Abstract
Rurioctocog alfa (recombinant factor VIII: Advate®) is available for the control of bleeding among patients with hemophilia A in Japan. To evaluate the perioperative safety and hemostatic efficacy of Advate®, a postmarketing surveillance was conducted in Japanese patients undergoing surgery in a real-world setting. A total of 74 surgical procedures performed in 58 subjects aged 0–75 years, including three females, were studied. A hemostatic efficacy rating of “excellent” or “good” was reported in 73/74 surgical procedures (98.6%). Perioperative bleeding was successfully controlled by Advate® in five subjects with positive FVIII inhibitors (2.4-9.1 BU/mL). Advate® was administered at higher initial bolus doses (114-385 IU/kg) and at higher rates by subsequent initial continuous infusion (8.3-15 IU/kg/hour) in the five subjects with inhibitor than in the subjects without inhibitor (n = 47; mean initial bolus dose: 53.4 IU/kg; subsequent mean initial continuous infusion: 3.8 IU/kg/h). Adverse drug reactions were reported in 7/74 (9.5%) procedures, two of which were the development of de novo FVIII inhibitors. Overall, the perioperative use of Advate® in a real-world setting was found to be safe and effective among Japanese patients with hemophilia A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rurioctocog alfa (Advate®, Shire) is a third-generation (plasma/albumin-free) recombinant anti-hemophilic factor VIII (FVIII), whose marketing approval in Japan was granted in October 2006 with the indication for the control of bleeding among patients with hemophilia A [1, 2]. In clinical practice, Advate® has been used for regular replacement therapy (hereinafter referred to as regular prophylaxis), on-demand control of episodic bleeding, and perioperative bleeding management in pediatric and adult patients with hemophilia A [3].
Hemophilia A is a congenital bleeding disorder caused by the deficiency of a functional coagulation FVIII, which is often characterized by frequent bleeding into joints with pain, swelling, and disability [4]. In some cases, intracranial hemorrhage can be life threatening or fatal [5]. The average life expectancies of patients with hemophilia A had been far below normal before FVIII replacement therapies became commercially available [6]. However, the advent of plasma-derived coagulation factors and subsequent recombinant FVIII (rFVIII) concentrates has enabled patients with hemophilia A to have near normal male life expectancies with improved quality of life [7].
In the growing and aging patient population with hemophilia A, hemophilia-related comorbidities (such as hemophiliac arthropathy) and age-related comorbidities (such as cardiovascular diseases and cancer) are expected to be more common [8, 9]. These comorbidities often require surgical interventions where patients with hemophilia A face a risk of excessive bleeding.
To control bleeding during surgery, two methods of administration are currently used: single or intermittent bolus infusion (BI) and continuous infusion (CI) after loading bolus infusion [10, 11]. For maintenance of plasma FVIII level, intermittent BI results in remarkable changes in FVIII between peaks and troughs according to the interval of BI. In contrast, CI is advantageous in keeping steady-state plasma FVIII level over time, thereby avoiding a low trough level, which may potentially cause excessive bleeding during the perioperative period. CI could reduce the total consumption of FVIII by eliminating unnecessary high peaks compared to intermittent BI infusion [12, 13].
Excessive and/or uncontrolled bleeding are potential risks during the perioperative period for patients with hemophilia A who undergo surgery. In addition, the most serious complication after surgical procedures is the formation of inhibitors, neutralizing alloantibodies against FVIII [14, 15]. FVIII inhibitors attenuate the therapeutic effect of infused FVIII and can result in serious bleeding unless proper treatment is provided.
To investigate the safety and efficacy of hemostatic treatment with Advate® during the perioperative period in patients with hemophilia A undergoing surgery, a postmarketing surveillance (PMS) on the use of Advate® was undertaken in Japan under the conditions of postapproval routine clinical practice.
Materials and methods
Study design
This PMS was conducted in an open-label, multicenter, prospective/retrospective, uncontrolled, non-interventional manner and in accordance with the Japanese ordinance for Good Post-Marketing Study Practice [16]. It was designed to evaluate the safety and perioperative hemostatic efficacy of Advate® in Japanese patients with congenital hemophilia A, who were to undergo surgery between November 2010 and June 2013. The observation period started on the day of the preoperative infusion of Advate® and ended either on the day of the postoperative discharge or when the treating physician considered that postoperative treatment was completed.
Subjects
Patients of any age with hemophilia A who had been scheduled to undergo surgery during the study period were registered, irrespective of sex, treatment regimen, type of surgical procedure to be performed, or severity of hemophilia A. If a patient was to undergo more than one surgical procedure, the patient was registered again.
Statistical analyses
The sample size calculation was not based on statistical considerations. Given the limited number of patients with congenital hemophilia A who were undergoing surgery in Japan during the study period, the original plan was to register at least 30 subjects.
For the evaluation of perioperative hemostatic efficacy of Advate®, the attending surgeon or physician in charge of bleeding control was responsible for the hemostatic efficacy rating using the four-point rating scale, which comprised “excellent,” “good,” “fair,” and “none” (Table 1). The evaluation of perioperative hemostatic efficacy consisted of two phases (i.e., intraoperative and postoperative), based on which the overall hemostatic efficacy was determined. The overall hemostatic efficacy was defined as the lesser rating of the intra- and postoperative hemostatic efficacy.
Safety was assessed based on the occurrence of adverse drug reactions (ADRs). To investigate the occurrence of ADRs in subjects who received at least one dose of Advate®, all adverse events were recorded and assessed for the relationship to the use of Advate® with the assessment of seriousness (serious or non-serious) and expectedness (expected or unexpected). Those ADRs not listed in the Japanese package insert throughout the PMS period were defined as unexpected ADRs.
Inhibitor titers were expressed in terms of Bethesda units (BU/mL). A positive inhibitor was determined according to the cutoff value of the local laboratory.
All data analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Subjects
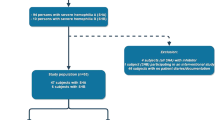
Between November 2010 and December 2011, 75 surgical procedures of 59 subjects (56 male patients with hemophilia A, 1 female patient, and 2 female carriers of hemophilia A) were registered at 10 medical institutions in Japan. One procedure was excluded as an FVIII concentrate other than Advate® was exclusively administered for tooth extraction. Fifty-eight subjects received perioperative treatment with Advate® in 74 surgical procedures. At baseline, the mean (standard deviation: SD) age was 36.0 (19.3) years with a median of 40.5 years (range 0–75), while the mean (SD) body weight was 54.3 (21.0) kg with a median of 56.0 (range 5.7–110) kg.
Surgical procedures and mode of administration
Multiple surgical procedures were performed in 11 subjects (19.0%) as follows: 2 surgeries (n = 7), 3 surgeries (n = 3), and 4 surgeries (n = 1). Fifty-seven surgical procedures were performed in severe subjects (77.0%), 1 was performed in a moderate subject (1.4%), and 16 were performed in mild (21.6%) subjects including 1 female patient and 2 carriers. The most commonly performed surgical procedures were orthopedic in nature (n = 41), followed by vascular (n = 10), gastroenterological (n = 9), dermatologic (n = 5), oral/dental (n = 5), obstetrical/gynecological (n = 2), urological (n = 1), and ophthalmic (n = 1) procedures (Table 2).
Advate® was administered by CI in 51/74 (68.9%) procedures. Most orthopedic (92.7%), vascular (60.0%), and urological (100%) surgeries were performed by CI, whereas the majority of other surgeries were performed by BI. Sixty-nine surgical procedures were performed in subjects who had no preoperative inhibitor. Of these, 22 were treated only by BI: single BI in 9 procedures and 2 BIs in 13 procedures; the mean (SD) initial BI dose was 44.6 (13.6) IU/kg (range 13.5–75.0). CI was used for the rest of the 47 surgical procedures; the mean (SD) loading bolus dose, 53.4 (11.8) IU/kg (range 27.0–99.0); the mean (SD) initial CI rate, 3.8 (0.9) IU/kg/h (range 2.7–6.5).
Six male subjects had a history of FVIII inhibitors, of whom 5 tested positive again and 1 tested negative prior to surgery. The preoperative FVIII inhibitor titers of the 5 subjects ranged from 2.4 to 9.1 BU/mL (Table 3). Case 1 was a 4-month-old previously untreated patient who developed a spinal epidural hematoma, for which removal surgery was performed (registered as first surgery). On postoperative (PO) day 18, FVIII inhibitor (2.46 BU/mL) was detected. On PO day 19, the subject with the inhibitor subsequently underwent spondylodesis (registered as second surgery). Advate® was administered by BI twice daily for 7 consecutive days and once daily for another 2 days. The other 4 subjects aged 1–17 years (Cases 2–5) received loading bolus doses of 114–385 IU/kg, followed by CI (initial rate: 8.3-15 IU/kg/h) for central venous catheterization (Cases 2, 3, and 4) or central venous catheter removal (Case 5). On the preoperative days and/or on PO day 1, plasma FVIII levels were recovered to 50–124% (Cases 2–5). In Case 3, the plasma FVIII level decreased to < 1% on PO day 5 with the FVIII inhibitor titer increased to 6.6 BU/mL. In Case 5, the plasma FVIII level decreased to < 1% on PO day 6 with the FVIII inhibitor titer increased to 25.3 BU/mL (Table 4). No additional bypassing agents were administered to the 5 subjects perioperatively. All of the 5 subjects achieved hemostasis with an overall hemostatic efficacy rating of “excellent.”
Efficacy
An overall hemostatic efficacy rating of either “excellent” or “good” was given to 57/58 (98.3%) subjects of 73/74 (98.6%) surgeries: 60 (81.1%) surgeries rated “excellent” and 13 (17.6%) rated “good.” Blood transfusion was required in 12/74 (16.2%). Intraoperative blood loss was < 200 mL in 52 procedures (70.3%), 200 to ≤ 400 mL in 9 procedures (12.2%), 400 < to ≤ 600 mL in 7 procedures (9.5%), and > 600 mL in 6 procedures (8.1%). None of the subjects was concomitantly treated with FVIII products other than Advate® or bypassing agents.
One subject was rated “fair.” This was a 43-year-old male subject who had undergone total joint arthroplasty for hemophilic arthropathy of the right knee. The subject received the loading BI of 52.6 IU/kg and subsequent initial CI at 4.4 IU/kg/h. Although the subject experienced an intraoperative blood loss of 144 mL, the surgery was completed with no transfusion and no clinical complications. The subject was discharged on PO day 19.
All female subjects (n = 3) achieved hemostasis with an overall hemostatic efficacy rating of “excellent.” The 29-year-old female subject with mild hemophilia A underwent nephrectomy of the right kidney for a renal tumor, with a loading bolus dose of 40 IU/kg followed by CI (infusion rate: 2.8 to 4.0 IU/kg/h). The intraoperative blood loss was 47 mL. No blood transfusion was performed. The 26-year-old subject and the 33-year-old subject, both of whom were carriers of hemophilia A, underwent perineotomy at vaginal delivery, for which they received a single bolus dose of 27.3 IU/kg and 13.5 IU/kg, respectively. Both subjects experienced a small amount of intraoperative blood loss.
Safety
Of the 74 surgical procedures performed, 7 events of ADRs were reported in 7 procedures (9.5%). Of the 7 ADRs, 3 events were serious ADRs and 4 events were non-serious ADRs. The serious ADRs included 2 events of the development of de novo FVIII inhibitor and 1 event of an increase in FVIII inhibitor titersFootnote 1. The non-serious ADRs were 2 events of anemia, 1 event of postoperative anemia, and 1 event of abnormal liver function. No perioperative death was reported. No ADRs were considered unexpected.
There were 2 subjects (4-month-old and 22-year-old) who developed de novo FVIII inhibitor during perioperative treatment with Advate®. The 4-month-old male subject was a previously untreated patient with severe hemophilia A, who underwent removal of spinal epidural hematoma (first surgery). On the day of the surgery, the subject received a loading bolus dose of 43.9 IU/kg followed by CI (infusion rate: 5.4 IU/kg/h) until PO day 4 and a daily bolus dose until PO day 10. On PO day 18 (12 EDs), FVIII inhibitor (2.46 BU/mL) was detected. The second surgery of spondylodesis was covered by intermittent BI of Advate® (Case 1 in Tables 3 and 4). Immune tolerance induction therapy was then implemented. The inhibitor consequently decreased to 0.3 BU/mL at 20 months after the first surgery. The 22-year-old male subject with severe hemophilia A had ≥ 151 EDs with other FVIII products prior to registration. The subject had an infected pilonidal cyst, for which debridement and epidermization were performed. On the day of the surgery, the subject received two bolus doses of Advate® (42.4 and 14.1 IU/kg), followed by bolus doses of 28 IU/kg for 7 days and 14 IU/kg for 2 days. On PO day 19 (11 EDs after surgery), FVIII inhibitor (1.94 BU/mL) was detected, which became undetected spontaneously 17 days later.
Discussion
The present study evaluated the safety and hemostatic efficacy of Advate® in perioperative bleeding management among 58 Japanese subjects (aged 0–75 years) with congenital hemophilia A who underwent a total of 74 surgical procedures in a real-world postmarketing setting. The perioperative hemostatic efficacy of Advate® in 73/74 surgical procedures was shown to be effective. The result was comparable to the results of the previous Advate® surgery study [10] and to the findings on other commercially available rFVIII concentrates [17, 18].
Orthopedic surgery was performed most commonly in this study, accounting for 55.4% (41/74) of all surgical procedures performed. Total joint arthroplasty accounted for 56.1% (23/41) of all orthopedic procedures. Given that 77.0% (57/74) of subjects had severe hemophilia A and the mean (median) age of the subjects was 36.0 (40.5) years, it can be inferred that these subjects had developed severe arthropathy during the period when prophylactic treatment had not been standard care.
The peak level of plasma FVIII > 100% with a trough above 50–80% during major surgeries is recommended in the treatment guideline for hemophilia A patients without inhibitors [19]. The guideline recommends CI for hemostatic control during major surgical procedures in hemophilia patients without inhibitors. In the present study, CI was applied to 68.9% (51/74) of surgical procedures performed, most of which were orthopedic in nature. CI was also applied to the majority of vascular surgeries: central venous catheterization, central venous catheter removal, and radial arteriorrhaphy. The mean loading bolus dose of 53.4 IU/kg followed by the mean initial CI with 3.8 IU/kg/h (n = 47) would reflect that the treating physicians attempted to achieve a target plasma FVIII above 100% as stated in the guideline. Although the type of surgical procedure was not predefined as major or minor in this study, most surgical procedures that required CI would have been classified as major if defined in terms of risk of excessive bleeding during surgery, whereas 23 procedures treated by BI were considered to be minor surgeries because only 1 or 2 BIs were required perioperatively.
Five subjects with a positive FVIII inhibitor at registration underwent surgery with FVIII replacement dosing in addition to a neutralizing dose. The large initial bolus doses (114–385 IU/kg for patients with 2.4–9.1 BU/mL of inhibitor) were considered to be elected as total replacement doses including the inhibitor-neutralizing dose (Table 4). In 4 subjects (Cases 2–5), subsequent CIs started with initial rates of 8.3–11.5 IU/kg/h, which were higher than the mean (SD) initial CI rate of 3.8 (0.9) IU/kg/kg in surgeries of 47 non-inhibitor subjects. Although the rationale for the higher setting of CI rates during the perioperative phase in the inhibitor-positive subjects was not recorded by the treating physicians, a higher rate of CI would be required due to larger FVIII clearance by the production of inhibitor antibody even after neutralization with a large initial dose. The plasma FVIII levels were kept at 53 to 86% during PO day 1–4, although the available data are sparse and all 4 cases resulted in effective control of bleeding. It is suggested that surgeries such as central venous catheterization for patients with relatively low-titer inhibitors (< 10 BU/mL) could be managed by a large dose of FVIII replacement therapy. It should be noted that vigilant plasma FVIII monitoring will be required in the postoperative hemostatic management with FVIII, especially for inhibitor-positive patients.
The formation of inhibitory alloantibodies to FVIII is a major obstacle of hemophilia treatment. Inhibitors are likely to develop in certain environmental settings such as intensive FVIII treatment, inflammation, and infection [20]. Inflammation may provoke antibody formation by the concurrent presence of cytokine release arising from injured tissues, which is considered a ‘danger signal’ [21]. Surgery can trigger the development of inhibitors, as a danger signal causing tissue damage and injury during the procedure with intensive FVIII administration [22]. In severe hemophilia A patients, inhibitors predominantly occur early in treatment; during the first 50 EDs. Of 2 de novo inhibitors reported in the present study, 1 case of 4-month-old severe hemophilia A had risk factors: a PUP case (0 ED) at registration and intensive treatment of ≥ 5 EDs during the perioperative period. Another case of a 22-year-old developed low-titer transient inhibitor despite having more than 150 EDs with other FVIII products at registration but had intensive perioperative Advate® administration for 10 days, resulting in inhibitor formation at 11 EDs with Advate®.
Conclusion
Although the types of surgical procedures performed were mostly orthopedic in nature, this PMS covered a wide range of the surgical spectrum, from ophthalmic to gastroenterological surgeries, and resulted in ratings of “excellent” or “good” hemostatic efficacy by the treating physician for all but one surgery. The results demonstrated that perioperative bleeding control with Advate® is well tolerated and effective in Japanese patients with hemophilia A in a real-world postmarketing setting.
Notes
The increase in FVIII inhibitor titers was coded into two discrete serious ADRs (i.e., FVIII inhibition and condition aggravated) by the sponsor, which was reported in the reexamination application of Advate® PMS (a special investigation on surgery).
References
Tarantino MD, Collins PW, Hay CR, Shapiro AD, Gruppo RA, Berntorp E, et al. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia. 2004;10:428–37.
Blanchette VS, Shapiro AD, Liesner RJ, et al. on behalf of the rAHF-PEM Clinical Study Group. Plasma and albumin-free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated paediatric patients. J Thromb Haemost 2008; 6:1319-26.
Dhillon S. Octocog Alfa, Antihaemophilic Factor (Recombinant), Plasma/Albumin Free Method (ADVATE®) A Review of its use in the management of patients with haemophilia A. Drugs. 2012;72:987–1007.
Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol. 2007;136:777–87.
Tabibian S, Motlagh H, Naderi M, Dorgalaleh A. Intracranial hemorrhage in congenital bleeding disorders. Blood Coagul Fibrinolysis. 2017. https://doi.org/10.1097/MBC.0000000000000660.
Mejia-Carvajal C, Czapek EE, Valentino LA. Life expectancy in hemophilia outcome. J Thromb Haemost. 2006;4:507–9.
Oldenburg J, Dolan G, Lemm G. Haemophilia care then, now and in the future. Haemophilia. 2009;15(Suppl 1):2–7.
Franchini M, Mannucci PM. Co-morbidities and quality of life in elderly persons with haemophilia. Br J Haematol. 2010;148:522–33.
Canaro M, Goranova-Marinova V, Berntorp E. The ageing patient with haemophilia. Eur J Haematol. 2015;94(Suppl 77):17–22.
Négrier C, Shapiro A, Berntorp E, Pabinger I, Tarantino M, Retzios A, et al. Surgical evaluation of a recombinant factor VIII prepared using a plasma/albumin-free method: efficacy and safety of Advate in previously treated patients. Thromb Haemost. 2008;100:217–23.
Batorova A, Martinowitz U. Continuous infusion of coagulation factors. Haemophilia. 2002;8:170–7.
Batorova A, Martinowitz U. Intermittent injections vs. continuous infusion of factor VIII in haemophilia patients undergoing major surgery. Br J Haematol. 2000;110:715–20.
Dingli D, Gastineau DA, Gilchrist GS, Nichols WL, Wilke JL. Continuous factor VIII infusion therapy in patients with haemophilia A undergoing surgical procedures with plasma-derived or recombinant factor VIII concentrates. Haemophilia. 2002;8:629–34.
Fischer K, Lassila R, Peyvandi F, Calizzani G, Gatt A, Lambert T, et al. Inhibitor development in haemophilia according to concentrate. Four-year results from the European HAemophilia Safety Surveillance (EUHASS) project. Thromb Haemost. 2015;113:968–75.
Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127:379–91.
Santagostino E, Lentz SR, Misgav M, Brand B, Chowdary P, Savic A, et al. Safety and efficacy of turoctocog alfa (NovoEight®) during surgery in patients with haemophilia A: results from the multinational GuardianTM clinical trials. Haemophilia. 2015;21:34–40.
Mahlangu JN, Ragni M, Gupta N, Rangarajan S, Klamroth R, Oldenburg J, et al. Long-acting recombinant factor VIII Fc fusion protein (rFVIIIFc) for perioperative haemostatic management in severe haemophilia A. Thromb Haemost. 2016;116:1–203.
Fujii T, Amano K, Atsumi T, Ishiguro A, Ohira K, Okamoto K, et al. Treatment guideline for hemophilia without inhibitor: 2013 update. Japanese J Thromb Hemost. 2013;24:619–39.
Gouw SC, van den Berg HM. The multifactorial etiology of inhibitor development in hemophilia: genetics and environment. Semin Thromb Hemost. 2009;35:723–34.
Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5.
Eckhardt CL, van der Bom JG, van der Naald M, Peters M, Kamphuisen PW, Fijnvandraat K. Surgery and inhibitor development in hemophilia A: a systematic review. J Thromb Haemost. 2011;9:1948–58.
Acknowledgements
This manuscript is dedicated to the memory of our esteemed colleague Dr. Hideji Hanabusa, MD, whose untimely passing away in October 2016 left a permanent void. He touched the lives of many as a mentor, scholar, collaborator, and friend. Dr. Hanabusa was instrumental in the development of this product, and the creation and interpretation of the data included herein, and a co-author of this manuscript. We thank Shire PMS and PV teams for the support in data clarification. We recognize with gratitude the patients and institutions that participated in the studies: IMSUT Hospital, Institute of Medical Science, The University of Tokyo; Nara Medical University Hospital; Tokyo Medical University Hospital; St. Marianna University School of Medicine Hospital; Nagoya University Hospital; Ogikubo Hospital; Hospital of the University of Occupational and Environmental Health; Kurume University Hospital; Hyogo College of Medicine Hospital; Mie University Hospital.
Author information
Authors and Affiliations
Contributions
KN, HT, MS, AY, TM, JT, MT, KF, and AS collected and interpreted data, and revised the manuscript. WE analyzed the statistics, interpreted data, and revised the manuscript. HU, HT, and MA interpreted data and drafted and revised the manuscript. All authors had full editorial control of the manuscript and provided their written approval forms for the content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Keiji Nogami has received grants from Baxalta and funding for research from Baxalta, Bayer, Novo Nordisk, Bioverativ, Chugai, and honoraria from Baxalta, Bayer, Novo Nordisk, Bioverativ, CSL Behring, and Chugai outside the submitted work. Hideyuki Takedani has received honoraria from Baxalta/Shire, Bayer, Biogen, Bioverativ, Chugai Pharmaceutical, CSL Behring, Kaketsuken, Novo Nordisk, and Pfizer and has received a grant from CSL Behring. Midori Shima has received personal fees and grants from Baxalta Bayer, Novonordisk, CSL Behring, Chugai, and Pfizer; personal fees from Bioverativ and Roche; grants from Kaketsuken outside the submitted work. Akira Yoshioka has received honoraria from Baxalta, Japan Red Cross, Daiichi Sankyo, and Bayer outside the submitted work. Tadashi Matsushita has received personal fees from Baxalta for the submitted work, grants and personal fees from Bayer, Baxalta, Novo Nordisk, Kaketsuken, and Biogen-idec outside the submitted work. Junki Takamatsu has declared no conflict of interest. Masashi Taki is an advisory board member for Bioverativ, Chugai, CSL Behring, and Novo Nordisk and an investigator in clinical trials conducted by Baxalta/Shire, Bioverativ, Chugai, CSL Behring, Novo Nordisk, and Octapharma; has received research supports from CSL Behring and speaker’s fee from Baxalta/Shire, Bayer, Bioverativ, Novo Nordisk, and Pfizer. Katsuyuki Fukutake has received grants and personal fees from Baxalta outside the submitted work and holds concurrent posts as a professor for the Department of Molecular Genetics of Coagulation Disorders supported by CSL Behring without additional salary; is an advisory committee member of Chugai Pharmaceutical, and consultant of Chugai Pharmaceutical; has received research funding from Bayer, Biogen/Bioverativ, Kaketsuken, Novo Nordisk, and Pfizer; has received honoraria for consulting, speaking or advising from Bayer, Biogen/Bioverativ, Chugai Pharm./Roche, CSL Behring, Japan Blood Products, Kaketsuken, MSD, Novo Nordisk, Octapharma, and Pfizer. Akira Shirahata has declared no conflict of interest. Werner Engl, Haruhiko Uchikawa, Hiroshi Takagi, and Morio Arai are full-time employees of Shire (formerly Baxalta).
About this article
Cite this article
Nogami, K., Takedani, H., Shima, M. et al. Perioperative safety and hemostatic efficacy of Advate® in patients with hemophilia A in a postmarketing surveillance in Japan. Int J Hematol 108, 22–29 (2018). https://doi.org/10.1007/s12185-018-2434-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2434-2