Abstract
We investigated the safety and efficacy of mycophenolate mofetil (MMF) in the prevention and treatment of graft-versus-host disease (GVHD) using a nationwide retrospective survey in Japanese children undergoing hematopoietic stem cell transplantation (HSCT). Overall, 141 children undergoing allogeneic HSCT for hematological malignancy (n = 84), non-malignancy (n = 52), and solid tumors (n = 5) were administered MMF orally (median 8 years; range 0–15 years; 89 males and 52 females) during 1995–2011. Donors were primarily unrelated and mismatched related. In the GVHD prophylaxis group, 29% and 8.6% of patients developed grade II–IV and III–IV GVHD, respectively. Of the 32 evaluable patients, 16% developed chronic [limited (n = 4) and extensive (n = 1)] GVHD. In the acute GVHD treatment group, 61% had decreased grade. In the chronic GVHD treatment group, 36% had improved symptoms. Combined immunosuppressant was reduced or discontinued in 61% patients. Major adverse events (AEs) were neutropenia (4.3%), infection (3.5%), thrombocytopenia (2.1%), myelosuppression (2.1%), and diarrhea (1.4%). MMF dosage was reduced in two children due to grade ≥ 3 AEs; two children died from infection. MMF thus may be well tolerated in children, and may be an effective option for prophylaxis and treatment of acute and chronic GVHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the curative therapy for hematological malignancy, solid tumors, and non-malignancy in children. The supportive care for children undergoing HSCT widely improved its application and resulted in better outcome [1]; however, significant comorbidities such as graft-versus-host diseases (GVHD) limit its prevalence. The first-line treatment for acute GVHD (aGVHD) is corticosteroids [2], but non-responders suffer significant comorbidity. Among those who survived earlier transplant-related complications, 20–50% of children develop chronic GVHD (cGVHD) [3]. However, therapeutic options are limited in patients who are resistant to steroids [4]; thus, there are unmet needs for evidence-based therapy.
Mycophenolate mofetil (MMF) is a reversible inhibitor of inosine monophosphate dehydrogenase inhibitor that is highly selective for suppression of T- and B-cell growth [5,6,7]. MMF has been utilized as prophylaxis and treatment for acute and chronic GVHD in children with limited evidence. In Japan, MMF is currently only approved as an immunosuppressant for organ transplantation, although a nationwide retrospective survey revealed the efficacy and safety of using MMF in > 1000 patients who received HSCT from related [8] and unrelated donors [9]. However, the former report included only a few children aged > 12 years, the latter included only adult patients, and the actual situation using MMF in children undergoing HSCT, especially from unrelated donors, is unclear. Thus, this study aimed to evaluate the safety and efficacy of using MMF as prophylaxis and therapy for GVHD in children undergoing HSCT using a nationwide survey in the Japanese population.
Materials and methods
Study design
Data on the use of MMF after allogeneic HSCT from related [8] and unrelated [9] donors were collected as described in these reports. Questionnaires were sent to 228 adult and pediatric transplant institutes registered in the Japan Society for Hematopoietic Cell Transplantation (JSHCT) as detailed in the previous reports and data from patients aged 15 or younger were extracted. From 28 institutes, 141 children undergoing HSCT were identified to have received MMF for prophylaxis and treatment of GVHD, and were included in this study. Data regarding the purpose of treatment, dosage, duration of treatment, presence or absence of subjective symptoms of GVHD, GVHD grade and stage (before and after treatment), decrease or increase in concomitant immunosuppressants, effects, adverse events (AEs), and outcomes were collected. Basic information for each transplantation, such as HLA disparity based on low-resolution typing, was extracted from the Transplant Registry Unified Management Program (TRUMP) system, a registry used to store Japanese patient outcomes [10]. The combination of recipient HLA homozygote and donor HLA heterozygote was regarded as 2-loci mismatch when they do not share the same HLA type. The combination of recipient HLA homozygote and donor HLA homozygote was also regarded as 2-loci mismatch if these types were different. Several demographic data were not available because of lack of patient entry into the TRUMP system. The effects of MMF to subjective symptoms (none, disappearance, improvement, no change, and exacerbation) and the use of steroids (none, withdrawal, dose reduction, no change, and dose increase) were assessed by physicians in each institution. AEs were evaluated by the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE ver. 4.0). This study was approved by the ethical committees of the JSHCT, Nagoya University Graduate School of Medicine, and Japanese Red Cross Nagoya First Hospital.
Statistics
Continuous variables were summarized as mean and standard deviations, and categorical variables were summarized as percentage. The two-sided t test was used to compare continuous variables between the two groups, and the analysis of variance was used to compare more than two groups. Probabilities of neutrophil recovery, platelet recovery, aGVHD, and cGVHD were compared using cumulative incidence, and transplant-related mortality was analyzed using the cumulative incidence. P < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using JMP Pro (ver. 13.0, SAS Institute Inc., NC, USA), except for the analysis of cumulative incidence considering the competing risks using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (ver. 3.4.0, the R Foundation for Statistical Computing, Vienna, Austria) [11].
Results
Patient characteristics
A total of 141 children who underwent HSCT received MMF from October 1995 to August 2011 (Table 1): 89 (63%) boys and 52 (37%) girls, with a median age of 8 (range 0–15) years at the time of transplantation. Eighty-four (60%) patients were diagnosed with hematological malignant diseases, 5 (3.5%) with solid tumors, and 52 (37%) with non-malignant diseases. The graft source was the bone marrow (BM) from unrelated donors in 75 (53%) patients and cord blood (CB) from unrelated donors in 34 (24%) patients. The questionnaires were supposed to target children receiving allogeneic HSCT from unrelated donors; however, the study actually included 24 (17%) children undergoing HSCT from related donors whose HLAs were mostly mismatched. Eight (5.7%) children lacked data on stem cell source. Among the 75 children who received unrelated BM, 39 received 6/6 serologically HLA-matched BM, 22 received 5/6 matched BM, 11 received 2 mismatched BM, and 3 received 3 mismatched BM in the graft-versus-host direction. Among the 34 children who received unrelated CB, 10 received 6/6 serologically HLA-matched CB, 9 received 5/6 matched CB, and 15 received ≥ 2 mismatched CB in the graft-versus-host direction. This study included 3 groups; 35 children (25%) received MMF for GVHD prophylaxis, 62 (44%) received MMF for aGVHD treatment, and 44 (31%) received MMF for cGVHD treatment.
Route, dosage, intervals, and duration of MMF administration
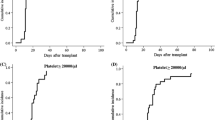
All patients received MMF orally. The duration of its administration was significantly different between the prophylaxis and treatment groups: 14–345 (median 37) days in the GVHD prophylaxis group, 10–2825 (median, 147) days in the aGVHD treatment group, and 4–1482 (median 234) days in the cGVHD treatment group (P < 0.01). The initial MMF dosage was 190–1600 (median 532) mg/m2/day in 103 patients who possessed full data on height and weight, and the dosage in each group is summarized in Fig. 1. The initial median dosage was 539 mg/m2/day in the prophylaxis group, 707 mg/m2/day in the aGVHD treatment group, and 681 mg/m2/day in the cGVHD treatment group, which was not significantly different among the three groups (P = 0.42). MMF was administered in two divided doses in 114 patients, three divided doses in 26 patients, and once daily in one patient.
Prophylaxis of GVHD using MMF
Among the 141 children receiving MMF, 35 (25%) received MMF for GVHD prophylaxis (Table 1). All patients in this cohort achieved engraftment; neutrophil engraftment was achieved within the median of 17 days for BM recipients and 21 days for CB recipients. Ten patients (29%) developed grade II–IV aGVHD, and three (8.6%) with grade III–IV aGVHD. The affected organ was mainly the skin and intestine (Fig. 2a). The cumulative incidence of developing grade II–IV aGVHD 100 days after transplantation was 28% (Fig. 2b). No significant differences were found in the incidence of grade II–IV aGVHD between the unrelated HLA-matched, mismatched, and related mismatched recipients. MMF dose was not significantly different between children with grade ≤ I and grade II–IV aGVHD, respectively (median 532 vs. 546 mg/m2; P = 0.76). Five of the 32 evaluable children developed cGVHD: four (13%) developed limited cGVHD, and one (3.1%) developed extensive cGVHD (Table 2). Two children experienced grade II aGVHD before the onset of cGVHD. The patient who developed extensive cGVHD received serologically 5/6 matched CB in the graft-versus-host direction for congenital immunodeficiency and died of cGVHD 20 months after transplantation.
Incidence of acute graft-versus-host disease (GVHD) in the prophylaxis group. a Affected organs in children who developed acute GVHD. The skin and gut were among the most common affected organs. b The cumulative incidences of grade II–IV and grade III–IV acute GVHD 100 days after hematopoietic stem cell transplantation
MMF for aGVHD treatment
Sixty-two children (44%) received MMF for aGVHD treatment in this study. This group included 14 children who used MMF to treat both aGVHD and cGVHD. Before starting the MMF, 48 children had grade II–IV aGVHD, and 28 had grade III–IV aGVHD. The first-line therapy for aGVHD was tacrolimus plus steroids (n = 51), steroids only (n = 8), and others (n = 3; tacrolimus only, cyclosporin A plus steroid, and a combination of tacrolimus, cyclosporin A, and steroid). After starting the MMF, 38 children (61%) had decreased GVHD grade (Fig. 3a). A decrease by one grade was observed in 18 patients (29%), and a decrease of two or more grades was noted in 20 patients (32%). Improvement in skin stages was observed in 40 children (65%), intestine in 17 (27%), and liver in 8 (13%; Fig. 3a). Symptoms worsened in two patients (3.2%); gut GVHD worsened in these patients (from stage 2 to 3 and from stage 0 to 2), but they stayed in the same grade (III and IV). Combined immunosuppressants were reduced in 35 patients (57%) and discontinued in 11 patients (18%). Five children (8.1%) had to increase the first-line immunosuppressants, namely tacrolimus and/or steroid, although they continued to receive MMF. Among the 14 children who used MMF to treat cGVHD and aGVHD, six had extensive cGVHD, and one improved to limited cGVHD afterward. Among the eight children with limited cGVHD, three became free of cGVHD. No exacerbation of cGVHD was noted in these 14 children.
Response to acute and chronic graft-versus-host disease (GVHD) in the treatment group. a After starting mycophenolate mofetil (MMF) for the treatment of acute GVHD, improvement in the skin stage was observed most frequently. b After the initial administration of MMF for chronic GVHD treatment, 16 patients (36%) had improved subjective symptoms. c Changes in the dosage of combined immunosuppressants after administering MMF for the treatment of acute and chronic GVHD
MMF for cGVHD treatment
A total of 44 children (31%) received MMF for the treatment of cGVHD. This group does not include those who also received MMF for treating aGVHD. Before the treatment, 14 patients had limited cGVHD, and 30 had extensive cGVHD. The concomitant therapy for cGVHD was tacrolimus plus steroid (n = 20), steroid only (n = 16), tacrolimus (n = 5), and others (n = 3; cyclosporin A plus steroid, cyclosporin A, and no other immunosuppressants). After starting the MMF, 16 patients (36%) had improved subjective symptoms (Fig. 3b). Six out of 14 children with limited cGVHD (43%) improved, and 10 out of 30 patients with extensive cGVHD (33%) improved. Notably, no one experienced exacerbation of cGVHD after starting the MMF administration at the time of answering the questionnaire, although one patient needed increment of concomitant steroid to ameliorate cGVHD. Concomitant immunosuppressants were reduced in 17 (41%) and discontinued in 10 patients (24%), respectively.
AEs regarding MMF administration in children undergoing HSCT
AEs with an NCI-CTCAE grade of ≥ 3 possibly associated with MMF were reported in 14 patients (10%). The common AEs were neutropenia (n = 6, 4.3%), infection (n = 5, 3.5%), thrombocytopenia (n = 3, 2.1%), myelosuppression (n = 3, 2.1%), and diarrhea (n = 2, 1.4%; Table 3). One patient developed grade 4 acute kidney injury (AKI) 20 days after starting the MMF for treating extensive cGVHD; however, the renal function finally improved without changing the dosage. Regarding renal AEs of any grade, two patients were reported to develop grades 1 and 2 AKI 9 and 42 days after starting MMF for the treatment of aGVHD and GVHD prophylaxis, respectively. The former with grade 1 AKI improved after decreasing the MMF dosage, whereas the latter with grade 2 AKI did not improve even after decreasing the dosage.
Five out of 45 children (11%) whose MMF dosage was 300–600 mg/m2/day developed AEs grade of ≥ 3, and seven out of 38 (18%) who received MMF of 600–1200 mg/m2/day developed AEs. The MMF dose tended to be higher in patients with AEs as compared with those without AEs, but was not statistically significant (median 713 vs. 572 mg/m2/day; P = 0.519). The MMF dosage of two patients had to be reduced because of AEs grade of ≥ 3, but no exacerbation of other symptoms was observed after the reduction. Two patients (1.4%) were reported to die from AEs possibly associated with MMF, i.e., primarily infections.
Transplant outcomes
Among the 141 patients, 30 (21%) died after transplantation as of 2011. Transplantation-related mortality was 31%, with a median follow-up of 5 years, which was not significantly different among the GVHD prophylaxis and treatment groups (Supplemental Figure). The main causes of death were disease relapse (n = 9, 3.0%), cGVHD (n = 6, 2.0%), aGVHD (n = 3, 1.0%), infection (n = 3, 1.0%), and secondary malignancy (n = 3, 1.0%). Of the eight deaths (23%) in the prophylactic group, the main causes were disease relapse (n = 3) and GVHD (n = 3). Fifteen deaths (24%) were reported in the aGVHD treatment group, with GVHD (n = 4), infection (n = 3), and disease relapse (n = 3) as the main causes. Seven deaths (16%) were reported in the cGVHD treatment group, with disease relapse (n = 3) and cGVHD (n = 2) as the main causes. Of the six children whose primary cause of death was reported to be cGVHD, two were in the prophylaxis group, two were in the aGVHD treatment group, and two were in the cGVHD treatment group. Among the 15 patients with available data in the cGVHD treatment group, 12 had a Karnofsky performance status of ≥ 90%, two with 60–90%, and one with 50% as of 2011.
Discussion
This retrospective study demonstrated that MMF in combination with other immunosuppressive therapies seems to be safe and effective as GVHD prophylaxis and treatment for aGVHD and cGVHD in children receiving HSCT from unrelated donor. MMF has been practically used in the HSCT field, especially when using reduced intensity conditioning; however, large prospective studies defining the optimal dose and combination of other immunosuppressants are lacking in children.
MMF has been utilized in children with prophylaxis and treatment of GVHD [12,13,14,15,16]. Although most of the reports were retrospectively designed and conducted in a small number of patients, MMF in combination with other immunosuppressive agents seems to be less toxic in children when compared with adults and may be effective for both prophylaxis and treatment of GVHD. A nationwide survey including 716 adult patients who received unrelated HSCT in Japan reported that the incidences of grade II–IV aGVHD and cGVHD in the prophylactic group were 38% and 28%, respectively [9]. These incidences seem to be higher than those from our cohort (29% for the aGVHD incidence and 14% for the cGVHD incidence). Interestingly, grade ≥ 3 infection was observed in 9.5%, which seems to be higher than our cohort, although the incidence of grade 3–4 neutropenia was 2.7% and did not seem to be higher than that in the pediatric cohort. A pilot study on the pharmacokinetics of MMF demonstrated that the concentrations of MPA, the active form of MMF, could vary among the different age groups [15, 17]. This could explain the different efficacy and AE demographics of MMF in younger children.
Our current survey demonstrates that the efficacy rate of MMF is approximately 60% for the treatment of aGVHD and cGVHD. The number of patients in our aGVHD cohort treated with MMF seems to be the largest in pediatric HSCT. When treating aGVHD with MMF, the best responses were observed in patients with skin aGVHD, which is consistent with those in a previous report that 14 of 15 patients with skin aGVHD showed improvements [18]. The overall improvement in aGVHD grade was noted in 11 of 17 patients (65%) in this report, which is consistent with our results (61%). A retrospective report from a single institute in Japan treating aGVHD revealed that 11 of 14 children (79%) with steroid-refractory grade II–IV aGVHD achieved a complete response within 8 weeks, and the toxicity was tolerable [19]. These children were not included in our study, and the higher response rate in that study may be due to the bias because of different sample sizes. The role of MMF for the treatment of aGVHD in children undergoing allogeneic HSCT may be defined in future prospective studies.
During the initial use of MMF for the treatment of cGVHD, 20 children had already received tacrolimus plus steroid, 16 received steroids, and five received tacrolimus in our study; however, after the MMF administration, symptoms related to cGVHD improved with other immunosuppressants in 17 cases (39%), and the treatment was discontinued in 10 cases (23%). This result is consistent with those from a smaller study in children that reported a significant reduction of steroids in 45% of children undergoing HSCT and discontinuation in 27% [20].
Previous reports demonstrated that the incidence of renal damage attributable to MMF (0–13%) was lower than that reported with other immunosuppressants like calcineurin inhibitors [18, 21, 22]. Our analysis revealed that the incidence of grade 4 AKI was 0.7% (n = 1). Thus, MMF would be especially useful in patients highly at risk for developing AKI. MMF has been reported to increase the risk for opportunistic infections, particularly viral diseases [23]. Our current study showed that 5 of 141 children (3.5%) who experienced infection were thought to be related to MMF overall, and three of them were in the GVHD prophylaxis group, in which neutropenia before engraftment could have influenced the rate of the opportunistic infection. Notably, none of the patients discontinued the use of MMF because of AEs, and only two patients needed a reduced MMF dosage because of AEs, which did not result in exacerbation of symptoms afterward.
Our study has some limitations. As a retrospective study, incompleteness or inaccuracy of data and lack of control over the quality of measurements could result in biases. In the GVHD prophylaxis group, nearly half of patients were serologically 6/6 HLA-matched, and the rate seems to be comparable to those in the previous retrospective cohorts (Supplemental Table) [13, 15, 16, 24,25,26,27]. Seven out of eight children who received HSCT from related donors were HLA mismatched, which may adversely affect the GVHD incidence. Nevertheless, the incidence of grade II–IV GVHD was comparable to existing reports that include many of those who received HSCT from matched related donors (Supplemental Table).
In conclusion, our nationwide retrospective cohort suggests that MMF is tolerable and effective as prophylaxis and treatment of GVHD in children undergoing allogeneic HSCT. Prospective randomized studies including the pharmacokinetics are necessary to determine the optimal MMF dose and combination therapy for GVHD in children.
References
Kato K, Sakaguchi H, Muramatsu H, Sekiya Y, Kawashima N, Narita A, et al. Danaparoid reduces transplant-related mortality in stem cell transplantation for children. Pediatr Transplant. 2018;22:e13099.
Dhir S, Slatter M, Skinner R. Recent advances in the management of graft-versus-host disease. Arch Dis Child. 2014;99:1150–7.
Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin N Am. 2010;57:297–322.
Okamoto S, Teshima T, Kosugi-Kanaya M, Kahata K, Kawashima N, Kato J, et al. Extracorporeal photopheresis with TC-V in Japanese patients with steroid-resistant chronic graft-versus-host disease. Int J Hematol. 2018;108:298–305.
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118.
Miyamoto T, Takashima S, Kato K, Takase K, Yoshimoto G, Yoshida S, et al. Comparison of cyclosporine and tacrolimus combined with mycophenolate mofetil in prophylaxis for graft-versus-host disease after reduced-intensity umbilical cord blood transplantation. Int J Hematol. 2017;105:92–9.
Nakane T, Nakamae H, Yamaguchi T, Kurosawa S, Okamura A, Hidaka M, et al. Use of mycophenolate mofetil and a calcineurin inhibitor in allogeneic hematopoietic stem-cell transplantation from HLA-matched siblings or unrelated volunteer donors: Japanese multicenter phase II trials. Int J Hematol. 2017;105:485–96.
Iida M, Fukuda T, Ikegame K, Yoshihara S, Ogawa H, Taniguchi S, et al. Use of mycophenolate mofetil in patients received allogeneic hematopoietic stem cell transplantation in Japan. Int J Hematol. 2011;93:523–31.
Iida M, Fukuda T, Uchida N, Murata M, Aotsuka N, Minagawa K, et al. Mycophenolate mofetil use after unrelated hematopoietic stem cell transplantation for prophylaxis and treatment of graft-vs.-host disease in adult patients in Japan. Clin Transplant. 2014;28:980–9.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9.
Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–58.
Urban C, Benesch M, Sykora KW, Schwinger W, Lackner H. Non-radiotherapy conditioning with stem cell transplantation from alternative donors in children with refractory severe aplastic anemia. Bone Marrow Transplant. 2005;35:591–4.
Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–43.
Styczynski J, Tallamy B, Waxman I, van de Ven C, Milone MC, Shaw LM, et al. A pilot study of reduced toxicity conditioning with BU, fludarabine and alemtuzumab before the allogeneic hematopoietic SCT in children and adolescents. Bone Marrow Transplant. 2011;46:790–9.
Zhang D, Renbarger JL, Chow DS. Pharmacokinetic variability of mycophenolic acid in pediatric and adult patients with hematopoietic stem cell transplantation. J Clin Pharmacol. 2016;56:1378–86.
Basara N, Blau WI, Römer E, Rudolphi M, Bischoff M, Kirsten D, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant. 1998;22:61–5.
Inagaki J, Kodama Y, Fukano R, Noguchi M, Okamura J. Mycophenolate mofetil for treatment of steroid-refractory acute graft-versus-host disease after pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2015;19:652–8.
Busca A, Saroglia EM, Lanino E, Manfredini L, Uderzo C, Nicolini B, et al. Mycophenolate mofetil (MMF) as therapy for refractory chronic GVHD (cGVHD) in children receiving bone marrow transplantation. Bone Marrow Transplant. 2000;25:1067–71.
Basara N, Blau WI, Kiehl MG, Römer E, Rudolphi M, Bischoff M, et al. Efficacy and safety of mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant recipient. Transplant Proc 1998;30:4087-4089
Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84:681–5.
Minagawa K, Yamamori M, Katayama Y, Matsui T. Mycophenolate mofetil: fully utilizing its benefits for GvHD prophylaxis. Int J Hematol. 2012;96:10–25.
Burroughs LM, Storb R, Leisenring WM, Pulsipher MA, Loken MR, Torgerson TR, et al. Intensive postgrafting immune suppression combined with nonmyeloablative conditioning for transplantation of HLA-identical hematopoietic cell grafts: results of a pilot study for treatment of primary immunodeficiency disorders. Bone Marrow Transplant. 2007;40:633–42.
Chen HR, Ji SQ, Wang HX, Yan HM, Zhu L, Liu J, et al. Humanized anti-CD25 monoclonal antibody for prophylaxis of graft-vs-host disease (GVHD) in haploidentical bone marrow transplantation without ex vivo T-cell depletion. Exp Hematol. 2003;31:1019–25.
Windreich RM, Goyal RK, Joshi R, Kenkre TS, Howrie D, Venkataramanan R. A pilot study of continuous infusion of mycophenolate mofetil for prophylaxis of graft-versus-host-disease in pediatric patients. Biol Blood Marrow Transplant. 2016;22:682–9.
Militano O, Ozkaynak MF, Mehta B, van deVen C, Hamby C, Cairo MS. Mycophenolate mofetil administered every 8 hours in combination with tacrolimus is efficacious in the prophylaxis of acute graft versus host disease in childhood, adolescent, and young adult allogeneic stem cell transplantation recipients. Pediatr Blood Cancer. 2018;65:e27091.
Acknowledgements
The authors would like to thank the following collaborating institutions for collecting patient data: Hokkaido University, Sapporo Medical University, Jichi Medical University, Narita Red Cross Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Medical Dentinal University, Yokohama City University, Kanagawa Cancer Center, Tokai University, Niigata University, Kanazawa University, Shinshu University, Hamamatsu Medical University, Shizuoka Children’s Hospital, Kyoto University, Kinki University, Osaka University, Osaka City University, Osaka City General Hospital, Osaka Women’s and Children’s Hospital, Matsushita Memorial Hospital, Kobe University, Okayama University, Hiroshima University, Yamaguchi University, Kagawa Children’s Hospital, Kyushu University, and Kyushu Cancer Center (listed from north to south).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no relevant conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
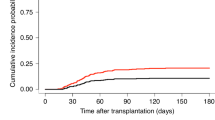
Supplemental Figure
: The cumulative incidence of transplantation-related morality. The overall transplantation-related mortality (TRM) rate was 0.31, with a median follow-up of 5 years. The TRM rate was 0.28 in the GVHD prophylaxis group, 0.30 in the acute GVHD treatment group, and 0.35 in the chronic GVHD treatment group. TRM was not significantly different among the GVHD prophylaxis and treatment groups. (PDF 426 KB)
About this article
Cite this article
Kawashima, N., Iida, M., Suzuki, R. et al. Prophylaxis and treatment with mycophenolate mofetil in children with graft-versus-host disease undergoing allogeneic hematopoietic stem cell transplantation: a nationwide survey in Japan. Int J Hematol 109, 491–498 (2019). https://doi.org/10.1007/s12185-019-02601-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02601-5