Abstract
Hematopoietic stem cell transplantation (HSCT) and immunosuppressive therapy (IST) with antithymocyte globulin (ATG) and cyclosporine (CsA) have been widely accepted as the standard first-line treatments for severe aplastic anemia (SAA). However, most of the patients with SAA had a slim chance to access these strategies in developing countries. Here, we reported 10-year results in a cohort of 232 patients with SAA who received a novel IST of CsA, levamisole, and danazol (CsA&LMS-based regimen). The cumulative incidence of response was 52.1% at 6 months, 66.4% at 12 months, and 77.1% at 24 months. The 10-year overall survival (OS) and failure-free survival was 60.2% and 48.3%, respectively. Positive predictors of OS in multivariate analysis were higher pretreatment ANC, younger age, higher pretreatment absolute reticulocyte count (ARC), and response within 6 months. The probability of CsA&LMS discontinuation was 50.2% at 10 years. With a slow CsA&LMS taper, the actuarial risk for relapse was only 9.5%. The cumulative incidence of MDS/AML was 8.2% at 10 years. The long-term follow-up information demonstrated that the CsA&LMS regimen could be a promising strategy for patients with SAA in developing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aplastic anemia (AA) is identified as an immune-mediated bone marrow failure, characterized by “anhematopoiesis” and peripheral blood pancytopenia [1]. Hematopoietic stem cell transplantation (HSCT) is curative in a large majority of patients with severe AA (SAA) younger than 40 years old. Immunosuppressive therapy (IST) with antithymocyte globulin (ATG) and cyclosporine (CsA) can effectively restore the blood-cell production in most SAA patients who are > 40 years of age or younger patients without HLA-compatible sibling donors. Therefore, HSCT and IST have been the standard first-line treatments for SAA patients in the last few decades.
AA occurs more frequently in Asia than in the West, with 2- to 3-fold higher incidence rates [2]. Unfortunately, most SAA patients in developing countries have little chance of these first-line treatments because of unavailable HLA-matched donors or old age (> 50 years old). In addition, high costs, long hospitalization periods, and insufficient health insurance services remain problematic. More than 2/3 AA patients in our center could only bear CsA [3]. And, recent prospective study in China revealed that less than 1/4 SAA patients could receive the guideline-recommended first-line treatments of ATG or HSCT [4]. As the advantage of adding CsA to horse ATG (h-ATG) was evident in patients with SAA, the major challenge for clinicians was exploring a novel immunomodulatory agent to replace h-ATG in SAA [5].
Levamisole (LMS), a time-honored agent, was originally used as a synthetic antihelminthic agent. Subsequently, its broad immunomodulatory effects were further demonstrated in vitro and in vivo [6,7,8]. In addition, levamisole was discovered to favor marrow restoration between courses of chemotherapy [9]. And, levamisole was reported to enhance granulocyte colony formation partly through modulating the release of colony-stimulating activity [10]. Moreover, a recent study revealed that levamisole could reshape the bone marrow microenvironment by suppressing adipogenesis of AA-derived mesenchymal stem cells through ZFP36L1-peroxisome proliferator-activated receptor gamma coactivator 1 beta (PPARGC1B) axis [11]. Therefore, we postulated the complementary and synergistic interaction between LMS and CsA might improve the response and survival in AA. In addition, danazol which was reported to have potential interactions with CsA and increased telomere activity in human hematopoietic cells was added to the CsA&LMS-based regimen for maximum restoration of blood-cell production [12, 13].
Previously, we reported the encouraging short-term effectiveness of the CsA&LMS-based regimen in a cohort of 261 SAA patients [14]. For an informed decision of this novel IST, long-term observation of survival, relapse, and evolution was essential. Here, we reported 10-year outcomes of this cohort of SAA patients to clarify the potential and problems of the CsA&LMS-based regimen.
Methods
Patients and follow-up
From December 2008 to September 2013, 261 newly diagnosed SAA patients receiving the CsA&LMS-based regimen were included in this study. Details concerning the patient characteristics and the treatment protocol were published previously [14].
Follow-up information was asked from patients and their attending physicians, including complete blood counts, treatments, transfusion history, adverse events, and general health status. Data on relapse, clinical or laboratory evidence for paroxysmal nocturnal hemoglobinuria (PNH), myelodysplastic syndrome (MDS), leukemia, and solid tumors were also collected. This study was approved by the Ethics Committees of the Institute of Hematology, in accordance with the guidelines of the Declaration of Helsinki (Ethics number: KT2014005-EC-1); all patients gave written consent to publish follow-up results anonymously.
Protocol details
The details of the CsA&LMS regimen were previously reported [14]. In brief, the CsA and LMS were alternatively administrated every other day. The initial dose of CsA was 3 mg/kg/day in adults and 5 mg/kg/day in children (6–18 years old). Oral LMS was alternatively administrated at a dose of 150 mg per day in adults and 2.5 mg/kg per day in children (< 40 kg) in three divided doses. Both CSA and LMS were continued for at least 12 months. Tapering always started after a stable maximal response lasting for 6 or more months with a very slow tapering rate of 25% reduction in dose for every 3–4 months according to the response. All patients received oral danazol (5.0–10.0 mg/kg/day), folic acid (0.1–0.2 mg/kg/day), and vitamin B12 (10 μg/kg, twice a week) for more than 12 months. The dose of danazol was adjusted according to the serum transaminase levels.
Definitions
SAA was defined by the presence of bone marrow hypocellularity (less than 30%) and satisfying two of the three following criteria: absolute neutrophil count (ANC)<0.5 × 109/L; absolute reticulocyte count (ARC)<60 × 109/L; platelet count<20 × 109/L [15]. Very severe AA (VSAA) was further defined by initial ANC less than 0.2 × 109/L [16].
Hematological response was defined as no longer meeting the criteria for SAA without transfusion and recombinant human granulocyte colony-stimulating factor (rhuG-CSF). Complete response (CR) was determined by satisfying all the three following peripheral blood count criteria: hemoglobin> 110 g/L; ANC> 1.5 × 109/L; platelet count> 80 × 109/L [17]. Partial response (PR) was considered when the peripheral blood counts no longer meet the criteria for SAA without transfusion and rhuG-CSF. No response was identified as still meeting the criteria for SAA, transfusion dependency, or death within 6 months.
Time to response was calculated from the first day of treatment to the day patients obtained PR. All PRs were counted as events, including late response but not the remission induced by salvage therapy. When calculating response kinetics, only response achieved within 24 months after a single course of treatment was included. Patients who reached response after salvage treatment and those who died without response were censored at the time of salvage therapy or death.
Relapse was indicated by the requirement for blood transfusion. Time to relapse was calculated from the day the patient achieved response to the day of relapse. When calculating the time to relapse, patients who died and those who survived at last follow-up without relapse were censored at the time of death or last follow-up.
Evolution into MDS was defined by the presence of dysplasia in at least two bone marrow lineages with or without characteristic abnormal karyotype. The clinical PNH was diagnosed by at least two times positive Ham test or more than 20% glycosylphosphatidyl-anchored protein (GPI-AP)-deficient clone in two series, and associated with clinical or laboratory evidence of hemolysis.
Overall survival (OS) was determined from the first day of the CsA&LMS-based regimen to the day of death, transplantation, or last follow-up (February 2019). Failure-free survival (FFS) was defined as survival with response. Time to treatment failure (TTTF) was calculated from the first day of treatment to salvage therapy for NR, relapse, clonal evolution, solid tumor, or disease-related death, whatever came first. For nonresponders who received no salvage therapy and finally died, 6 months were considered as TTTF. Early mortality was defined as deaths within 3 months after initiation of CsA&LMS-based treatment.
Statistical analysis
Two group comparisons were made using the chi-square test or Fisher’s exact test as appropriate. Cox regression was used to estimate the hazard ratios of covariates for OS and FFS. The probability of survival, relapse, and clonal evolution were calculated using the Kaplan-Meier method and compared using the log-rank test. All significance tests were 2-sided. Calculations were performed using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) statistical software packages.
Results
Follow-up
Updated information was collected from all the 261 SAA patients. During observation, 29 patients were lost follow-up. Therefore, 232 patients were included for the calculation of long-term outcomes. Of the 232 patients, 68 (29.3%) cases were VSAA, including 7 hepatitis-associated AA patients. The median age for all patients was 24 (range 5–77, 132 male and 100 female) years. The median observation time of surviving patients was 6.8 (range, 1.0–10.1) years.
Response
Of this cohort, 107 (46.1%, 93 PR + 14 CR) and 132 (56.9%, 93 PR + 39 CR) cases achieved response at 6 months and 12 months, respectively. Subgroup analysis demonstrated that the response in patients with higher ANC (> 0.2 × 109/L) was more frequent than that in VSAA patients at 6 months (55.5% vs 23.5%, P < 0.001). And, when the deaths within 6 months were excluded, the difference between the two groups remained significant (58.3% vs 45.7%, P = 0.006).
Blood counts of 14 patients improved more than 12 months and up to 25 months after initiation of treatment. Therefore, the overall response rate was 62.9% (146/232, 57 PR + 89 CR) during the observation time. The median time from initiation of treatment to PR was 3.0 months (range 0.5–25.0) and to CR was 16 months (range 3.0–64.0). Young patients (≤ 20 years) had a significantly higher probability of CR than those old ones (47.3% vs 32.6%, P = 0.03). And, the achievement of CR was more frequent in patients with short disease duration (≤ 60 days) than those with long one (45.5% vs 30.3%, P = 0.02). In addition, the advantage of CR was more evident in patients with higher pretreatment ANC (> 0.2 × 109/L) or higher pretreatment platelets (> 7 × 109/L) than those with lower ones (44.5% vs 23.5%, P = 0.003; 45.7% vs 32.3%, P = 0.02). Univariate analysis revealed that the likelihood of CR was not associated with sex, etiology, and pretreatment ARC. Multivariate analysis demonstrated that short duration and higher pretreatment ANC remained favorable factors for achieving CR.
Kinetics of response
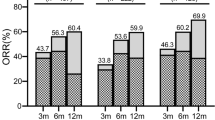
Up to 39 (16.8%) patients achieved response after more than 6 months, 18 of these patients achieved CR and maintained stable CR 1.4 to 8.7 years after initial treatment. Only 2 patients responded after more than 24 months. Therefore, the probability of remission was calculated by the information from those patients who responded within 24 months of treatment. The actuarial response rates for this cohort were 52.1% at 6 months, 66.4% at 12 months, and 77.1% at 24 months (Fig. 1a), respectively. As seen in Fig. 1b, the slope of response curve was steeper in patients with higher pretreatment ANC than that in VSAA patients (P = 0.03).
Survival and failure-free survival
The overall survival (OS) of all patients was 60.2% (95% CI 50.2–68.7%) at 10 years (Fig. 2a). Rapid response was strongly associated with long-term survival. OS was up to 96.3% for patients who responded within 6 months while 29.1% for nonresponders (Fig. 2b). There was a significant survival advantage of patients with higher initial ANC over that of VSAA patients (70.8% vs 33.1%; Fig. 2c). And, patients with higher pretreatment ARC (> 20 × 109/L) or higher pretreatment PLT (> 7 × 109/L) harbored higher OS rates than those with lower ones (81.1% vs 46.6% and 63.1% vs 55.5%, respectively; Fig. 2d, e). In addition, younger patients (≤ 40 years) had significantly better survival than older ones (68.0% vs 34.7%, Fig. 2f). Interestingly, the survival in patients with a long disease duration (> 60 days) was superior to that with shorter ones (61.9% vs 57.7%, P = 0.006). In fact, most (88.1%) of the patients with a long duration were those patients with initial ANC > 0.2 × 109/L. Therefore, when we further stratified patients by pretreatment ANC, the significance disappeared in both groups (P = 0.3 and P = 0.6, respectively). In multivariate analysis, severity of the disease, patients’ age, higher pretreatment ARC, and response within 6 months remained favorable factors of OS.
The actuarial 10-year FFS of all patients was 48.3% (95% CI 41.1–55.1%) (Fig. 3a), it is evident that treatment failures occurred more frequently in the VSAA group than those in patients with higher initial ANC (58.2% vs 24.4%; Fig. 3b). In addition, patients with higher pretreatment ARC or PLT harvested higher 10-year FFS rates than those with lower ones (66.5% vs 35.3% and 54.7% vs 42.6%, respectively; Fig. 3c, d). Positive predictors of FFS in multivariate analysis were higher pretreatment ARC and ANC.
In this cohort, 80 (34.5%) patients died with a median interval of 0.5 years (range 0.02–9.0) after initiation of treatment. All these deaths were nonresponders at 3 months including infections in 46 patients, hemorrhage episodes in 19 ones, MDS or AML in 10 ones, lung cancer in 1 case, and unconfirmed causes in 4 ones. Twenty-three (9.9%) patients died within 3 months. Causes of early mortality were hemorrhage in 4 patients and infections in 19 patients.
LMS discontinuation and relapse
In this cohort, 89 (38.4%) patients reached CR during the observation time. Of all the CRs, 71 (79.8%) patients successfully discontinued the CsA&LMS-based regimen without relapse and clonal evolution. The cumulative probability of CsA&LMS-regimen discontinuation in all patients was 37.1% (95% CI 27.5–46.6%) at 5 years, 47.2% (95% CI 38.0–55.8%) at 7 years and 50.2% (95% CI 41.0–58.8%) at 10 years after initiation of treatment (Fig. 4a).
Blood counts deteriorated to fulfill the criteria of relapse in 12 (8.2%) of 146 responders. The secondary treatments and finally outcomes in patients who relapsed were displayed in Table 1. The actuarial risk for relapse was 9.5% (95% CI 1.4–27.7%) at 10 years (Fig. 4a). Unexpectedly, no relapse was observed in the VSAA group. The median time from response to relapse was 3.8 (range 0.7–6.4) years.
Adverse events
Short-term toxicities were previously reported [14]. During the long-term follow-up, no delayed LMS-associated toxicities were observed. CsA-associated toxicities were modest: impairment of renal function with creatine values up to 164.3 μmol/L occurred in 9 patients; gingival hyperplasia was developed in 7 patients; hypertrichosis was observed in 1 patient; involuntary movement was reported in 2 patients. All these toxicities were mild except for 1 patient with the highest creatine level who had the dose of CsA adjusted. Danazol-associated adverse events were more frequent and required intervention: impairment of liver function with transaminase values up to 683 U/L in 40 patients including grade 3 (NCI-CTC V3.0) in 8 patients, grade 2 in 15 patients, and grade 1 in 17 patients. For patients with grade 3, danazol was discontinued followed by intensive liver-protective treatment. After the transaminase values recovered to normal for at least 2 weeks, danazol was re-administrated at half of the initial dose, then adjusted by transaminase level to achieve the optimal tolerable dose (median 6.6 mg/kg/day, range 5.5–8.2 mg/kg/day). For patients with grade 2, the dose of danazol was reduced and the final median dose was 7.1 (range 5.0–9.0) mg/kg/day. For patients with grade 1, the dose of danazol was continued while the liver-protective treatment was strengthened for 2 weeks. Other adverse effects were mild or modest acne (18 patients), gynecomastia (2 patients), and hepatic adenoma (2 patients).
Clonal and malignant diseases
In this cohort, clonal or malignant diseases developed in 21 (9.1%) patients: PNH in 7 patients, MDS in 7 patients, leukemia in 5 patients, and solid tumor in 2 patients. PNH was observed 1.8–4.8 years after initiation of treatment. The probability of PNH evolution was 4.5% (95% CI 0.1–22.9%). All patients had previously reached remission: 4 patients with CR and 3 ones with PR. The actuarial risk for MDS or leukemia was 8.2% (95% CI 0.9–26.1%) at 10 years. The median interval from treatment to the confirmation of MDS or leukemia was 2.4 years (range 1.3–7.8). Seven of 12 patients had previously been in response: 4 patients in CR and 3 patients in PR. According to the French-American-British (FAB) classification, leukemia in 5 patients was all classified as AML-M5. All of them died rapidly including 1 patient who relapsed after HSCT.
Cytogenetic abnormalities developed in 12 (5.2%) patients. Seven patients evolved into MDS or leukemia and died rapidly after supportive care or unsuccessfully secondary treatment: 5 patients with monosomy 7 and 2 ones with complex karyotypes. The remaining 5 patients developed transiently cytogenetic abnormalities (complex karyocytes, 9q+, 6p+, +Y and +8, respectively) without the presence of bone marrow dysplasia and maintained stable blood counts (CR in 3 patients and PR in 2 patients). The secondary treatments and final outcomes of patients who developed clonal diseases were illustrated in Table 1.
Discussion
Although improvements had been achieved in treatments for SAA by combining ATG with CsA or adding eltrombopag to standard IST, the options for SAA patients who were not ineligible for ATG or HSCT remained limited [5, 18]. The disappointing response rate of CsA alone for SAA patients was impressive: only 11.6% of patients responded at 3 months [19]. In addition, CsA-associated toxicities were frequent when a full dosage was used for a long term, especially nephrotoxicity which was observed in 42–56% of patients [19, 20]. Therefore, a novel CsA&LMS-based regimen was probed for those patients. A former report clarified the effectiveness of danazol for AA patients who were refractory to standard IST [21]. And then, danazol was accepted as a therapeutic option for AA patients when neither HSCT nor IST was available [22]. Thus, danazol was added to CsA&LMS-based regimen to maximize blood-cell production in SAA. Previously, we reported the encouraging short-term results of the novel regimen for this SAA cohort [14], while questions remained about the long-term benefits. This report extended the previous analysis and provided long-term information for physicians and patients to determine alternative treatment strategies for SAA.
The actuarial incidence of response was 52.1% at 6 months and 66.4% at 12 months in this study, which was comparable with recent international clinical trials concerning standard IST with cumulative response of 52.0% at 6 months and 65.0% at 12 months [23]. And, the superiority of response to this novel IST was much more evident than treatment with CsA alone [19, 24]. Subgroup analysis revealed a remarkable response rate of 67.1% in patients with higher pretreatment ANC at 12 months. And, a rapid response was also observed in these patients. Giving that rapid response may result in less use of medical resources, better prognosis, and thereby less costs, we suggested patients with higher initial ANC (> 0.2 × 109/L) as the best candidates for this novel IST. In addition, from the shape of response curve, we observed only 16.8% of patients responded more than 6 months. Moreover, late remission (> 6 months) was significantly associated with poor survival. Therefore, we suggested 6 months as a checkpoint to consider salvage or alternative treatment strategies.
The 10-year OS of all patients was 60.2% which was comparable with the long-term follow-up results of standard IST from large American and German trials [5, 15]. And, the actuarial FFS rate was 48.3% which was not inferior to that of standard IST [5, 25]. All these encouraging results were observed probably because most (164 of 232) of the patients in this cohort were those with higher pretreatment ANC in which the actuarial OS and FFS rates were up to 70.8% and 58.2%, respectively. High early mortality was observed in VSAA which further confirmed the unsuitability of this combined regimen for VSAA. In addition, younger age (≤ 40 years), pretreatment ARC> 20 × 109/L, and higher pretreatment platelet (> 7 × 109/L) were favorable prognostic factors for long-term outcomes, which gave us guidance for optimal patient management and individual therapeutic monitoring.
A former study demonstrated a reduced incidence of relapse at 5 years from 30 to 13% when CsA tapering was prolonged [26, 27]. Subsequently, a study conducted in children revealed the incidence of relapse was only 7.6% in the slow CsA taper group compared with 60% in the rapid taper group [28]. Although a recent study conducted in adult-based population provided different results, the actuarial risk of relapse remained high to 32% [29]. In this study, a slow CsA&LMS tapering schedule was performed and the actuarial risk of relapse was only 9.5% at 10 years; moreover, approximately half of patients successfully discontinued agents at last follow-up, which implicated the mechanism of persistent pathological immunity in SAA and highlighted the essence of a slow CsA&LMS taper schedule. One may argue the CsA-associated toxicities after long-term exposure. In fact, CsA was administrated every other day alternatively with LMS in this regimen. Therefore, CsA-associated toxicities were rare and modest. Only 9 (3.9%) patients developed nephrotoxicity in this cohort. In addition, a minority of relapse was observed in patients with CR which gave us an indication that the immunosuppressive effectiveness in patients with CR was thorough and might be curative. Therefore, how best to improve the CR rate needs to be further explored.
The incidence of clonal evolution, especially evolution into MDS/AML is another concern in the long-term follow-up. The risk for MDS/AML evolution was reported between 8 and 15% in previous studies [5, 15, 30]. Long-term use of granulocyte colony-stimulating factor and nonresponders at 6 months were considered as risk factors for MDS/AML evolution [31]. In this report, the actuarial risk for evolution into MDS/AML was only 8.2% at 10 years. This might be contributed to the relatively mild and persistent immunosuppressive effectiveness of this novel IST which alleviated the hematopoietic stress and deprived the opportunity for expansion of malignant clones. In addition, majority (8/12) of MDS/AML evolution occurred in patients without complete response, especially nonresponders. Therefore, we highly recommended immediate salvage or alternative treatments for nonresponders.
In this long-term follow-up, 71 (30.6%) of all patients achieved normal peripheral blood counts without relapse and clonal evolution, no longer need treatment, and did not have treatment-associated adverse effects. They were all alive and well; some of them may be cured. The same as standard IST, this novel regimen cured only a minority of patients. In most patients, the CsA&LMS regimen converted a life-threatening disease to a chronic disease. But this conversion eased the medical burden of the government and provided most of SAA patients long-term survival with improved quality of life.
In conclusion, LMS and CsA could be a promising strategy for SAA patients with long-term benefits including desirable response, convenient administration, acceptable side effects, low costs, favorable survival, and infrequent relapse and clonal evolution, especially when they are ineligible for ATG and HSCT in developing countries.
References
Young NS, Calado RT, Scheinberg P (2006) Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 108(8):2509–2519
Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Leaverton PE, Shapiro S, Young NS (2006) The epidemiology of aplastic anemia in Thailand. Blood 107:1299–1307
Li YM, Li XX, Ge ML, Shi J, Qian LS, Zheng Y, Wang J (2011) Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol 90:529–537
Zhu XF, He HL, Wang SQ, Tang JY, Han B, Zhang DH et al (2019) Current treatment patterns of aplastic anemia in China: a prospective registry study. Acta Haematol 15:1–9
Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H (2003) Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood 101:1236–1242
Ramot B, Biniaminov M, Shoham C, Rosenthal E (1976) Effect of levamisole on E-rosette-forming cells in vivo and in vitro in Hodgkin’s disease. N Engl J Med 294:809–811
Hersey P, Ho K, Werkmeister J, Abele U (1981) Inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures of T and B cells by levamisole in vitro and in vivo. Clin Exp Immunol 46:340–349
Stevenson HC, Green I, Hamilton JM, Calabro B, Parkinson DR (1991) Levamisole: known effects on the immune system, clinical results, and future applications to the treatment of cancer. J Clin Oncol 9:2052–2066
Lods JC, Dujardin P, Halpern GM (1976) Levamisole and bone-marrow restoration after chemotherapy. Lancet 1:548
Senn JS, Lai CC, Price GB (1980) Levamisole: evidence for activity on human haemopoietic progenitor cells. Br J Cancer 41:40–46
Liu LL, Liu L, Liu HH, Ren SS, Dou CY, Cheng PP, Wang CL, Wang LN, Chen XL, Zhang H, Chen MT (2018) Levamisole suppresses adipogenesis of aplastic anemia-derived bone marrow mesenchymal stem cells through ZFP36L1-PPARGC1B axis. J Cell Mol Med 22:4496–4506
Borrás-Blasco J, Rosique-Robles JD, Peris-Marti J, NavarroRuiz J, Gonzalez-Delgado M, Conesa-Garcia V (1999) Possible cyclosporin-danazol interaction in a patient with aplastic anemia. Am J Hematol 62:63–64
Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA (2009) Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114:2236–2243
Wang M, Li XX, Shi J, Shao YQ, Ge ML, Huang JB, Huang Z, Zhang J, Nie N, Zheng Y (2015) Outcome of a novel immunosuppressive strategy of cyclosporine, levamisole and danazol for severe aplastic anemia. Int J Hematol 102:149–156
Rosenfeld S, Follmann D, Nunez O, Young NS (2003) Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA 289:1130–1135
Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT et al (1988) Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anemia (SAA): a report of the EBMT SAA working party. Br J Haematol 70:177–182
Li XX, Shi J, Ge ML, Shao YQ, Huang JB, Huang ZD, Zhang J, Nie N, Zheng Y (2013) Outcomes of optimized over standard protocol of rabbit antithymocyte globulin for severe aplastic anemia: a single-center experience. PLoS One 8:e56648
Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, Valdez J, Lotter J, Feng X, Desierto M, Leuva H, Bevans M, Wu C, Larochelle A, Calvo KR, Dunbar CE, Young NS (2017) Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med 376:1540–1550
Gluckman E, Esperou-Bourdeau H, Baruchel A, Boogaerts M, Briere J, Donadio D, Leverger G, Leporrier M, Reiffers J, Janvier M (1992) Multicenter randomized study comparing cyclosporine-A alone and antithymocyte globulin with prednisone for treatment of severe aplastic anemia. Blood 79:2540–2546
Maschan A, Bogatcheva N, Kryjanovskii O, Shneider M, Litvinov D, Mitiushkina T, Timakov A, Timakova M, Samotchatova E, Rumiantsev A (1999) Results at a single Centre of immunosuppression with cyclosporine A in 66 children with aplastic anemia. Br J Haematol 106:967–970
Chuhjo T, Yamazaki H, Omine M, Nakao S (2008) Danazol therapy for aplastic anemia refractory to immunosuppressive therapy. Am J Hematol 83(5):387–389
Jaime-Pérez JC, Colunga-Pedraza PR, Gómez-Ramírez CD, Gutiérrez-Aguirre CH, Cantú-Rodríguez OG, Tarín-Arzaga LC, Gómez-Almaguer D (2011) Danazol as first-line therapy for aplastic anemia. Ann Hematol 90(5):523–527
Bacigalupo A, Oneto R, Schrezenmeier H, Hochsmann B, Dufour C, Kojima S, Zhu X, Chen X, Issaragrisil S, Chuncharunee S, Jeong DC, Giammarco S, van Lint MT, Zheng Y, Vallejo C (2018) First line treatment of aplastic anemia with thymoglobuline in Europe and Asia: outcome of 955 patients treated 2001-2012. Am J Hematol 93:643–648
Leonard EM, Raefsky E, Griffith P, Kimball J, Nienhuis AW, Young NS (1989) Cyclosporine therapy of aplastic anemia, congenital and acquired red cell aplasia. Br J Haematol 72:278–284
Tichelli A, Schrezenmeier H, Socié G, Marsh J, Bacigalupo A, Dührsen U (2011) A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood 117:4434–4441
Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J et al (2000) Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy – the European Group for Blood and Marrow Transplantation experience. Semin Hematol 37:69–80
Locasciulli A, Bruno B, Rambaldi A, Saracco P, Dufour C, Finelli C et al (2004) Treatment of severe aplastic anemia with antilymphocyte globulin, cyclosporine and two different granulocyte colony-stimulating factor regimens: a GITMO prospective randomized study. Haematologica 89:1054–1061
Saracco P, Quarello P, Iori AP, Zecca M, Longoni D, Svahn J, Varotto S, del Vecchio G, Dufour C, Ramenghi U, Bacigalupo A, Locasciulli A, Bone Marrow Failure Study Group of the AIEOP (Italian Association of Paediatric Haematology Oncology) (2008) Cyclosporin A response and dependence in children with acquired aplastic anemia: a multicentre retrospective study with long-term observation follow-up. Br J Haematol 140:197–205
Scheinberg P, Rios O, Scheinberg P, Weinstein B, Wu CO, Young NS (2014) Prolonged cyclosporine administration after antithymocyte globulin delays but does not prevent relapse in severe aplastic anemia. Am J Hematol 89:571–574
Sheinberg P, Young NS (2012) How I treat acquired aplastic anemia. Blood 120:1185–1196
Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y, Kaneko T, Takano T, Ikuta K, Tsukimoto I, Japan Childhood Aplastic Anemia Study Group (2002) Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood 100:786–790
Acknowledgments
The authors would like to thank all the doctors and nurses in the Therapeutic Centre of Anemic Diseases and the researcher team of the Clinical Laboratory Centre for their professional assistance.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81700120, No. 81770119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huo, J., Li, X., Shao, Y. et al. Long-term follow-up of a novel immunosuppressive strategy of cyclosporine alternatively combined with levamisole for severe aplastic anemia. Ann Hematol 99, 1727–1734 (2020). https://doi.org/10.1007/s00277-020-04153-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04153-9