Abstract
Rurioctocog alfa (recombinant factor VIII: Advate®) is available for the control of bleeding in patients with hemophilia A in Japan. To evaluate the inhibitor development, safety, and efficacy of rurioctocog alfa, a non-interventional and observational postmarketing surveillance was conducted on 352 previously treated Japanese patients aged 1–76 years with ≥ 4 exposure days under the conditions of routine clinical practice. A post-hoc comparison of the mean annualized bleeding rates which required treatment with rurioctocog alfa detected a statistically significant difference (P < 0.0001) between patients treated on regular prophylaxis (8.5 bleeds/year) and patients treated on an on-demand basis (36.6 bleeds/year). Favorable prophylactic and on-demand hemostatic efficacy (“excellent” or “good”) were shown in 88.5–100% of patients across all treatment regimens. A total of 22 events of adverse drug reactions were reported in 13 male patients. Of the 352 patients, 3 (0.9%) patients, all of whom had ≤ 50 exposure days before enrollment, developed de novo FVIII inhibitor. No deaths or allergic reactions were reported. Rurioctocog alfa was found to be well-tolerated and effective among patients with hemophilia A in a postmarketing routine clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophilia A is an X-linked recessive bleeding disorder which is often characterized by excessive and recurrent spontaneous bleeding into the musculoskeletal system without apparent trauma due to the lack of sufficient amount of clotting factor VIII (FVIII) [1]. The long-term consequence of recurrent bleeding without a proper treatment is associated with an increased morbidity and decreased quality of life (QOL), most often due to painful and disabling hemophilic arthropathy [2, 3].
The primary aim of hemophilia care is the prevention and treatment of recurrent bleeding by replacing the missing clotting FVIII, thereby protecting joints from developing hemophiliac arthropathy [4]. Regular replacement therapy (“regular prophylaxis”) with FVIII to infants and young children who have no joint damages has been shown to be effective in preventing recurrent bleeding, protecting joints from bleeding, and preserving normal joint functions [5]. The secondary prophylaxis, starting in adolescent and adult is also effective in reducing annual number of bleeds and days of lost from work/school [6, 7]. Therefore, regular prophylaxis has been recommended as the standard of care in the treatment of severe patients with hemophilia A [8].
The most serious complication in the treatment of hemophilia A is the formation of inhibitory alloantibodies against infused FVIII (i.e., FVIII inhibitor) which is associated with considerable bleeding-related morbidity and mortality [9]. FVIII inhibitors develop in the range of 15–32% in previously untreated patients (PUPs) and 0.9–2.9% in previously treated patients (PTPs) during administration of FVIII products. The results of several PUP (baseline FVIII ≤2%) studies demonstrated a median of 9–12 exposure days (ED) until inhibitor development [10].
Rurioctocog alfa (Advate®: Baxalta, part of Shire, Lexington, MA, USA) is a third-generation recombinant anti-hemophilic FVIII (rFVIII), which has been used for regular prophylaxis, on-demand control of bleeding episodes, and perioperative management of bleeding in patients with hemophilia A in Japan since its approval in 2006 [11].
A postmarketing surveillance (PMS) was undertaken from February 2007 through June 2012 to collect data on the development of FVIII inhibitor and to investigate the safety and efficacy of the rFVIII in patients with hemophilia A under the conditions of a long-term postapproval routine clinical practice in Japan.
Materials and methods
Study design
This was an open-label, multicenter, prospective/retrospective, uncontrolled, non-interventional, observational study conducted in accordance with the Japanese ministerial ordinance for Good Post-marketing Study Practice [12]. It was designed to evaluate the safety and prophylactic/on-demand hemostatic efficacy of the rFVIII in PTPs with congenital hemophilia A. All patients were followed for 2 years or longer.
Patients
Patients with congenital hemophilia A who had previously been treated with any FVIII concentrates (≥ 4 EDs) were defined as PTPs. Patients were eligible for the study irrespective of age, sex, and severity of hemophilia A. Those patients who had < 4 EDs were not considered PTPs and were ineligible. Patients were prospectively enrolled if they started to receive the rFVIII after the PMS contract agreement was made by the medical institutions. On the other hand, those who had already started receiving the rFVIII at the time of PMS contract agreement were enrolled retrospectively.
Statistical analyses
The sample size calculation was based on an anticipated incidence of inhibitor formation among severe PTPs, which has been reported to be approximately 1–3% [4]. The original plan was to enroll at least 300 patients to detect 1 case of inhibitor formation with a probability of 95% or higher. All data analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Treatment modalities
As this study was non-interventional and observational in nature, which was conducted in postmarketing routine clinical practice, the treatment modalities were determined by the treating physicians and could be changed at any time at the discretion of the treating physicians during the study according to the clinical course of hemophilia A.
Efficacy
To evaluate the prophylactic and on-demand hemostatic efficacy of the rFVIII, the annualized bleeding rate (ABR) that required treatment with the rFVIII was calculated for each patient and summarized descriptively for each treatment group. The distribution of bleeding events during regular prophylaxis is a right-skewed distribution with a considerable proportion of patients with zero events that can be adequately described by the negative binomial distribution [13]. A post-hoc statistical comparison was, therefore, performed in terms of the mean ABRs between the regular prophylaxis group and the on-demand group using a negative binomial model. The model accounted for the fixed effect of the treatment regimen as well as the logarithm of the observation period for efficacy as an offset. This analysis was also performed in the subset of patients with severe hemophilia A (baseline FVIII level < 1%). A difference with a P value of < 0.05 was considered to indicate a statistically significant difference in the mean ABRs between the two treatment groups.
In addition to ABR, the prophylactic and on-demand hemostatic efficacy of treating bleeds with the rFVIII was evaluated using a predefined four-point efficacy rating scale. The efficacy ratings were comprised of “excellent,” “good,” “fair,” and “none,” which were defined as follows:
-
1.
Excellent hemostatic efficacy of the rFVIII was comparable or superior to that of former FVIII concentrates. In other words, hemostasis was achieved with less frequent administration or less amount of the rFVIII administered. The frequency of bleeding episodes was comparable to former FVIII concentrates or reduced during prophylactic treatment.
-
2.
Good hemostatic effect of the rFVIII was satisfactory even though the efficacy was slightly lower than that of former FVIII concentrates. In other words, the frequency and the amount of the rFVIII administered were comparable to those of former FVIII concentrates; however, more frequent administration or an additional dose of the rFVIII was required in some cases, and/or the frequency of bleeding episodes slightly increased during prophylactic treatment to achieve hemostasis.
-
3.
Fair hemostatic efficacy of the rFVIII was apparently lower than that of former FVIII concentrates. In other words, more frequent administration or an additional dose of the rFVIII was required in a number of bleeding episodes and/or during prophylactic treatment to achieve hemostasis.
-
4.
None it was difficult to achieve hemostasis with the rFVIII on a daily basis. An additional medication or FVIII concentrate was required to achieve hemostasis.
The treating physicians gave a hemostatic rating on prophylactic and/or on-demand treatment using the aforementioned criteria every 6 months. To perform a conservative evaluation on the prophylactic and/or on-demand hemostatic efficacy, the worst rated hemostatic evaluation among 6-months-period evaluations of each patient was selected for the analysis of hemostatic outcomes. Treatment outcomes were considered favorable if rated “excellent” or “good” by the treating physicians. Descriptive statistics was provided for the hemostatic efficacy outcome results. Narratives were provided for those patients whose overall hemostatic efficacy was rated “none”.
Safety
Safety was assessed based on the occurrence of adverse drug reactions (ADRs). To investigate the occurrence of ADRs in patients who received at least 1 dose of the rFVIII, all adverse events were recorded and assessed for the relationship to the use of the rFVIII with the assessment of seriousness (serious or non-serious) and expectedness (expected or unexpected). Those ADRs not listed in the Japanese package insert throughout the PMS period were defined as unexpected ADRs. Narratives were provided for those patients who experienced unexpected serious ADRs. All reported ADRs were summarized using preferred terms of the Japanese version of the Medical Dictionary for Regulatory Activities version 18.1.
The assessment of immunogenicity was based on the first-time detection of a positive FVIII inhibitor (i.e., the development of de novo FVIII inhibitor). FVIII inhibitor was measured at the discretion of the treating physicians at local laboratories. Inhibitor titer was expressed in Bethesda units (BU/mL). A positive inhibitor was determined according to the cut-off value of the local laboratory. FVIII inhibitor was classified as either low-titer (< 5 BU/mL) or high-titer (≥ 5 BU/mL) [14].
Results
Patients
A total of 396 patients aged 1–76 years were enrolled at 101 medical institutions in Japan. A total of 12 (3.0%) patients were excluded during the enrollment due to the following reasons: erroneous enrollment (n = 4), transfer to other hospitals (n = 3), no follow-up with the absence of the treating physician (n = 2), no visit to medical institutions (n = 2), and protocol violation (n = 1). As a result, 384 patients from 88 medical institutions were included in the safety and efficacy analyses. Of the 384 patients, 32 patients (8.3%) were further excluded from the analyses due to the data unreliability problem caused by electronic data capture (EDC) system failures, which resulted in the inclusion of 352 patients from 88 medical institutions in the safety and efficacy analyses.
The demographics and characteristics of the patients are presented in Table 1. Of the 352 patients, 266 patients (75.6%) were patients with severe hemophilia A, whereas 63 (17.9%) were moderate and 21 (6.0%) were mild disease; for the remaining 2 (0.6%) patients, the disease severity was unknown. At the time of the enrollment, the majority of the patients (84.7%) had received FVIII concentrates for ≥ 151 EDs. More than one-half of the patients (59.4%) had arthropathy. The overall mean age (standard deviation: SD) was 25.8 (7.5) years. The overall mean (SD) weight was 50.0 (20.4) kg. Baseline FVIII levels and age are presented by treatment groups in Table 2.
Treatment modalities
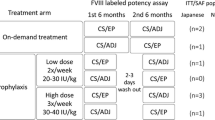
Patients (n = 352) were retrospectively allocated to the following 4 treatment groups according to their treatment modalities during the study: regular prophylaxis group (n = 173), on-demand group (n = 105), on-demand-to-regular-prophylaxis group (n = 22), and other-regimens group (n = 52). The regular prophylaxis group included treatment regimens with once to 3 times weekly administration of the rFVIII. The on-demand-to-regular-prophylaxis group included patients who were initially treated on-demand and then treated on regular prophylaxis. The other-regimens group (n = 52) comprised patients who switched treatment modalities from on-demand treatment to regular prophylaxis or vice versa more than once during the study (n = 20), those who received the rFVIII for surgery (n = 14), those who showed FVIII inhibitor before or after enrollment (n = 9), and those who were initially treated on regular prophylaxis and then switched to on-demand treatment (n = 9).
Observation period, frequency of prophylactic injection, and the prophylactic dosing
The study period was from February 2007 through June 2012. The overall mean follow-up period was 697.2 days (1.9 years) (n = 352). The mean (median) frequencies of prophylactic injection of the rFVIII were 2.9 (2.9) times a week in the regular prophylaxis group and 2.2 (1.8) times a week during prophylaxis in the on-demand-to-regular-prophylaxis group. The mean (median) initial prophylactic doses were 21.7 (19.2) IU/kg in the regular prophylaxis group and 22.8 (19.2) IU/kg at the start of prophylaxis in the on-demand-to-regular-prophylaxis group.
Annualized bleeding rate (ABR)
Summary statistics of ABR that required treatment with the rFVIII are presented for each treatment group in Table 3. Overall, the mean ABRs ranged from 8.5 to 36.6 bleeds/year across the treatment groups. For the subset of the patients with severe hemophilia A, the mean ABRs ranged from 8.9 to 37.9 bleeds/year across the treatment groups. The median ABR was 4.0 in the regular prophylaxis treatment group (both in overall patients and in severe patients). The post-hoc comparison of mean ABRs between the regular prophylaxis group (8.5 bleeds/year) and the on-demand group (36.6 bleeds/year) using a negative binomial model found a statistically significant difference (P < 0.0001). There was also a statistically significant difference between the regular prophylaxis group (8.9 bleeds/year) and the on-demand group (37.9 bleeds/year) in the subset of the patients with severe hemophilia A (P < 0.0001).
Prophylactic and on-demand hemostatic outcomes
Prophylactic and/or on-demand hemostatic treatment outcomes are presented by treatment group in Table 4. The proportions of patients with favorable outcomes (“excellent” or “good”) ranged from 88.5 to 100% of the patients across the treatment groups. There were 2 patients (one each in the regular prophylaxis and in the other-regimens group) whose hemostatic efficacy outcomes were rated “none”. The first case was a 53-year-old male patient who had a maximum of 175 BU/mL inhibitor prior to enrollment and 5 BU/mL at enrollment (Table 5, Case 6). The patient underwent an immune tolerance induction (ITI) therapy with the rFVIII, resulted in discontinuation after 5 months with remaining 33 BU/mL, and the hemostatic efficacy was rated “none”. The second case was a 10-year-old male patient who was treated on regular prophylaxis 3 times a week. Although the patient had no bleeding during the first 18 months, he thereafter experienced one breakthrough bleeding episode, for which the prophylactic treatment efficacy of the rFVIII was rated “none”.
Safety
The safety of the rFVIII was assessed in 352 patients. Of the 352 patients, 13 patients (3.7%) experienced 22 events of ADRs (7 events of serious ADRs and 15 events of non-serious ADRs). Of the 7 events of serious ADRs, 2 events (cerebral hemorrhage and abnormal hepatic function) were unexpected ADRs. The cerebral hemorrhage was reported in a 62-year-old male patient with severe hemophilia A who had comorbidities of hepatitis C infection, chronic gastritis, hypertension, and convulsion prior to enrollment. The patient developed the cerebral hemorrhage while switching from Reconate® to the rFVIII (rurioctocog alfa) and the event was, therefore, considered related to the rFVIII. The patient recovered from the cerebral hemorrhage with dysarthria. Abnormal hepatic function was reported in a 10-year-old male patient with severe hemophilia A who showed an increase in hepatic enzymes (AST: 36 IU/L and ALT: 70 IU/L). The treating physician reported an improvement in the hepatic enzymes 2 days after the onset (the outcome enzyme levels not recorded). Other serious ADRs were 4 events of FVIII inhibition (the development of FVIII inhibitor) and 1 event of aggravated conditionFootnote 1.
Non-serious ADRs were 2 events of increased blood alkaline phosphatase and 1 event each of anemia, increased aspartate aminotransferase, increased blood lactate dehydrogenase, decreased blood pressure, decreased hematocrit, decreased hemoglobin, decreased platelet count, increased platelet count, headache, abnormal hepatic function, hyperamylasemia, decreased red blood cell count, and increased white blood cell count. The reported ADRs were mostly related to changes in clinical laboratory test results.
FVIII inhibitor
Of the 318 patients without a history of FVIII inhibitor, 3 (0.9%) patients, all of whom had ≤ 50 EDs before enrollment, developed de novo FVIII inhibitor. All were low titer (0.7–1.9 BU/mL) and 2 of 3 were transient inhibitor (Table 6). The first case was an 8-year-old male patient with 1% baseline FVIII. The patient developed de novo FVIII inhibitor (1 BU/mL) 8 months after starting regular prophylaxis with the rFVIII, which became undetected 4 months later during regular prophylactic treatment. The second case was a 21-year-old male patient with 10% baseline FVIII. The patient developed de novo FVIII inhibitor (1.9 BU/mL) after 4 exposure days of the rFVIII for the treatment of the right elbow joint hemorrhage, for which the patient was discontinued from the study. The third case was a 30-year-old male patient with 1.7% baseline FVIII. The patient developed de novo FVIII inhibitor (0.7 BU/mL) 17 months after the initial on-demand dosing of the rFVIII, which became undetected 7 months later.
A total of 34 patients had history of positive FVIII inhibitor prior to enrollment. The most recent inhibitor titers at enrollment (0.7–5 BU/mL) were recorded in 6 of the 34 patients (Table 5). All but one 53-year-old patient (Table 5, Case 6: the case rated “none” in hemostatic efficacy mentioned above) had low-titer inhibitors (0.7–3.2 BU/mL), and the hemostatic efficacy of prophylaxis or on-demand treatment were favorable (“excellent” or “good”). The proportion of favorable outcomes in the remaining 28 patients was 89.3% across the treatment modalities.
Discussion
The clinical benefit of prophylactic treatment over on-demand treatment was reflected in the reduction of the mean ABR (8.5 vs. 36.6, P < 0.0001). The median ABR was 4.0 for both the overall patient group (n = 173) and the subset of patients with severe hemphilia A (n = 147) who were treated with prophylaxis only. In a Post-Authorization Safety Surveillance (PASS) of the rFVIII conducted in Europe and in the US (n = 512), the overall median ABR was 2.6 in patients treated with regular prophylaxis only (n = 297) [15]. On the other hand, in a meta-analysis of Advate®-PASS studies conducted in regions/countries including Australia, Europe, Japan, Italy, and the Unites States (n = 1188), the median ABRs of the patients on regular prophylaxis during the study (≥twice/week) was 1.66 [16]. The differences in the median ABRs of prophylaxis groups across the countries/regions are considered partly attributable to the differences in eligibility criteria specified in the protocols which were based on country-specific regulatory guidance. In the PMS conducted in Japan, all patients were eligible irrespective of age, sex, the severity of hemophilia A, and inhibitor titers at enrollment. On the other hand, patients with mild hemophilia A were ineligible in the EU and in the US protocols and patients with positive inhibitors (≥1 BU/mL) were also ineligible in the US protocol. In addition, the potential difference in the treatment regimen (dose and frequency) and/or adherence with regular prophylaxis in the routine management according to countries/regions might have resulted in the variety of ABRs. More recently, Khair, et al. reported the results of the prospective long-term Advate® Hemophilia A Outcome Database (AHEAD) cohort study which collected real-world data from 522 patients with severe and moderate hemophilia A from 21 countries. Median ABR was 2.2 at year 3 visit [17]. Overall, the median ABR of 4.0 in the prophylaxis group in this PMS was higher than those ABRs in other studies. The mean (median) frequencies of prophylactic injection in this PMS were 2.9 (2.9) times a week in the prophylaxis group, which were nearly complied with the standard regimen (3 times a week or every other day). On the other hand, the mean (median) prophylactic doses were 21.7 (19.2) IU/kg, which were at the lower end of those recommended in the treatment guidelines: 20–50 IU/kg per dose in the Japanese treatment guideline [18] and 25–40 IU/kg per dose in the WFH guideline [4]. In addition, the patients’ adherence to treatment was not assessed. These practices during the PMS period (2007–2012) might have contributed to the relatively high ABR in the prophylaxis group in this real-world collection of Japanese PMS. Another important point to keep in mind is that personalized prophylaxis taking into account the patients’ age, joint status, physical activity, PK-parameters, etc. is considered to be the standard of care in hemophilia nowadays, while such approach was not yet a regular practice at the time of this PMS. Although the result of this PMS should be carefully interpreted as the treatment modalities were classified and assessed in a post-hoc manner, the regular prophylaxis was nevertheless deemed to have a benefit over on-demand treatment in the prevention of bleeding in the cohort of Japanese patients who had previously been treated for ≥ 4 EDs.
Favorable prophylactic/hemostatic outcomes (“excellent” or “good”) were shown in approximately 89–100% of the patients across the treatment groups. In this PMS, 90.8% (157/173) of the patients treated with prophylaxis only and 93.3% (98/105) of the patients treated on-demand only were rated with favorable prophylactic/on-demand hemostatic outcomes, which were similar to the results from the Advate®-PASS study in EU and in the US with 95.9% (185/193) for prophylaxis only and 95.4% (166/174) for on-demand treatment only [14].
This PMS observed 3 cases of de novo low-titer FVIII inhibitors (1.0, 0.7, and 1.9 BU/mL) in patients with moderate or mild disease (baseline FVIII of 1%, 10%, and 1.7%). Two (1.0 and 0.7 BU/mL) were transient as disappearing during the study. All the 3 de novo inhibitor patients had ≤50 EDs at enrollment, which is associated with inhibitor formation [19]. A systematic review and meta-analysis study showed that only 43 (0.99%) cases developed de novo inhibitor in the total of 4323 PTPs from 33 independent PTP cohorts. The authors concluded a low overall rate of de novo inhibitors [20]. During clinical trials with the rFVIII in 108 PTPs, a low-titer inhibitor (2.0 BU/mL) was observed in 1 (0.9%) patient after 26 EDs [21]. According to the EU and US PASS study, the incidence of de novo inhibitor formation was 1/348 (0.29%) in PTPs with FVIII ≤2% who had no history of inhibitor and were treated with the rFVIII for > 50 EDs. Among the subset of severe PTPs with FVIII < 1% without a history of inhibitors, the incidence was 1/287 (0.35%) [14]. No inhibitor was reported in 129 PTPs during 2 years in the Japanese Recombinate Post-marketing Surveillance [22]. None of the patients with severe disease (n = 266) in the present study developed de novo inhibitor, which is comparably low to those observed in the other studies.
A total of 22 ADRs were reported in 13 patients during the PMS. The majority of ADRs was related to changes in clinical laboratory test results with very low frequencies, and no ADRs were considered specific to the Japanese patient population.
Limitations
The findings from this PMS should be noted with the following limitations:
-
1.
28 out of 34 patients (82.4%) with a history of FVIII inhibitor did not have their most recent records on the FVIII inhibitor at enrollment.
-
2.
The treatment modalities were classified in a post-hoc manner.
-
3.
Any bleeds not requiring treatment might have not been recorded.
-
4.
The treatment modalities could be changed at any time at the discretion of the treating physicians according to the clinical condition.
-
5.
A total of 44 patients were excluded from the analyses for the reasons as described in the results. Of the 44 excluded patients, 32 were excluded because of inadvertently missing data due to a temporal EDC system failure which had been noticed later. The missing data could not be retrieved as the treating physicians had left their institutions or the PMS contracts had expired.
Conclusion
The strength of this PMS was the long-term evaluation of the safety and efficacy associated with the prophylactic and/or on-demand use of the rFVIII in a large-scale postmarketing routine clinical practice involving 352 patients irrespective age, sex, and the severity of hemophilia A. The incidence of development of FVIII inhibitor appeared to be similar to previously reported estimates in PTPs. The prophylactic and on-demand use of the rFVIII was shown to be effective in the majority of the patients across the treatment groups. The use of the rFVIII in PTPs for the treatment of hemophilia A was, therefore, considered to be well-tolerated and effective in postmarketing daily clinical practice in Japan.
Notes
FVIII inhibition was reported twice in 1 patient. After the 1st FVIII inhibitor development, the patient experienced an increase in FVIII inhibitor titer during the study, which was coded into 2 discrete serious ADRs as “FVIII inhibition” and “aggravated condition” by the sponsor (drug use investigation on PTPs in the re-examination application of Advate® PMS study).
References
Heim M, Wershavski M, Martinowitz U, Chechick A, Azaria M. Elbow joint, crutches and locomotion: special reference to persons with haemophilia. Haemophilia. 2000;6:556–61.
Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–56.
Oymak Y, Yildirim AT, Yaman Y, Gurcinar M, Firat A, Cubuckcu D, et al. The effectiveness of tools for monitoring hemophilic arthropathy. J Pediatr Hematol Oncol. 2015;37:e80-5.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47.
Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44.
Tagliaferri A, Franchini M, Coppola A, Rivolta GF, Santoro C, Rossetti G, et al. Effects of secondary prophylaxis started in adolescent and adult haemophiliacs. Haemophilia. 2008;14:945–51.
Collins P, Faradji A, Morfini M, Enriquez MM, Schwartz L. Efficacy and safety of secondary prophylactic vs. on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-months crossover study. J Thromb Haemost. 2010;8:83–9.
Jiménez-Yuste V, Auerswald G, Benson G, Lambert T, Morfini M, Remor E, et al. Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: the past, the present and the future. Blood Transfus. 2014;12:314–9.
Franchini M, Mannucci PM. Inhibitors of propagation of coagulation (factors VIII, IX and XI): a review of current therapeutic practice. Br J Clin Pharmacol. 2011;72:553–62.
Peerlinck K, Hermans C. Epidemiology of inhibitor formation with recombinant factor VIII replacement therapy. Haemophilia. 2006;12:579–90.
Dhillon S. Octocog alfa, antihaemophilic factor (recombinant), plasma/albumin free method (Advate®): a review of its use in the management of patients with haemophilia A. Drugs. 2012;72:987–1007.
Pharmaceutical Administration. and Regulations in Japan (individual chapters) Chapter 4 Post-Marketing Surveillance Of Drugs. http://www.jpma.or.jp/english/parj/pdf/2018.pdf
den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–4.
White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560.
Oldenburg J, Goudemand J, Valentino L, Richards M, Luu H, Kriukov A, et al. Postauthorization safety surveillance of ADVATE [antihaemophilic factor (recombinant), plasma/albumin-free method] demonstrates efficacy, safety and low-risk for immunogenicity in routine clinical practice. Haemophilia. 2010;16:866–77.
Iorio A, Marcucci M, Cheng J, Oldenburg J, Schoenig-Diesing C, Matovinovic E, et al. Patient data meta-analysis of post-authorization safety surveillance (PASS) studies of haemophilia A patients treated with rAHF-PFM. Haemophilia. 2014;20:777–83.
Khair K, Mazzucconi MG, Parra R, Santagostino E, Tsakiris DA, Hermans C, et al. Pattern of bleeding in a large prospective cohort of haemophilia A patients: a three-year follow-up of the AHEAD (Advate in HaEmophilia A outcome Database) study. Haemophilia. 2018;24:85–96.
Fujii T, Amano K, Atsumi T, Ishiguro A, Ohira K, Okamoto K, et al. Treatment guideline for hemophilia without inhibitor: 2013 update. Jpn J Thromb Hemost. 2013;24:619–39.
McMillan CW, Shapiro SS, Whitehurst D, Hoyer LW, Rao AV, Lazerson J. The natural history of factor VIII:C inhibitors in patients with hemophilia A: a national cooperative study. II. Observations on the initial development of FVIII:C inhibitors. Blood. 1988;71:344–8.
Xi M, Makris M, Marcucci M, Santagostino E, Mannucci PM, Iorio A, et al. Inhibitor development in previously treated hemophilia A patients: a systematic review, meta-analysis, and meta-regression. J Thromb Haemost. 2013;11:1655–62.
Tarantino MD, Collins PW, Hay CR, Shapiro AD, Gruppo RA, Berntorp E, et al. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia. 2004;10:428–37.
Fukutake K, Arai M, Inaba H, Hanabusa H, Miyama J, Takamatsu J, et al. A multi-center post-marketing surveillance study of recombinant factor VIII (Recombinate) in previously treated patients with hemophilia A. Jpn J Thromb Hemost. 2005;16:650–63.
Acknowledgements
This manuscript is dedicated to the memory of our esteemed colleague Dr. Hideji Hanabusa, MD, whose untimely passing in October 2016 left a permanent void. He touched the lives of many as a mentor, scholar, collaborator, and friend. Dr. Hanabusa was instrumental in gaining approval for this product in Japan, and the creation and interpretation of the data included herein, and would have been a co-author of this manuscript. We thank Shire PMS and PV teams for the support of data clarification. We recognize with gratitude the patients and institutions that participated in the studies: Aichi Sannomaru Hospital, Aihara Internal Medicine and Pediatric Clinic, Asahikawa Medical University Hospital, Chiba Children’s Hospital, Dokkyo Medical University Hospital, Ehime University Hospital, Fukui-ken Saiseikai Hospital, Gunma University Hospital, Hasegawa Pediatric Clinic, Hayashi Pediatric Clinic, Higashiosaka City Medical Center, Hirano Internal Medicine, Hiroshima University Hospital, Hitachi General Hospital, Hokkaido Cancer Center, Hospital of the University of Occupational and Environmental Health Japan, Hyogo College of Medicine Hospital, Ibaraki Children’s Hospital, Iizuka Family Clinic, Iou Hospital, Ishinkai Yao General Hospital, Ishiyama Internal Medicine Clinic, Itoigawa Sogo Hospital, Iwaki Kyouritsu Hospital, Iwate Prefectural Miyako Hospital, Izumiotsu Municipal Hospital, Japanese Red Cross Morioka Hospital, Jichi Medical University Hospital, Jichi Medical University Saitama Medical Center, Jusendo General Hospital, Kagawa National Children’s Hospital, Kagawa University Hospital, Kanagawa Children’s Medical Center, Kariya Toyota General Hospital, Kitasato University Hospital, Kochi Health Sciences Center, Komatsu Municipal Hospital, Kumamoto University Hospital, Kurume University Hospital, Kyoto Okamoto Memorial Hospital, Kyushu Medical Center, Kyushu University Hospital, Matsudo City General Hospital, Mie Chuo Medical Center, Mie University Hospital, Miyoshi Central Hospital, Nagasaki University Hospital, Nagoya City University Hospital, Nagoya University Hospital, Nanbu Medical Center / Nanbu Child Medical Center, Nara Medical University Hospital, Nihonkai General Hospital, Niigata Saiseikai Sanjo Hospital, Nippon Medical School Tama Nagayama Hospital, Odate Municipal General Hospital, Ogaki Municipal Hospital, Ogikubo Hospital, Oita Memorial Hospital, Osaka City General Hospital, Osaka National Hospital, Rakusai Newtown Hospital, Saga University Hospital, Saitama Children’s Medical Center, Sakou Internal Medicine, Saku Central Hospital, Sanaikai Genaral Hospital, Sapporo Tokushukai Hospital, Sendai Medical Center, Sendai Nishitaga Hospital, Shibuya Children’s Clinic, Shizuoka Children’s Hospital, Shizuoka Medical Center, Soka Municipal Hospital, St. Marianna University School of Medicine Hospital, St. Marianna University School of Medicine Yokohama City Seibu Hospital, Tatebayashi Kosei Hospital, The Jikei University Hospital, Tokuyama Central Hospital, Tokyo Dental College Ichikawa General Hospital, Tokyo Medical University Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Women’s Medical University Medical Center East, Toyokawa City Hospital, University Hospital Kyoto Prefectural University of Medicine, Watari Hospital, Yakumo General Hospital, Yokohama City University Medical Center, and Yuri Kumiai General Hospital.
Funding
This research was funded by Baxalta (part of Shire, Lexington, MA, USA).
Author information
Authors and Affiliations
Contributions
KF, MT, TM, KN, MS, AY, JT, and AS collected and interpreted data, and revised the manuscript. WE analyzed the statistics, interpreted data, and revised the manuscript. HU, HT, and MA interpreted data and drafted and revised the manuscript. All authors had full editorial control of the manuscript and provided their approvals for the content of the manuscript prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
Katsuyuki Fukutake has received grants and personal fees from Shire outside the submitted work and holds concurrent posts as a professor for the Department of Molecular Genetics of Coagulation Disorders supported by CSL Behring without additional salary; is an advisory committee member of Chugai Pharmaceutical and consultant of Chugai Pharmaceutical; has received research funding from Bayer, Biogen/Bioverativ, Kaketsuken, Novo Nordisk, and Pfizer; has received honoraria for consulting, speaking or advising from Bayer, Biogen/Bioverativ, Chugai Pharm./Roche, CSL Behring, Japan Blood Products, Kaketsuken, MSD, Novo Nordisk, Octapharma, and Pfizer. Masashi Taki has received grants and personal fees from CSL Behring outside the submitted work, personal fees from Shire, Bayer, Bioverativ, Chugai, Kaketsuken, Pfizer, and Novo Nordisk outside the submitted work. Tadashi Matsushita has received personal fees from Shire for the submitted work; grants and personal fees from Bayer, Shire, Novo Nordisk, Kaketsuken, and Biogen-idec outside the submitted work. Keiji Nogami has received grants from Shire and funding for research from Shire, Bayer, Novo Nordisk, Bioverativ, Chugai, and honoraria from Shire, Bayer, Novo Nordisk, Bioverativ, CSL-Behring, Chugai outside the submitted work. Midori Shima has received personal fees and grants from Shire, Bayer, Novo Nordisk, CSL-Behring, Chugai, and Pfizer; personal fees from Bioverativ and Roche; grants from Kaketsuken outside the submitted work. Akira Yoshioka has received honoraria from Shire, Japan Red Cross, Daiichi Sankyo, and Bayer outside the submitted work. Junki Takamatsu and Akira Shirahata have declared no conflict of interest. Werner Engl, Haruhiko Uchikawa, Hiroshi Takagi, and Morio Arai are full-time employees of Shire; Werner Engl and Morio Arai own Shire stock.
About this article
Cite this article
Fukutake, K., Taki, M., Matsushita, T. et al. Inhibitor development, safety and efficacy of Advate® among previously treated patients with hemophilia A in a postmarketing surveillance in Japan. Int J Hematol 109, 336–345 (2019). https://doi.org/10.1007/s12185-018-02574-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-02574-x