study protocol comprised 20 mg/body DEX on days 1 and 2, and 10 mg/body on days 3–5 [high-dose (HD-) DEX group]. However, the protocol was amended in December 2012, because CMV infection and antigenemia recurrently occurred, as described in the “Safety” section. The dose of DEX was changed to 8 mg/body intravenously or orally on days 1 and 2 [low-dose (LD-) DEX group] in accordance with the 2010 JSCO clinical practice guideline for antiemesis [10]. While 15 patients were treated by RBD with HD-DEX, 18 were treated by RBD with LD-DEX. The dose reduction of bendamustine was performed in 1 patient at the fourth cycle of chemotherapy because of lasting grade 3 neutropenia. The completion rates of the first three cycles and all six cycles were 94% (31/33) and 61% (20/33), respectively; 87% (13/15) and 47% (7/15) in the HD-DEX group, and 100% (18/18) and 72% (13/18) in the LD-DEX group, respectively. Reasons for the treatment discontinuation were diagnosis and complication of other malignancies (e.g., gastric cancer, myelodysplastic syndrome, and desmoid tumor) after the treatment initiation (n = 4), prolongation of neutropenia and thrombocytopenia (n = 3), CMV retinitis (n = 2), discretion of the attending physicians (n = 2), patients’ request (n = 2), reactivation of hepatitis B (n = 1), and interstitial pneumonia (n = 1), redundantly. Two patients with FL were treated with rituximab maintenance therapy following RBD.
Efficacy
In this study, all 33 eligible patients were evaluable for response (Table 2). The ORR across all histological types was 88% (29/33) with 58% (19/33) attaining CR/CRu and 30% (10/33) attaining PR. SD and PD were 6% (2/33) for each. We observed no significant difference in the ORR between the HD-DEX and LD-DEX groups (12/15 vs. 17/18, P = 0.2335, Fisher’s exact test).
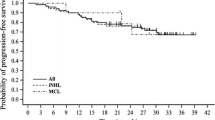
A median follow-up time for all patients was 37 months. At the time of the database cut-off, the median PFS and OS were not reached. The 3-year PFS and OS rates were 75.5 ± 8.1% (standard error, SE) and 85.5 ± 6.8%, respectively (Fig. 1a, b). Table 3 presents factors affecting the PFS and OS which were assessed by the univariate analysis. The FL International Prognostic Index (FLIPI) 2 at the time of enrollment [12] in patients with FL significantly associated with the PFS (P = 0.005, log-rank test; Fig. 1c). The number of RBD cycles and the dose of DEX correlated with the OS. The OS of the group treated with five or six cycles was significantly prolonged than that treated with four or fewer cycles (P = 0.033, log-rank test; Fig. 1d) and the OS of the group treated with LD-DEX was significantly prolonged than that treated with HD-DEX (P = 0.020, log-rank test). Patients’ characteristics listed in Table 1 were similar between the group treated with five or six cycles and that treated with four or fewer cycles by the Fisher’s exact test. Moreover, both the ORR and CRR were similar between these two groups (21/24 vs. 8/9, P = 0.7048 and 15/24 vs. 4/9, P = 0.2934, respectively, Fisher’s exact test). The other clinical factors listed in Table 1 were not significantly associated with the PFS and OS.
Survival curves by the Kaplan–Meier method. a Progression-free survival (PFS) and b overall survival (OS) curves for all eligible patients (n = 33); c PFS curves for patients with follicular lymphoma (FL) (n = 22) categorized according to the FL International Prognostic Index 2 at the time of enrollment. Solid line, dotted line, and dashed line indicate low- (n = 2), intermediate- (n = 15), and high-risk patients (n = 5), respectively (P = 0.005, log-rank test); d OS curves for all eligible patients (n = 33) categorized the number of treatment cycles of chemotherapy. Solid line, patients treated with five or six cycles (n = 24); dotted line, patients treated with four or less cycles (n = 9) (P = 0.033, log-rank test)
Safety
We evaluated safety in all 33 eligible patients. Table 4 presents hematological and nonhematological toxicity.
In this study, the leading toxicity was myelosuppression. The incidence of all grades of leukocytopenia, anemia, and thrombocytopenia was 94% (31/33), 64% (21/33), and 76% (25/33), respectively. Severe leukocytopenia, neutropenia, and lymphocytopenia were frequently observed. The incidence of grade 3–4 leukocytopenia, neutropenia, and lymphocytopenia was 55% (18/33), 67% (22/33), and 91% (30/33), respectively. The incidence of grade 3–4 anemia and thrombocytopenia was less frequent [3% (1/33), and 9% (3/33), respectively]. Although neutropenia and thrombocytopenia of most patients were reversible, the treatment of 3 patients was discontinued because of the delay of treatment over 3 weeks for the prolongation of neutropenia and thrombocytopenia.
One of the most frequent nonhematological toxicities was CMV antigenemia. The regular monitoring of CMV antigenemia was performed in 14 patients of the HD-DEX group and in 13 patients of LD-DEX group. Ten patients developed CMV antigenemia > 1/100,000 WBCs, 6 patients (40%) in the HD-DEX group, and 4 patients (22%) in the LD-DEX group. Overall, 5 patients treated with HD-DEX developed CMV antigenemia > 10/100,000 WBCs, of whom 2 developed grade 2 retinitis after 3 and 5 cycles of the treatment. Of these patients, three were treated with valganciclovir and two were treated with ganciclovir and immunoglobulin preparation. The protocol treatment of the 2 patients with retinitis was discontinued at the discretion of the attending physician. Because of these recurrent viral antigenemia and infection, the protocol was amended, and the dose of DEX was reduced as described above. No patient treated with LD-DEX developed CMV antigenemia > 10/100,000 WBCs and CMV infection. The incidence of antigenemia over this threshold in patients treated with LD-DEX was significantly lower than that in patients treated with HD-DEX (0/18 vs. 5/15, P = 0.0127, Fisher’s exact test). In contrast, no significant difference was observed in its incidence between the two groups of grade 0–3 and grade 4 lymphocytopenia (3/18 vs. 2/15; P = 0.5905, Fisher’s exact test).
Table 4 lists other nonhematological toxicities. No patient developed grade 4 or 5 toxicity. The incidence of rash was 33% (11/33), including 12% (4/33) with grade 3 toxicity. We observed no significant difference in the incidence of rash between HD-DEX and LD-DEX groups (5/15 vs. 6/18; P = 0.6427, Fisher’s exact test). The incidence of GI toxicity (nausea and vomiting) was not significantly different between the two groups (3/15 vs. 7/18; P = 0.2144, Fisher’s exact test).
Five patients died within the study observation period. All the patients were treated with HD-DEX. The causes of death of these patients were recurrence or progression of FL (n = 2) and MCL (n = 2), and complication of gastric cancer (n = 1).
Discussion
This multicenter phase II study established the efficacy and adverse effects of RBD for RR indolent B-NHL and MCL. Some previous studies have reported the effects of various chemotherapy regimens combined with rituximab for RR indolent B-NHL and MCL. Representative salvage chemotherapies combined with rituximab for these RR lymphomas were high-dose cytarabine-containing regimens [13], ifosfamide-containing regimens [14], purine analogs [15,16,17], and bendamustine. Reportedly, the CHASER regimen (rituximab, cyclophosphamide, high-dose cytarabine, etoposide, and DEX) produces exceptionally high ORR and CRR. Oki et al. reported that ORR and CRR of RR indolent B-NHL treated with CHASER regimen were 100% (17/17) and 94% (16/17), respectively [13]. Although RBD has relatively equal ORR to combination chemotherapy of rituximab and purine analogs, including cladribine and fludarabine [15, 16], the CRR of RBD was superior to that of the combination of rituximab and fludarabine (R-Flu) reported by Savage et al. [17]. A recent phase III study of RB compared with R-Flu for patients with RR indolent B-NHL and MCL reported that the PFS, ORR, and CRR for patients treated with RB were superior to those for patients treated with R-Flu [6]. In this study, the ORR and CRR were relatively equal to those of previous studies of RB. Further investigation is warranted to elucidate the therapeutic effect of the addition of DEX to RB for the population comprising RR indolent B-NHL and MCL.
In this study, FLIPI2 at the time of enrollment in patients with FL was significantly associated with the PFS. Both FLIPI and FLIPI2 are prognostic indexes for patients with FL at diagnosis [12, 18], and have been reported before bendamustine became common as a treatment for indolent B-NHL. To date, prognostic factors in patients treated with RB remain unclear. On the other hand, a few studies have investigated prognostic factors in patients with RR FL. Montoto et al. reported that FLIPI was of prognostic significance in the RR setting [19]. Our result suggests that FLIPI2 might have a prognostic value in the RR setting or patients treated with RB(D). In addition, the number of cycles of RBD was significantly correlated with the OS. In contrast, patients’ characteristics at the enrollment, ORR, CRR, and PFS were statistically similar between the groups divided by the number of cycles of RBD. Considering these results, it remained unclear whether the difference in the OS could be because of the effect itself according to the number of treatment cycles or not. The dose of DEX was also significantly correlated with the OS. The all five patients who died within the study observation period were treated with HD-DEX. However, because the causes of death of these patients were lymphoma itself (n = 4) and gastric cancer (n = 1), which were unrelated to complications of the HD-DEX, this result might be only a statistical causal connection and might not necessarily show an actual causal connection.
The leading adverse event of this study was myelosuppression. The incidence rates of neutropenia and thrombocytopenia following RBD were lower than those following high-dose cytarabine-containing regimens, including CHASER [13]. In this study, consistent with the previous reports of bendamustine therapy [1, 20], while severe lymphocytopenia was frequent, and thrombocytopenia and anemia were less frequent.
The most frequent nonhematological adverse events were CMV antigenemia and rash in this study. Ohmachi et al. conducted a phase II study of RB for RR diffuse large B-cell lymphoma (DLBCL) in Japan, in which 20 mg DEX was administered intravenously on days 1–3 and 10 mg orally on days 4 and 5 for the antiemetic prophylaxis [9]. While the incidence of CMV infection was 0–1.5% in the previous studies of RB, it was 10.2% in their study. They discussed that HD-DEX might be accountable for the high incidence of CMV infection. This study revealed that 5 patients with CMV antigenemia > 10/100,000 WBCs, of whom 2 patients developed CMV retinitis, were all treated with HD-DEX. Reportedly, lymphocytopenia is characteristic of hematological toxicity of bendamustine with or without rituximab, and could be relevant to the development of CMV reactivation in some previous studies [20, 21]. In a phase II study for RR DLBCL, all patients with CMV infection were exhibited grade 4 lymphocytopenia [9]. In this study, however, no significant difference was observed in the incidence of CMV antigenemia between the two groups of grade 0–3 and grade 4 lymphocytopenia. The incidence rates of rash and GI toxicities were not significantly different between the HD- and LD-DEX groups. Based on these findings, this study suggests that LD-DEX (8 mg, 2 days) in accordance with the guideline for antiemesis could be tolerable and feasible in RBD.
In conclusion, RBD was effective for RR indolent B-NHL and MCL. The frequent adverse events of RBD were myelosuppression, rash, and CMV antigenemia. Particularly, it is necessary to be careful to occurrence of CMV antigenemia and infection when DEX is added to RB.
References
Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N, Japanese Bendamustine Lymphoma Study Group, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–64.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, NCI Sponsored International Working Group, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–53.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, International Harmonization Project on Lymphoma, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:3383–9.
Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–9.
Rummel M, Kaiser U, Balser C, Stauch M, Brugger W, Welslau M, Study Group Indolent Lymphomas, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016;17:57–66.
Matsumoto K, Takayama N, Aisa Y, Ueno H, Hagihara M, Watanabe K, Keio BRB Study Group, et al. A phase II study of bendamustine plus rituximab in Japanese patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma previously treated with rituximab: BRB study. Int J Hematol. 2015;101:554–62.
Ogura M, Ando K, Taniwaki M, Watanabe T, Uchida T, Ohmachi K, Japanese Bendamustine Lymphoma Study Group, et al. Feasibility and pharmacokinetic study of bendamustine hydrochloride in combination with rituximab in relapsed or refractory aggressive B cell non-Hodgkin’s lymphoma. Cancer Sci. 2011;102:1687–92.
Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31:2103–9.
Takeuchi H, Saeki T, Aiba K, Tamura K, Aogi K, Eguchi K, et al. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016;21:1–12.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press; 2008.
Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–62.
Oki Y, Ogura M, Kato H, Kikuchi A, Taji H, Kagami Y, et al. Phase II study of a salvage regimen using cyclophosphamide, high-dose cytarabine, dexamethasone, etoposide, and rituximab in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Cancer Sci. 2008;99:179–84.
Stewart DA, Duan Q, Carlson L, Russell JA, Bahlis NJ, Duggan P, et al. A prospective phase II study of RICE re-induction, then high-dose fludarabine and busulfan, followed by autologous or allogeneic blood stem cell transplantation for indolent b-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2011;11:475–82.
Nagai H, Ogura M, Kusumoto S, Takahashi N, Yamaguchi M, Takayama N, et al. Cladribine combined with rituximab (R-2-CdA) therapy is an effective salvage therapy in relapsed or refractory indolent B-cell non-Hodgkin lymphoma. Eur J Haematol. 2011;86:117–23.
Robak T, Smolewski P, Urbanska-Rys H, Gora-Tybor J, Blonski JZ, Kasznicki M. Rituximab followed by cladribine in the treatment of heavily pretreated patients with indolent lymphoid malignancies. Leuk Lymphoma. 2004;45:937–44.
Savage DG, Cohen NS, Hesdorffer CS, Heitjan D, Oster MW, Garrett TJ, et al. Combined fludarabine and rituximab for low grade lymphoma and chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:477–81.
Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Montoto S, López-Guillermo A, Altés A, Perea G, Ferrer A, Camós M, et al. Predictive value of follicular lymphoma international prognostic index (FLIPI) in patients with follicular lymphoma at first progression. Ann Oncol. 2004;15:1484–9.
Saito H, Maruyama D, Maeshima AM, Makita S, Kitahara H, Miyamoto K, et al. Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B-cell and mantle cell lymphoma. Blood Cancer Jounal. 2015;5:e362.
Hasegawa T, Aisa Y, Shimazaki K, Nakazato T. Cytomegalovirus reactivation with bendamustine in patients with low-grade B-cell lymphoma. Ann Hematol. 2015;94:515–7.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Junya Kuroda received grants from Eisai Co., Ltd. and Chugai Pharmaceutical Co., Ltd., and Masafumi Taniwaki received grant from Chugai Pharmaceutical Co., Ltd. The remaining authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsumoto, Y., Kobayashi, T., Shimura, Y. et al. Combined rituximab, bendamustine, and dexamethasone chemotherapy for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma: a multicenter phase II study. Int J Hematol 110, 77–85 (2019). https://doi.org/10.1007/s12185-019-02650-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02650-w