Abstract
For refractory or relapsed acute myeloid leukemia patients, allogeneic hematopoietic stem cell transplantation is the only curative treatment option, but the disease must be in remission before this can be attempted. “Salvage” therapy regimens containing high-dose cytarabine plus fludarabine or cladribine with or without anthracyclines or plus mitoxantrone and etoposide fail in 30–50% of cases. We report the outcome of 14 patients treated with a clofarabine-based treatment administered after at least one failed fludarabine-based “salvage” attempt in a “real life” (outside a clinical trial) context. No death related to the clofarabine-based treatment was observed. Four of the 14 patients (29%) reached complete remission and one (7%) achieved a reduction of marrow blasts to fewer than 10%. Three of these five patients were successfully transplanted and have shown a long-term survival. The small number of this group of patients does not permit the identification of clinical features clearly related to a favorable outcome, but we note that all the three long-term survivals were FLT3 wild type. Clofarabine-based “salvage therapy” in patients with very poor expectancy is feasible even after a fludarabine-based salvage attempt, albeit with success only in a small percentage of cases (3/14 = 21%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of non-promyelocytic acute myeloid leukemia (AML) over the past 20 years has been based on induction remission chemotherapy, centered on cytarabine in combination with an anthracycline, often using a so-called “3+7” regimen [1]. Complete remission (CR) is obtained approximately in 70% [2–4] of younger patients and in about 50% of elderly patients [5]. Refractoriness is seen in about 30-40% of cases. Furthermore, relapse after CR occurs in another 30–40% of cases. Patients with relapse/refractory (R/R) AML have a poor prognosis. For these patients, allogeneic hematopoietic stem cell transplantation (HSCT) is the only treatment option with a reasonable potential for a long-term disease control. However, in order to reduce treatment mortality and avoid relapse after transplantation, the disease must be in remission before this can be attempted. A standard remission re-induction or “salvage therapy” before HSCT is not clearly defined. Ideally, it should induce high remission rates with a tolerable toxicity profile. Based on the evidence, in pre-clinical and clinical studies, R/R AML treatment is centered on the association of a first (i.e., cytarabine) and a second-generation nucleoside analog (i.e., fludarabine, cladribine), [6–8] with or without an anthracycline. Another salvage regimen is based on the association of mitoxantrone, etoposide and high-dose cytarabine (MEC) [9, 10]. An experience in 46 R/R AML patients reports the ability of the FLAG IDA regimen (fludarabine, cytarabine, G-CSF, idarubicin) to induce CR in 52% of cases [11], with a mortality rate of about 6%. The CLAG regimen (cladribine, high-dose cytarabine, and G-CSF) was compared with the MEC regimen [12] with a CR rate of 37.9% for CLAG and 23.8% for MEC. As it can be easily inferred from these reports, a huge proportion (30–50%) of R/R AML patients cannot have a sufficient reduction in disease burden to allow performing HSCT, even after the “salvage therapy”.
Clofarabine is a next-generation nucleoside analog, synthesized with the aim to improve the drug class effectiveness and to reduce the neurologic dose-limiting-toxicity of cytarabine [13, 14]. It was initially tested and finally approved by FDA and EMA on R/R Acute Lymphoblastic Leukemia (ALL) in children [15]. Clofarabine has been also shown to be effective in R/R AML in several clinical studies, alone and in association with cytarabine [16–22]. As a single agent in R/R AML, clofarabine was able to obtain a CR rate of 42% and an ORR of 55% [17]. The association with cytarabine is particularly interesting, since clofarabine can increase the accumulation of cytarabine triphosphate (ara-CPT), especially when administrated 4 h before cytarabine. Clonogenic assays revealed that this combination produced the synergistic killing of myeloid leukemic cells [14]. Forty-seven patients with AML, refractory to at least two prior induction regimens including high-dose cytarabine, or relapsed and refractory to re-induction therapies with high-dose cytarabine [18], were treated with 22.5 mg/m2 clofarabine, followed by cytarabine 1 g/m2 after 3 h, for 5 days. CR and ORR rates were 51 and 62%, respectively. Among the 24 patients obtaining CR, 13 (28%) underwent allogeneic SCT. No reports in our knowledge are been published yet about the outcome of a clofarabine-based course after the failure of a salvage therapy containing fludarabine, even if clofarabine may be an interesting possibility to try to reduce the burden of leukemic disease, even in these cases. From a chemical point of view, the molecule of clofarabine could overcome the resistence to fludarabine through the halogenation in the 2-position of adenine and the substitution of a fluorine group in position C- 20 of the arabinofuranosyl moiety. These modifications enhance the resistance to phosphorolytic cleavage, increase the inhibition of DNA synthesis and extend the retention of clofarabine triphosphate in the leukemic blasts [13]. In this context, in our centre, a clofarabine-based regimen was employed in a “real life” (i.e., outside the clinical trials) group of R/R AML patients who did not reach a remission of disease even after a “salvage therapy” containing fludarabine, as a bridge to HSCT. We present the retrospective analysis of the outcome of these patients with the aim to evaluate the feasibility, toxicity and anti-leukemic activity of clofarabine in this subset.
Materials and methods
Study group
The data were retrospectively collected from the clinical records of a group of consecutive R/R AML patients, refractory after at least one attempt of “salvage therapy” containing fludarabine. They were all treated with a clofarabine-based regimen. Clofarabine was administered as “off label” drug with evidence of effectiveness on the disease shown by non-pivotal trials, with approval for each patient by the Hospital Review Board, under the responsibility of the prescribing hematologist. All patients had been fully informed about the expected potential toxicity related to the treatment, and had provided their consent according to the institutional guidelines. This program was offered to all “fit” patients, refractory to a previous salvage treatment. “Frail” or “unfit” patients, i.e., without adequate renal (creatinine clearance over 70 ml/min) or hepatic function (serum bilirubin < 1.5 ULN, GOT/GPT < 2.5 ULN), or an ECOG performance status ≤ 2, were excluded. The records analyzed refer to the period starting from October 2008 to January 2015.
The definition of “refractory AML” was reserved for patients who did not achieve CR after induction therapy. The term “relapsed AML” was used when patients relapsed after reaching first CR.
Response criteria
Response rates were defined in accord with the International Working Group criteria (IWG 2003) [23]. Complete response (CR) requires the presence of ≤5% blasts in a bone marrow with normal cellularity, no circulating blast cells, a neutrophil count greater than or equal to 1 × 109 L−1, and a platelet count greater than or equal to 100 × 109 L−1. A complete response with incomplete hematopoietic recovery (CRi) is similar to CR, but without recovery of platelets greater than or equal to 100 × 109 L−1, or of neutrophils greater than or equal to 1 × 109 L−1. A partial response (PR) consists of a blood count recovery as in CR, but with the persistence of 5–25% marrow blasts with a decrease of at least 50% of blasts.
Toxicity assessment
The evaluation of organ toxicity related to treatment with clofarabine was based on the recorded National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v3.0). Regimen-related grade I-IV toxicity was documented for the following organs/systems: liver, gastrointestinal, genitourinary, cardiopulmonary, neurologic, skin, and constitutional findings.
Statistical consideration
Overall response rate, duration of remission, and survival status at the end of the follow-up were considered as effectiveness endpoints. The overall survival (OS) and progression-free survival (PFS) were calculated from the first day of the clofarabine-based course until death. The Kaplan Meier method was used to describe OS and PFS. The variables supposed to influence the outcome were analyzed using significance testing such as the Fisher’s test, Wald’s test or Mc Fadden’s test.
Results
Study group
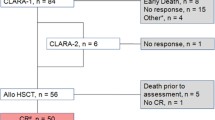
The clinical records of 14 R/R AML patients (6 males and 8 females) who underwent the above-described program were reviewed. Any patient was treated at least with one fludarabine-based course. The fludarabine containing regimens were: FLAIG (fludarabine 30 mg/m2 in 2 divided doses/day, on days 1–5; cytarabine 2000 mg/m2 in 2 divided doses/day on days 1–5; idarubicin 10 mg/m2 on days 1 and 3; G-CSF 175 μg/m2 on days 0–4) in 10 cases; FLAG (fludarabine 25 mg/m2/day on days 1–5; cytarabine 2000 mg/m2/day on days 1–5; G-CSF 175 μg/m2 on days 0–4) in 3 cases; FLAI (fludarabine 30 mg/m2/day on days 1–3, cytarabine 1000 mg/m2/day on days 1–3; idarubicin 5 mg/m2/day on days 1–3) in 1 case. They had received a median of 2 previous lines of chemotherapy. Eight patients have had just one attempt to "salvage", while the other six more than one attempt. The patients’ characteristics are summarized in Table 1. The median age was 53 years (range 16–68). Of the 14 patients, two had therapy-related AML, one had antecedent MDS, and the rest had de novo disease. Seven patients were relapsed and 7 were refractory to the induction therapy. With regard to biological risk, three of the 7 refractory AML patients (Table 2) had a normal karyotype, three had a complex karyotype (more than 2 abnormalities), and one had deletion of chromosome 5. Of these seven patients, six had wild-type NPM1, and only one patient had FLT3-ITD mutation. All the 7 relapsed AML patients (Table 3) had a normal karyotype; six had NPM1 mutation, and 2 patients had FLT3-ITD mutation.
Treatment dosing and supportive measures
All patients received combination therapy with cytarabine at 1 g/m2/day and clofarabine 22.5 mg/m2/day on days 1–5. During hospitalization, clinical status, laboratory parameters, vital signs, and adverse events were monitored daily. In all cases, the treating physicians provided supportive measures according to the medical needs. For all patients, anti-infective prophylaxis was administered at the onset of neutropenia and included levofloxacin (500 mg/day orally) and posaconazole oral solution (200 mg twice daily).
Response to clofarabine
After treatment with clofarabine, persistent disease was observed in 9 patients (64%). One of them had a reduction in the number of marrow blasts to less than 10%, but without meeting the criteria for CR or PR. One patient was subjected to conditioning for transplantation at the time of the hematological nadir after treatment with clofarabine, so response to clofarabine could not be assessed. CR was achieved in 2 (14%) patients. Two other patients (14%) achieved blasts clearance but with incomplete hematopoietic recovery (CRi). Three of these four patients underwent transplant after 87, 106, and 121 days after the beginning of the clofarabine therapy. The last one relapsed after 75 days from the beginning of clofarabine, before the transplant could be performed. The average time to transplant or relapse in these CR or CRi patients was 97 days. The protracted time to transplant is justified by the need of long-lasting resolution of infectious complications.
Transplant feasibility, regimens and outcome
Ten patients (71%) received HSCT after a median of 60 days (range: 10-121 days) from the treatment with clofarabine. Of these, 6 were non responders (of them 1 with marrow blast <10%), 3 were in remission (2 CR+1 CRi) and 1 was transplanted before hematologic recovery after clofarabine-based regimen. The choice of this transplant before recovery was made in exceptional circumstances in a very young patient (16 years old) who did not benefit by a previous double attempt of rescue (a fludarabine-based course and a high-dose cytarabine plus mitoxantrone course).
Based on the availability of suitable donors, HLA-matched transplantation (8 unrelated, 1 related donors) was performed in 9 cases and haploidentical transplantation in one case. The patients received one of the following conditioning regimens: CY-TBI (n = 6), cyclophosphamide 60 mg/kg/day on days −7 and −6 followed by total body irradiation (12 Gy) on days −3, −2 and −1; BU-FLU (n = 3), busulfan total dose of 16 mg/kg per os on days −8 to −4 and fludarabine 30 mg/m2/day on days −6 to −3; TrRaMM (n = 1, haploidentical transplantation): thiotepa 5 mg/kg on day −7, treosulfan 14 g/m2/day on days −6 to −4, fludarabine 30 mg/m2/day on days −6 to −3, ATG 10 mg/kg on days −4 to −2, rituximab 200 mg/m2 on day −1. The choice of the conditioning regimen was based on the available protocols, according to the physician’s preferences.
Of the 10 patients who received HSCT, seven (70%) relapsed with a median of 101 days after transplant (range 17–209 days) and died with a median of 141 days after transplant (range 53–287 days).
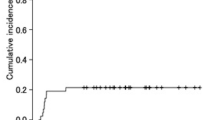
Considering the whole group of 14 patients, the median OS was 132 days (Fig. 1). Specific characteristics including relapse and survival details are provided in Tables 2 and 3 for refractory and relapsed AML patients, respectively. As of June 2016, 3 patients (21.4% of the total sample) are long-term survivors and in CR at 1288, 1402 and 2198 days after the beginning of the clofarabine-based treatment.
Characteristics of long-term survivals
Of the three long-time survivors, two were refractory to the induction therapy and have achieved CR and CRi, respectively, after treatment with clofarabine. The third relapsed after the induction/consolidation therapy. He did not reach CR or PR according to the WHO criteria but had a significant reduction in marrow blasts (<10%), after the clofarabine-based course. All of them were FLT3 wild type. The percentage of blasts at the time of transplantation (all survivors with <10% of blasts) and the presence of a low biological risk (in particular FLT3 wild type), might have an impact on survival, but the numbers are too low to demonstrate any statistical significances (Wald test, p = 0.1058 and the Mc Fadden test, p = 0.470).
Clofarabine toxicity
No deaths correlated to the clofarabine treatment were observed. The median duration of severe neutropenia and thrombocytopenia following the initiation of treatment with clofarabine was 27 days (range 24–37) and 36 days (range 28–56), respectively. Renal, liver, skin, and infectious toxicities after clofarabine administration are listed in Table 4. Patients reported a grade 1–2 in SGOT/SGPT in 36% of the cases and a grade 3–4 elevation in 21% of the cases. A grade 1–2 hyperbilirubinemia was observed in three of the 14 patients (21%), and only two patients (14%) developed a grade 1–2 renal insufficiency. Mild and reversible (grade 1–2) hand-foot skin toxicity was observed in only two patients (14%); no grade 3–4 cutaneous events were observed. Five of the 14 patients (36%) had mucositis, but total parenteral nutrition was only required in two cases (grade 3–4 mucositis). Thirteen of the 14 patients (93%) had at least one episode of febrile neutropenia: a microbiological source was identified in all but one of these patients, and two patients had severe sepsis that required intensive care management.
Discussion
Relapsed/refractory AML patients have a very poor prognosis. We have carried out a retrospective review of 14 patients with R/R AML, who had already failed the “salvage therapy”, and all of them had been already treated with a fludarabine-based course. Some of them had already tried even more than one “salvage” attempt. The risk assessment was also higher considering the lack of sibling donor (8/10 of the transplant made in this group were MUD, 1/10 was haploidentical donor). This is a “real life” context, where it would have been fully justified to candidate these patients to the best supportive care, trying to improve their quality of life, rather than the survival. However, the good reports about clofarabine in the AML setting encouraged us to use this drug as a further attempt to bridge these patients to transplant.
Based on the presented series, we can make some observations on the safety and effectiveness of the clofarabine treatment in the specific and, at least in our knowledge, unexplored subset of fludarabine pre-treated patients.
As regards safety, the clofarabine-based regimen in patients pre-treated with fludarabine was acceptable in terms of toxicities and it can be considered feasible. As a matter of fact, no patient death was observed. Infection related to febrile neutropenia was a common adverse event (93% of cases), but not unlike what is generally expected in a re-induction therapy for AML. Clofarabine liver toxicity is also a known side effect, but only a mild and reversible increase in serum bilirubin and transaminases occurred in this series. Another peculiar danger of the clofarabine treatment is hand-foot skin toxicity, but it has only been a mild and reversible event too.
As for the effectiveness, some considerations need to be done on the extent and the quality of response, the transplant feasibility, and the hypothetical factors predicting the response.
In the presented series, 4/14 of patients (29%) were in morphologically leukemia-free state (CR+CRi) after the clofarabine-based course. These results are not really satisfactory, but are not different from other recent reports referring to patients who had not received fludarabine before the clofarabine-based course [24, 25]. Interestingly, patients who reached CR or CRi had a considerable period of long-term remission before relapse or transplantation (97 days on average). Based on this, with all the limits on the type of study, we may presume that achieving remission with a clofarabine-based salvage therapy in this context, albeit it happens in a minority of cases, might also mean gaining the time necessary to perform transplantation. Two of these responding patients successfully bridged to allogeneic HSCT and are long-term survivors. The third underwent HSCT, but relapsed after transplantation and died. The fourth died before transplantation due to early disease relapse. Another patient successfully received HSCT and is a long-term survivor. He did not reach CR after clofarabine, but had a reduction in the bone marrow blast percentage count to less than 10%. We may, therefore, conclude that our approach actually gave a little chance of survival (about 20%) for patients with such a poor expectancy. This chance was not only offered patients who reach CR or CRi, but also those who had a significant reduction of the blast percentage. In other terms, the clinical target is the significant reduction of bone marrow blast counts, without necessarily the achievement of CR. This is a retrospective study without a comparative group. For instance, we do not know the results of a hypothetical group of patients with the same condition of refractoriness to fludarabine salvage, performing transplant without other attempts to induce remission. Anyway, based on the literature data, it is well known that the lower the leukemia burden prior to transplantation, the better is the outcome [26–28], and having a marrow blast count over 20% is a strong negative predictor of a long-term disease control. As the matter of fact in our, albeit small, series, 10/14 (more than 70%) really underwent allogeneic transplant, but no one without CR or at least a reduction of blast count under 10% was transplanted with success, as expected when the disease is fully active. On the other hand, since the “graft versus leukemia” (an immunological mechanism mediated by the donor T-cells against the allogeneic host tumor cells) is the actual basis of the success of transplant in a chemotherapy-resistant patient, it seems unlikely that a further chemotherapy course (clofarabine) before transplant may induce this kind of resistance.
About the hypothetical clinical features associated with a good outcome after clofarabine therapy, the small number of cases does not allow any assumptions. Anyway we observed that all long-term survivors had less than 10% marrow blasts and were FLT3 wild type. The long-term survival who did not reach the CR after the clofarabine containing course also carried a good prognostic molecular feature (NPM1 mutant and FLT3 wild type).
This small group of patients is certainly not enough to draw definitive conclusions.
However, a few tips can help us to understand what we can expect when we plan to propose a clofarabinebased treatment to a patient already refractory to an attempt of a “rescue” containing fludarabine. This approach seems to be feasible as a bridge to transplant. Anyway, the patient must share the choice to go on, after being fully and thoroughly informed about the small chance to successfully perform the transplant. The success in obtaining a long-term survival seems to be linked to the effectiveness of clofarabine in reducing the marrow blast percentage to less than 10% at least. In the presented series, this happens in 5 out of 14 cases. Only in these cases, it is correct to proceed with the transplant, otherwise it seems to be not feasible, and the palliation should be the best choice. In case of CR or CRi, the clofarabine treatment also seems to ensure the time needed for performing the transplant. In case of a significant reduction of the bone-marrow blast count even without a state of CR, the transplant may still have a chance of success, but no delay must be granted.
References
Schiffer CA. Acute myeloid leukemia in adults: where do we go from here? Cancer Chemother Pharmacol. 2001;48(suppl):S45–52.
Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction an consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol. 2009;27:5397–403.
Willemze R, Suciu S, Meloni G, Labar B, Marie JP, Halkes CJ, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMAAML-12 Trial. J Clin Oncol. 2014;32(3):219–28.
Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–36.
Gardin C, Turlure P, Fagot X, Thomas X, Terre C, Contentin T, et al. Postremission treatment of elderly patients with AML in first CR after IC: results of the multicenter randomized ALFA 9803 trial. Blood. 2007;109:5129–35.
Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Hołowiecki J, et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol. 2008;80:115–26.
Montillo M, Mirto S, Petti MC, Latagliata R, Magrin S, Pinto A, et al. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am J Hematol. 1998;58:105–9.
Parker JE, Pagliuca A, Mijovic A, Cullis JO, Czepulkowski B, Rassam SM, et al. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 1997;99:939–44.
Amadori S, Arcese W, Isacchi G, Meloni G, Petti MC, Monarca B, et al. Mitoxantrone, etoposide, and intermediate dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J Clin Oncol. 1991;9:1210–4.
Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high risk myelo high-risk myelodysplastic syndrome: a phase III trial (E2995). J Clin Oncol. 2004;22(6):1078–86.
Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, Rizzi R, et al. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003;82(4):231.
Price SL, Lancet JE, George TJ, Wetzstein GA, List AF, Ho VQ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: Is one better? Efficacy comparison between CLAG and MEC regimens. Leuk Res. 2011;35:301–4.
Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA. Synthesis and biologic activity of 2-fluoro-2-halo derivatives of 9-beta-d-arabinofuranosyladenine. J Med Chem. 1992;35:397–401.
Cooper T, Ayres M, Nowak B, Ganchi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55:361–8.
Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–9.
Burnett AK, Russell NH, Hunter AE, Milligan D, Knapper S, Wheatley K, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122:1384–94.
Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–9.
Scappini B, Gianfaldoni G, Caracciolo F, Mannelli F, Biagiotti C, Romani C, et al. Cytarabine and clofarabine after high-dose cytarabine in relapsed or refractory AML patients. Am J Hematol. 2012;87:1047–51.
Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–86.
Kantarjian HM, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–55.
Faderl S, Gandhi V, O’Brien S, Bonate P, Cortes J, Estey E, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–7.
Becker PS, Kantarjian HM, Appelbaum FR, Petersdorf SH, Storer B, Pierce S, et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol. 2011;155:182–9.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9.
Roberts DA, Wadleigh M, McDonnell AM, DeAngelo DJ, Stone RM, Steensma DP. Low efficacy and high mortality associated with clofarabine treatment of relapsed/refractory acute myeloid leukemia and myelodysplastic syndromes. Leuk Res. 2015;39(2):204–10.
Middeke JM, Herbst R, Parmentier S, Bug G, Hänel M, Stuhler G, et al. Clofarabine salvage therapy before allogeneic hematopoietic stem cell transplantation in patients with relapsed or refractory AML: results of the BRIDGE trial. Leukemia. 2016;30(2):261–7.
Schlenk RF, Döhner K, Mack S, Stoppel M, Király F, Götze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German–Austrian trial AMLHD98A. J Clin Oncol. 2010;28(30):4642–8.
Sierra J, Storer B, Hansen JA, Bierke JW, Martin PJ, Petersdorf EW, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA- matching, and marrow cell dose. Blood. 1997;89(11):4226–35.
Hemmati PG, Terwey TH, Na IK, Jehn CF, le Coutre P, Vuong LG, Dörken B, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: a single center analysis of long-term outcome. Eur J Hematol. 2015;95(6):498–506.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Molteni, A., Riva, M., Ravano, E. et al. Clofarabine-based chemotherapy as a bridge to transplant in the setting of refractory or relapsed acute myeloid leukemia, after at least one previous unsuccessful salvage treatment containing fludarabine: a single institution experience. Int J Hematol 105, 769–776 (2017). https://doi.org/10.1007/s12185-017-2198-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2198-0