Abstract
Purpose of Review
To review the most current diagnostic tools and treatment options for pyogenic and tubercular spine infection.
Recent Findings
Recent studies have focused on risk factors for failed nonoperative management in order to improve patient selection. Also, spine instrumentation and different grafting options have been safely utilized in the setting of an active infection without increasing the incidence of reoccurrence. However, the optimal surgical technique has yet to be established and instead should be patient specific.
Summary
Spine infections include a broad spectrum of disorders including discitis, vertebral osteomyelitis, and spinal epidural abscess. It is paramount to recognized spine infections early due to the potential catastrophic consequences of paralysis and sepsis. The management of spine infections continues to evolve as newer diagnostic tools and surgical techniques become available. Magnetic resonance imaging with contrast is the imaging study of choice and computed tomography-guided biopsies are crucial for guiding antibiotic selection. Antibiotics are the mainstay of treatment and surgery is indicated in patients with neurological deficits, sepsis, spinal instability, and those who have failed nonoperative treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
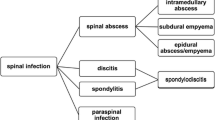
Spine infections represent a heterogeneous group of disorders that includes discitis, vertebral osteomyelitis (VO), and spinal epidural abscess (SEA). Although rare, the incidence of VO has steadily increased as a result of patients living longer with chronic, immunosuppressive comorbidities, and now accounts for 2 to 7% of all cases of osteomyelitis [1]. Despite a significant amount of literature on spinal infections, minimal high-level evidence exists to guide ideal medical and surgical management. In this article, we focus on the current literature for pyogenic spinal infections (PSI) and spinal tuberculosis in order to guide clinicians through the medical and surgical decision-making process.
Pyogenic Spinal Infections
Hematogenous spread of bacteria to the spine is the most common mechanism of contracting a primary PSI. The most common organism causing PSI is S. aureus, with methicillin-resistant Staphylococcus aureus (MRSA) accounting for over 40% of these cases [2]. The expansion of antibiotic-resistant bacteria is concerning as they increase the risk of failed nonoperative treatment. Graham reported a 12.7% incidence of gram-negative PVO, identifying E. coli, P. aeruginosa, H. influenza, and K. pneumonia on cultures [3]. Additionally, nearly 1 in 10 pyogenic vertebral osteomyelitis (PVO) cases are polymicrobial, emphasizing the importance of following cultures until finalized [2]. Risk factors for PSI include advanced age, immunosuppression (diabetes, malignancy, corticosteroids), IV drug use, indwelling central catheters, and recent spinal instrumentation [4]. Inoculation of the spine through hematogenous spread most commonly involves the lumbar spine (58%) followed by the thoracic (30%) and cervical spine (12%) [5].
Spinal Tuberculosis
M. tuberculosis is the most common etiology of vertebral granulomatous infection. Vertebral granulomatous infections are found in 10–20% of TB cases in developed nations and upwards of 20–41% in undeveloped nations [6]. Tubercular infection commonly spread from the metaphyseal regions of the vertebral body anteriorly beneath the anterior longitudinal ligament and extends in a cranial-caudal direction. The spread can be discontinuous, creating skip lesions and paravertebral abscesses (Fig. 1). Furthermore, immunocompromise has been found to increase the incidence of musculoskeletal lesions. While 3–5% of patients with pulmonary TB develop musculoskeletal lesions, this number substantially rises to nearly 60% in patients with HIV [7]. Compared to PSI, spinal tuberculosis displays a greater predisposition for thoracic spine involvement, deformity, and significant neurologic deficits [8].
Clinical Evaluation
The clinical picture of spinal infections can mimic malignancy, as constitutional symptoms such as malaise, night sweats, back pain, and weight loss are common presenting symptoms. Symptoms can also be misattributed to unremitting back pain from degenerative spinal disorders. As a result, the diagnosis of spinal infections can often be delayed and result in severe neurological complications [9]. Physical examination may reveal localized spinal tenderness over involved regions with limited range of motion. SEA is a relatively infrequent but potentially devastating spinal infection and an important pathology to rapidly identify. Davis found the classic triad of back pain, fever, and neurological deficit was present in only 13% of patients [10].
In cases of advanced spinal tuberculosis, gross kyphotic deformity may also be present (Fig. 2). Patient may also report dysphagia or dyspnea as a result of cervical spine infections spreading anteriorly and compressing surrounding structures. Clinicians should be aware that neurologic deficits can appear at multiple timepoints during the disease process, with 33% of deficits presenting in the first month, 40% between 4 weeks to 3 months, and 27% presenting after 3 months [11]. Pott’s paraplegia is a distinct case of paralysis in the setting of spinal tuberculosis resulting from anterior pathologic compression of the neural elements due to abscess extension into the spinal canal or from kyphotic collapse of the vertebrae with bony retropulsion. Due to the low specificity of many of these clinical findings and the potential catastrophic outcomes, a high level of suspicion and a thorough diagnostic workup is required.
Diagnostic Evaluation
Laboratory Findings
Some serological markers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are useful screening tools to differentiate nonspecific back pain from more serious etiologies [12]. CRP is often elevated along with ESR, but CRP is more sensitive and more effective in monitoring treatment response due to its shorter half-life. Although often used as a screening tool, white blood cell (WBC) count is unreliable and may be normal in up to 40% of patients [13]. Clinicians should closely follow inflammatory markers during the treatment of discitis and VO, as ESR > 55 mm/h and CRP > 2.75 after 4 weeks of antibiotic treatment is associated with treatment failure (odds ratio 5.15) [14]. Compared to PSI, TB is less frequently associated with elevated inflammatory markers. Blood cultures are not sensitive but should be obtained in all patients with a suspected spinal infection as positive results drive antibiotic selection and can predict poor outcomes of nonoperative treatment [15].
Plain Radiography
Plain radiographs are usually normal during the early phases of a spinal infection. Radiographic findings such as narrow disc space and destruction of the endplates may be evident; however, bony destruction may not be present for weeks. In contrast, during the early phases of infection, spinal tuberculosis may show vertebral body involvement with sparing of the disc space and is more commonly seen posteriorly. In chronic PVO and spinal tuberculosis, clinicians should also obtain upright 36″ AP and lateral imaging for surgical planning to evaluate for kyphotic deformity with potential sagittal imbalance.
Magnetic Resonance Imaging and Computed Tomography
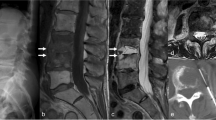
Contrast-enhanced MRI is the imaging modality of choice for diagnosing spinal infections. MRI can help show the extent of the infection, provide superior imaging of paraspinal soft tissue and epidural space, and assist with distinguishing tubercular spinal infections and PSI. MRI is also critical in the setting of neurologic deficit for planning surgical approach and levels of decompression and stabilization. If available, MRI scanning of the entire spine is optimal to evaluate for skip abscesses and other areas of neurologic compression (Fig. 3). For example, Ju et al. reported 22 skip lesions in 233 patients with SEA [16].
Forty-four-year-old with DM type 2 transfer presented with neck pain, arm weakness, and urinary incontinence with a diagnosis of C5-C6 spondylodiscitis (a). Follow-up imaging of the entire spine revealed a thoracic epidural (b) abscess extending down to the sacrum and L3-L4 spondylodiscitis (c). The patient underwent C5-C6 anterior cervical discectomy and fusion with autograft and C4-7 posterior cervical decompression and fusion with T4-S1 laminectomy (d). The patient later went back for an L3-L4 anterior lumbar interbody fusion and posterior lumbar instrumented fusion (e)
Early MRI changes for discitis and PVO commonly display increased T2 and decreased T1 signal intensity in affected discs and adjacent endplates/vertebrae and increased T2 signal in the paraspinal soft tissues. The early MRI findings of increased T2 disc signal along with contrast uptake within the disc are highly sensitive (70–100%) for diagnosis [2]. Findings on MRI that can help distinguish spinal tuberculosis from PSI include large, well-defined paraspinal abscess with thin rim enhancement and smooth margins, thoracic spine involvement, subligamentous extension to adjacent vertebra with preserved disc height, and multilevel involvement with skip lesion [17]. Anley and colleagues found that patients with both TB vertebral osteomyelitis (TBVO) and HIV have been found to demonstrate increased vertebral collapse (107% versus 75.3% for TBVO patients who are HIV negative) and large anterior epidural purulent collections [18]. CT scan be obtained to demonstrate the extent of osseous destruction and for preoperative planning, but soft tissue involvement and the epidural space are poorly evaluated.
Biopsy and Cultures
Despite the effectiveness of advanced imaging, diagnostic cultures are central to directing antimicrobial therapy. In patients without indications of urgent surgical treatment, tissue biopsy via CT-guided needle biopsy is often required. CT-guided spinal biopsy is a useful tool for making a definitive diagnosis and potentially avoiding the need for open surgery. CT-guided biopsies have a specificity of 99% but its sensitivity ranges from 52 to 91% [19, 20]. Biopsies of related soft tissue abscesses may offer higher diagnostic yields in patients with PVO with soft tissue abscesses; as Kim reported, culture positive rates of vertebral bodies and soft tissue were 39.7 and 63.5%, respectively [21]. If cultures cannot be successfully obtained percutaneously, open biopsy should be considered.
Unless the patient is septic, antibiotics should be held prior to obtaining cultures as studies have shown that empiric treatment has been associated with lower diagnostic yield [22,23,24]. While De Lucas et al. identified a causative organism in 60% of patients not previously treated with antibiotics, only 23% of patients who had received antibiotics had positive cultures [24]. If tuberculosis is suspected, acid fast bacilli (AFB) and cultures should be collected.
Treatment
Most spinal infections can be treated successfully with a combination of prolonged antibiotics and bracing. However, patients with a neurological deficit, sepsis, spinal instability, or those who have failed nonoperative treatment are best managed with a combination of medical and surgical treatment. Timely diagnosis and a multidisciplinary approach are essential to the management of any spinal infection.
Antibiotics are the mainstay of treatment. Generally, antibiotics should be administered after cultures are obtained. However, in the setting of severe sepsis, broad-spectrum antibiotics should be initiated empirically. The choice and duration of antibiotic should be tailored to the isolated organism and infectious disease specialist should be consulted to assist with antibiotic selection, dosing, route, and duration of therapy. Discitis and VO without an epidural abscess can commonly be medically managed, with surgery reserved for failed nonoperative treatment or progression of spinal deformity resulting in instability. Factors that suggest spinal instability include vertebral body collapse greater than 50%, greater than 20° of angulation, and greater than 5° of vertebral translation [25].
Discitis and Vertebral Osteomyelitis
The goals of surgery include early decompression, aggressive tissue debridement, and stabilization of the spine. The approach for the surgical management of discitis and VO is controversial and guided by clinical judgment. While an anterior approach allows direct exposure for debridement and reconstruction, the posterior approach allows for posterior instrumentation, debridement, and reconstruction with one approach but may limit direct access to pathology. As surgeons become more proficient with minimally invasive surgery (MIS) techniques, MIS posterior and direct lateral retroperitoneal approaches have been published for PSI with successful results [26,27,28]. The surgical approach for cervical discitis between anterior debridement and fusion or circumferential approaches is controversial. While Shousha and colleagues reported successful results with anterior alone surgery for cervical discitis, Ghobrial have suggested efficacy and low complication rates with circumferential (anterior discectomy and fusion with posterior instrumentation) treatment of cervical discitis and cervical epidural abscesses [29,30,31]. Additionally, significant controversy exists in the use of instrumentation, one-stage versus two-staged procedures, anterior versus posterior instrumentation, and the use of autograft versus allograft.
Despite controversy, multiple studies suggest favorable outcomes and low recurrent infection rates using instrumentation in the surgical treatment of spinal infection. Bydon and colleagues retrospectively reported on 118 patients undergoing debridement of PSI with or without instrumentation, noting similar rates of recurrent infection (8.3% for debridement and 9.8% for debridement with instrumentation) and re-operation (19.4% for debridement and 17.1% for debridement with instrumentation) [32]. Similarly, Carragee evaluated 32 immunocompromised patients with PSI treated with either anterior or posterior instrumentation and only reported one recurrence in 22 patients available for 10-year follow-up [33•].
Structural bone grafting can be used in these situations for anterior structural support after debridement, but fusion can be challenging in situations of multilevel grafting and in the setting of infection. Common autograft sources used in spinal surgery include tricortical iliac crest, rib, and fibular strut. Humeral or femoral allograft struts are other options for bone graft in the setting of an extensive anterior debridement and/or corpectomy. While allograft avoids the morbidity of donor site harvesting, autograft is theorized to have superior rates of incorporation. While some concern exists with introducing allograft into infected surgical fields, several small case series suggest similar recurrent infection rates and clinical outcomes when compared to autograft [34, 35]. Performing corpectomies with cage reconstructions, Lu et al. reported recurrent infections after initial management in one of 19 allograft cases compared with one of 17 autograft (rib or iliac crest) cases within an average follow-up of 21 months with no implant failures reported [35]. Despite several case series suggesting equivalency to autograft, surgeons should exercise caution with the use of allograft in surgical management of PVO.
The use of cages with bone graft placed into vertebral defects after debridement is a helpful adjunct in reconstruction. Using expandable titanium cages for reconstruction of large defects from multiple contiguous corpectomies, Robinson reported successful outcomes in 25 patients with no PVO recurrence and significant improvements in ODI and VAS scores at 36-month follow-up [36]. Kuklo reviewed their experience with single-stage anterior titanium cages and posterior instrumentation in 21 patients, noting 2 repeat operations, an average of 12.3° improvement in kyphosis, and no reported deaths or neurologic complications at an average follow-up of 44 months [37]. Sundararaj similarly reported successful outcomes in 32 patients with single-stage anterior debridement with cage placement and posterior instrumentation, with neurologic improvement in 10/13 (76.9%) and good or excellent clinical outcomes in 30/32 (93.8%) [38]. Shetty reviewed their institution’s experience of 27 posterior interbody fusions for spondylodiscitis, reporting no cases of cage migration, loosening, pseudarthrosis, or recurrence of infection at a mean follow-up of 30 months [39].
Postoperative cases of discitis are rare and can be initially managed with antibiotic therapy in the neurologically intact patient. Conversely, cases of postoperative spondylodiscitis with internal fixation require surgical debridement [40]. Failure of medical management and/or neurologic deficit can be treated with debridement of the disc space and posterior interbody fusion [41, 42].
Spinal Epidural Abscesses
The choice between operative and nonoperative treatment for SEA is controversial as well but tends to favor operative treatment due to the risk of neurologic injury with nonoperative management [43, 44]. Risk factors for medical management failure included documented MRSA infection, neurologic impairment, CRP > 115 mg/L, WBC > 12,500, and ring-like enhancement on advanced imaging. Even in patients not displaying risk factors, failure of medical management was reported between 8.3 and 17% [45]. Shah et al. retrospectively analyzed 367 patients who were treated nonoperatively for SEA and found independent predictors for failed nonoperative treatment included a motor deficit, sensory changes, pathological or compression fracture in affected levels, malignancy, diabetes, and posteriorly located abscesses with failure rates as high as 75% in patients with these predictors [46•].
Decompression should be performed promptly to avoid the risk of irreversible neurologic deficits. While Ghobrial’s study of 87 patients with SEA failed to find a significant benefit to early decompression (< 24 h), they suggested early surgery appeared to offer a benefit to patients presenting with neurological deficit [47•]. Rigamonti et al. demonstrated the risk of delayed treatment, finding that poor outcomes (death, incontinence, paraplegia) occurred in 9 of 19 patients (47%) treated after 24 h compared to only 1 of 10 patients (10%) treated promptly [48]. Patel et al. retrospectively reviewed 128 consecutive cases of SEA with 60% undergoing surgery and the rest were treated nonoperatively [15]. Over 40% of those treated nonoperatively required surgery and had significantly less improvement in motor scores than those treated with early surgery. While nonoperative treatment in neurologically intact patients with epidural abscesses is a treatment option, the risk of failed nonoperative treatment at around 25% and the potential for catastrophic neurologic deficits with delayed treatment supports early surgical management [46•]. Additionally, the etiology of the SEA has been shown to affect the results of surgical management. Zimmerer and colleagues reported on 16 primary SEA and 20 secondary SEA (16 of which were from discectomies), 34 of which underwent surgical management. Interestingly, 100% of the primary SEA improved with a single debridement, but 100% of the secondary SEA from discectomies required multiple debridements [49].
Spinal Tuberculosis
Multidrug therapy has dramatically improved the rate of success for treating spinal tuberculosis. The mortality rate of TBVO approached 30–50% prior to the advent of multidrug therapy, which has since dramatically reduced to less than 1% with current therapy [5]. A prolonged course of multidrug therapy (isoniazid, rifampicin, pyrazinamide, ethambutol, streptomycin) should be undertaken in neurologically intact patients with preserved cord space. Close follow-up is required since prolonged chemotherapy and compliance is crucial to the successful management. While young, immunocompetent patients may be appropriate candidates for an abbreviated 6-month course of medical therapy, multidrug resistant strains may require up to 2 years of treatment [7]. While the addition of bracing may provide pain relief and prevent progression of spinal deformity, worsening kyphosis and sagittal imbalance is major long-term concern and requires close observation [18]. Percutaneous pedicle screw and rod constructs have been utilized as a less invasive method to prevent the progression of kyphosis with medical management but can occasionally require complex osteotomies for reconstruction. Yang and colleagues managed 34 patients with spinal TB patients by combining local and systemic chemotherapy with percutaneous pedicle screws in adjacent vertebra. They reported 27 patients with excellent outcomes, 7 with fair outcomes, no neurological complications, no loss of Cobb angle, and only one patient developed an abscess requiring anterior debridement with bone graft fusion [50].
Surgical Treatment
Indication for surgery that have been cited in the literature include neurological deficit, failure to respond to medical therapy, spinal cord compression greater than 50% even without neurologic symptoms, gross spinal instability, greater than 4 involved vertebrae, focal kyphosis of 60° or more, pan-columnar involvement, large paraspinal and epidural abscesses, and severe pain [11, 51]. Prompt treatment within 3 months of neurologic deficit has been associated with improved neurologic outcomes, while greater number of levels involved, lower AIS grade, bladder and bowel dysfunction, and prolonged neurologic symptoms are associated with worse outcomes [52, 53••]. Halo traction can also be utilized preoperatively to assist with surgical correction of kyphotic deformity [54]. While it is the authors’ preference that neurologic deficits in the setting of spinal cord compression should be managed surgically in order to expedite recovery and prevent permanent neurological deficits, some studies have reported neurological improvement with medical management alone. In a retrospective follow-up study, Jin-Tao and colleagues found complete resolution of neurologic deficits in 44% of patients treated operatively compared to 16.7% of patients treated nonoperatively at 6 months; however, neurologic recovery rates were nearly identical at 28 months (91.7% versus 94.4%, respectively) [55]. Surgical treatment within 2 weeks of starting chemotherapy can be performed safely and effectively, with patients showing improvements in ESR at long-term follow-up irrespective of their preoperative response to chemotherapy [56].
Surgical management has evolved over the last several decades, with circumferential approaches and instrumentation becoming more common. Chandra noted these recent treatment trends have led to decreasing paraplegia, with a 32% rate prior to 2004 and 11% from 2004 to 2011 [51]. Controversies regarding the surgical management of TBVO include surgical approach, timing of procedure, and deformity correction procedures. Thoracotomies can provide optimal anterior visualization for TBVO involving the thoracic spine, but they can also compromise pulmonary function. In patients with poor pulmonary function, extrapleural and transpedicular surgical approaches are potential alternatives to thoracotomies. Additionally, there is a growing body of evidence to support the safety and efficacy of posterior approaches for thoracolumbar TBVO decompression and fusion. Wang et al. compared anterolateral or posterior transforaminal approaches for surgical debridement and strut graft placement through anterolateral or transforaminal approaches followed by posterior instrumentation. The authors demonstrated better correction of kyphotic deformity with circumferential approaches for thoracolumbar lesions; however, significant morbidity was associated with circumferential approaches including increased operative times, estimated blood loss (EBL), complications, and hospital length of stays [56]. A meta-analysis comparing anterior versus posterior-based approaches for TBVO demonstrated greater Cobb angle correction with posterior approaches, but there were no differences in operative time, length of stay, loss of correction, or time to fusion [57]. Another meta-analysis found a similar efficacy for posterior approaches compared to combined posterior-anterior (PA) approaches but once again there was an increased operative time, EBL, hospital length of stay, and complications in the PA group [58••]. Wang and colleagues assessed the minimum 5-year outcomes of three approaches including anterior, posterior, and combined, recommending posterior approaches over the other two approaches due to similar time to fusion and VAS scores, but less operative time, blood loss, and lower complication rate [58••]. Also, historically psoas abscesses required anterior approaches, but recent studies have demonstrated successful management of spinal tuberculosis with paraspinal or psoas abscesses with single-stage posterior debridement with instrumentation [59].
Surgical management consists of debridement of all caseous, purulent, and granulation tissue, sequestered bone and bone that is compressing neural structures. Following debridement, cages packed with bone graft or structural grafts should be used to fill the voids [11]. The optimal choice of graft for anterior column reconstruction is controversial, with both iliac crest and titanium cages demonstrating similar outcomes and fusion rates. Wang et al. assessed the long-term outcomes of anterior radical debridement and reconstruction using titanium mesh cages for TBVO [60]. At 6 years, correction of kyphosis was maintained with no failures of instrumentation. Also, all patients improve in VAS back pain scores, neurological function, and all demonstrated a solid bony fusion with complete eradication of their infection. Similarly, Yin and colleagues compared 36 cases of posterior instrumentation for lumbosacral TB with anterior placement of either iliac crest (n = 19) or titanium mesh (n = 17), and reported similar improvements in functional outcomes, eradication of infection, fusion rates, and lower complication rates in the titanium mesh group [61].
Although the cervical spine is less frequently affected by TB, cervical spine disease carries significant morbidity with risks of significant motor deficits including quadriparesis and retropharyngeal disease with respiratory compromise. Similar to thoracolumbar TBVO, patients with significant kyphosis, neurologic deficit, and multilevel involvement should be considered for operative management. Although Bhandari only noted 4 of their 42 patients required surgical decompression and stabilization for cervical TBVO, they reported duration of illness > 3 months, major motor deficit, bladder involvement, flexor spasms, significant spinal cord compression, and spinal extension of the abscess as significantly associated with poor outcomes [62]. Decompression and reconstruction should be performed at the site of significant spinal cord compression, which is commonly anterior due to kyphotic collapse and direct abscess extension into the spinal canal. Subaxial anterior cervical decompression and fusion with instrumentation is a safe and effective option for kyphotic cervical TBVO, with He reporting 25 patients (22 with multilevel involvement) with ~ 20° improvement in kyphosis, 100% fusion rate and resolution of TBVO, no graft or instrumentation related complications, and improvement in neurologic status in 90% (18/20) [63]. Surgeons should consider the addition of posterior instrumentation and fusion to augment anterior multilevel corpectomies and correction of significant kyphosis. For the kyphotic cervical spine, Pan and colleagues demonstrated that improvements in C2-7 sagittal vertical axis was the most important factor for improvement in NDI scores [64].
A topic of concern in the surgical management of spinal TB is adjacent multisegment disease (Fig. 4). Due to the significant destruction of the anterior and middle vertebral column of contiguous segments, there is an increased risk for severe kyphosis and spinal cord compression, which necessitate surgical intervention. Li et al. reviewed four surgical techniques for the treatment of adjacent multisegment spinal TB in 48 patients and discussed their relative indications. Single-stage anterior debridement, bone grafting, and anterior instrumented fusion was indicated in patients with two vertebral levels or less of significant bony destruction. Longer anterior constructs would experience a much greater amount of stress and increased risk for hardware failure; therefore, they recommended the addition of posterior pedicle screw instrumentation in cases involving three or more levels. Another technique described was a single-stage posterior debridement, bone grafting, and instrumentation, which was reserved when no extensive abscess or spinal cord compression was present, significant bony destruction was isolated to a single level, and the patient was in poor health. The last technique reported was a CT-guided percutaneous drainage with a delayed posterior approach, which was indicated for patients who could not tolerate open surgery and had no vertebral collapse or spinal cord compression [65]. Overall, all 48 patients were cured at final follow-up with graft union in 47 patients and concluded that individualized surgical techniques should be used based on extent of spinal involvement, the patient’s health, and surgeon’s experience. Zhang and colleagues also demonstrated excellent results using transpedicular debridement with posterior instrumentation and fusion for thoracic and lumbar TBVO in 59 patients. All patients had complete resolution of their disease and improved by one ASIA grade or more, and over 98% had radiographic evidence of a solid bony fusion [66].
Severe kyphotic deformity (greater than 60 degrees) is functionally disabling and can result in late-onset paraplegia [11]. Due to the rigidity and significant deformity, severe late-onset kyphosis typically requires a three-column osteotomy, such as pedicle subtraction osteotomies (PSO) and vertebral column resection (VCR), to effectively correct the deformity. For thoracic kyphosis, the VCR is the preferred method to correct sagittal imbalance because it allows for anterior decompression of the acute kyphotic bend [67]. Although posterior VCR can provide upwards of 58° of correction in post-infectious kyphosis, it is technically demanding and there is significant morbidity associated with the procedure including an average EBL of close to 3 L and neurological complications such as spinal cord and root level injuries [68]. However, Liu and colleagues demonstrated the long-term efficacy and safety of VCR for kyphosis in 28 patients with cured spinal TB. After an average follow-up of 8 years, there were no mortalities; no severe complications related to instrumentation; significant improvement in kyphosis and sagittal balance of 40° and 13 mm respectively; nearly half the patients showed an improvement of greater than one ASIA grade; and significant improvement in disability, pain, and patient satisfaction scores [69].
Conclusion
Spine infections include a heterogenous group of diseases including discitis, VO, and SEA. PSI are most commonly caused by S. aureus while granulomatous infectious are commonly caused by M. tuberculosis. Spine infectious typically have an insidious onset with nonspecific findings, which underlines the importance of having a high level of suspicion, particularly in patients with risk factors such as immunosuppression, IVDU, and recent travel to endemic areas. MRI with and without contrast is the imaging study of choice, but diagnostic culture via CT-guided or open biopsy is paramount for optimal treatment. Most spine infections can be treated with nonoperative treatment with antibiotics and bracing. Surgery is indicated for neurologic deficits, spine instability, progressive deformity, and failed nonoperative treatment. Additionally, clinicians should take into account risk factors that predispose to failed nonoperative treatment. The optimal surgical treatment depends on the location, extent of the infection, and degree of spine instability. While surgical treatment options include anterior, posterior, and combined approaches performed as a single-stage or two-stage procedure, the selection of approach and procedure should be tailored to the patient’s pathology and clinical status.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87(11):1454–8. https://doi.org/10.1302/0301-620X.87B11.16294.
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–7. https://doi.org/10.1016/j.semarthrit.2008.03.002.
Graham SM, Fishlock A, Millner P, Sandoe J. The management gram-negative bacterial haematogenous vertebral osteomyelitis: a case series of diagnosis, treatment and therapeutic outcomes. Eur Spine J. 2013;22(8):1845–53. https://doi.org/10.1007/s00586-013-2750-4.
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. Executive Summary: 2015 Infectious Diseases Society of America (IDSA) Clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults: Table 1. Clin Infect Dis. 2015;61(6):859–63. https://doi.org/10.1093/cid/civ633.
Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22(12):2787–99. https://doi.org/10.1007/s00586-013-2850-1.
Wu M, Su J, Yan F, Cai L, Deng Z. Skipped multifocal extensive spinal tuberculosis involving the whole spine: a case report and literature review. Medicine. 2018;97(3):e9692. https://doi.org/10.1097/MD.0000000000009692.
Rajasekaran S, Khandelwal G. Drug therapy in spinal tuberculosis. Eur Spine J. 2012;22(S4):587–93. https://doi.org/10.1007/s00586-012-2337-5.
Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, Reguera JM, Palomino-Nicás J, Martos F, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56(12):709–15.
Jean M, Irisson J-O, Gras G, Bouchand F, Simo D, Duran C, et al. Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors. Scand J Rheumatol. 2017;46(1):64–8. https://doi.org/10.3109/03009742.2016.1158314.
Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285–91. https://doi.org/10.1016/j.jemermed.2003.11.013.
Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39–49. https://doi.org/10.1097/BLO.0b013e318065b7c3.
Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47–55.
Kapeller P, Fazekas F, Krametter D, Koch M, Roob G, Schmidt R, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol. 1997;38(2):94–8. https://doi.org/10.1159/000113167.
Yoon SH, Chung SK, Kim K-J, Kim H-J, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19(4):575–82. https://doi.org/10.1007/s00586-009-1216-1.
Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14(2):326–30. https://doi.org/10.1016/j.spinee.2013.10.046.
Ju KL, Kim SD, Melikian R, Bono CM, Harris MB. Predicting patients with concurrent noncontiguous spinal epidural abscess lesions. Spine J. 2015;15(1):95–101. https://doi.org/10.1016/j.spinee.2014.06.008.
Harada Y, Tokuda O, Matsunaga N. Magnetic resonance imaging characteristics of tuberculous spondylitis vs. pyogenic spondylitis. Clin Imaging. 2008;32(4):303–9. https://doi.org/10.1016/j.clinimag.2007.03.015.
Anley CM, Brandt AD, Dunn R. Magnetic resonance imaging findings in spinal tuberculosis: Comparison of HIV positive and negative patients. Indian J Orthop. 2012;46(2):186–90. https://doi.org/10.4103/0019-5413.93688.
Pupaibool J, Vasoo S, Erwin PJ, Murad MH, Berbari EF. The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J. 2015;15(1):122–31. https://doi.org/10.1016/j.spinee.2014.07.003.
Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218(1):211–4. https://doi.org/10.1148/radiology.218.1.r01ja06211.
Kim C-J, Kang S-J, Choe PG, Park WB, Jang H-C, Jung S-I, et al. Which tissues are best for microbiological diagnosis in patients with pyogenic vertebral osteomyelitis undergoing needle biopsy? Clin Microbiol Infect. 2015;21(10):931–5. https://doi.org/10.1016/j.cmi.2015.06.021.
Kim C-J, Song K-H, Park WB, Kim ES, Park SW, Kim H-B, et al. Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother. 2012;56(4):2122–4. https://doi.org/10.1128/AAC.05953-11.
Wang Y-C, Wong C-B, Wang I-C, Fu T-S, Chen L-H, Chen W-J. Exposure of prebiopsy antibiotics influence bacteriological diagnosis and clinical outcomes in patients with infectious spondylitis. Medicine. 2016;95(15):e3343. https://doi.org/10.1097/MD.0000000000003343.
de Lucas EM, González Mandly A, Gutiérrez A, Pellón R, Martín-Cuesta L, Izquierdo J, et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28(3):315–20. https://doi.org/10.1007/s10067-008-1051-5.
Dietze DD, Fessler RG, Patrick Jacob R. Primary reconstruction for spinal infections. Neurosurg Focus. 1997;2(4):E2. https://doi.org/10.3171/foc.1997.2.4.2.
Madhavan K, Vanni S, Williams SK. Direct lateral retroperitoneal approach for the surgical treatment of lumbar discitis and osteomyelitis. Neurosurg Focus. 2014;37(2):E5. https://doi.org/10.3171/2014.6.FOCUS14150.
Turel MK, Kerolus M, Deutsch H. The role of minimally invasive spine surgery in the management of pyogenic spinal discitis. J Craniovertebr Junction Spine. 2017;8(1):39–43. https://doi.org/10.4103/0974-8237.199873.
Blizzard DJ, Hills CP, Isaacs RE, Brown CR. Extreme lateral interbody fusion with posterior instrumentation for spondylodiscitis. J Clin Neurosci. 2015;22(11):1758–61. https://doi.org/10.1016/j.jocn.2015.05.021.
Ghobrial GM, Franco D, Theofanis T, Margiotta PJ, Andrews E, Wilson JR, et al. Cervical Spondylodiscitis: Presentation, Timing, and Surgical Management in 59 Patients. World Neurosurg. 2017;103:664–70. https://doi.org/10.1016/j.wneu.2017.04.119.
Ghobrial GM, Viereck MJ, Margiotta PJ, Beygi S, Maulucci CM, Heller JE, et al. Surgical management in 40 consecutive patients with cervical spinal epidural abscesses: shifting toward circumferential treatment. Spine. 2015;40(17):E949–53. https://doi.org/10.1097/BRS.0000000000000942.
Shousha M, Heyde C, Boehm H. Cervical spondylodiscitis: change in clinical picture and operative management during the last two decades. A series of 50 patients and review of literature. Eur Spine J. 2015;24(3):571–6. https://doi.org/10.1007/s00586-014-3672-5.
Bydon M, De la Garza-Ramos R, Macki M, Naumann M, Sciubba DM, Wolinsky J-P, et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 2014;82(6):e807–14. https://doi.org/10.1016/j.wneu.2014.06.014.
• Carragee E, Iezza A. Does Acute placement of instrumentation in the treatment of vertebral osteomyelitis predispose to recurrent infection: long-term follow-up in immune-suppressed patients. Spine. 2008;33(19):2089–93. https://doi.org/10.1097/BRS.0b013e3181839b9c 32 consecutive immune compromised patients with pyogenic vertebral osteomyelitis who underwent single-stage debridement and acute placement of spinal instrumentation were prospectively observed for reoccurrence of infection for up to 10 years. A total of 22 patients had full follow-up without reoccurrence of infection. Only one patient had a reoccurrence that was successfully treated with debridement and retention of instrumentation. Four patients underwent removal of instrumentation due to suspected nonunion or infection but none had confirmed histological evidence of infection. Single-stage debridement and spinal instrumentation for PVO has a low risk of long-term recurrence.
Kim HW, Ryu J-I, Bak KH. The safety and efficacy of cadaveric allografts and titanium cage as a fusion substitutes in pyogenic osteomyelitis. J Korean Neurosurg Soc. 2011;50(4):348–56. https://doi.org/10.3340/jkns.2011.50.4.348.
Lu DC, Wang V, Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery. 2009;64(1):122–30. https://doi.org/10.1227/01.neu.0000336332.11957.0b.
Robinson Y, Tschoeke SK, Kayser R, Boehm H, Heyde CE. Reconstruction of large defects in vertebral osteomyelitis with expandable titanium cages. Int Orthop. 2009;33(3):745–9. https://doi.org/10.1007/s00264-008-0567-2.
Kuklo TR, Potter BK, Bell RS, Moquin RR, Rosner MK. Single-stage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. 2006;19(5):376–82. https://doi.org/10.1097/01.bsd.0000203945.03922.f6.
Sundararaj GD, Babu N, Amritanand R, Venkatesh K, Nithyananth M, Cherian VM, et al. Treatment of haematogenous pyogenic vertebral osteomyelitis by single-stage anterior debridement, grafting of the defect and posterior instrumentation. J Bone Joint Surg (Br). 2007;89(9):1201–5. https://doi.org/10.1302/0301-620X.89B9.18776.
Shetty AP, Aiyer SN, Kanna RM, Maheswaran A, Rajasekaran S. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. Int Orthop. 2016;40(6):1163–70. https://doi.org/10.1007/s00264-015-3063-5.
Wang X, Tao H, Zhu Y, Lu X, Hu X. Management of postoperative spondylodiscitis with and without internal fixation. Turk Neurosurg. 2015;25(4):513–8. https://doi.org/10.5137/1019-5149.JTN.9008-13.1.
Santhanam R, Lakshmi K. A Retrospective Analysis of the Management of Postoperative Discitis: A Single Institutional Experience. Asian Spine J. 2015;9(4):559–64. https://doi.org/10.4184/asj.2015.9.4.559.
Kucuk A, Karademir M, Tumturk A, Ulutabanca H, Ercal BD, Senol S, et al. Surgical strategies for spondylodiscitis due to lumbar disc surgery. Turk Neurosurg. 2017;27(1):95–8. https://doi.org/10.5137/1019-5149.JTN.14234-15.1.
Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim B-S. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. 2014;37(2):E4. https://doi.org/10.3171/2014.6.FOCUS14127.
Alton TB, Patel AR, Bransford RJ, Bellabarba C, Lee MJ, Chapman JR. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015;15(1):10–7. https://doi.org/10.1016/j.spinee.2014.06.010.
Tuchman A, Pham M, Hsieh PC. The indications and timing for operative management of spinal epidural abscess: literature review and treatment algorithm. Neurosurg Focus. 2014;37(2):E8. https://doi.org/10.3171/2014.6.FOCUS14261.
• Shah AA, Ogink PT, Nelson SB, Harris MB, Schwab JH. Nonoperative management of spinal epidural abscess: development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546–55. https://doi.org/10.2106/JBJS.17.00629 A total of 367 patients who underwent nonoperative treatment for spinal epidural abscess were retrospectively reviewed for risk factors of failed medical management. Six independent predictors of failed medical management were identified: motor deficit at presentation, pathological or compression fractures, active malignancy, diabetes mellitus, sensory changes, and dorsal location of abscess. The nomogram created can be used as a tool to weigh the risks and benefits of nonoperative and operative management.
• Ghobrial GM, Beygi S, Viereck MJ, Maulucci CM, Sharan A, Heller J, et al. Timing in the surgical evacuation of spinal epidural abscesses. Neurosurg Focus. 2014;37(2):E1. https://doi.org/10.3171/2014.6.FOCUS14120 Retrospective review of 62 consecutive cases of cervical spine epidural abscess with the ASIA motor score (0-100) as the primary outcome measure. Early surgery (average time to OR was 24 h) for cervical spine epidural abscess resulted in improved motor scores, while delayed surgery (average time to OR was 7 days) due to failed medical therapy resulted in worse motor scores. Early surgical decompression of cervical spinal epidural abscess is recommended.
Rigamonti D, Liem L, Sampath P, Knoller N, Namaguchi Y, Schreibman DL, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52(2):189–96 discussion 197.
Zimmerer SME, Conen A, Müller AA, Sailer M, Taub E, Flückiger U, et al. Spinal epidural abscess: aetiology, predisponent factors and clinical outcomes in a 4-year prospective study. Eur Spine J. 2011;20(12):2228–34. https://doi.org/10.1007/s00586-011-1838-y.
Yang H, Song F, Zhang L, Li N, Zhang X, Wang Y. Management of spine tuberculosis with chemotherapy and percutaneous pedicle screws in adjacent vertebrae: a retrospective study of 34 cases. Spine. 2016;41(23):E1415–20. https://doi.org/10.1097/BRS.0000000000001858.
Chandra SP, Singh A, Goyal N, Laythalling RK, Singh M, Kale SS, et al. Analysis of changing paradigms of management in 179 patients with spinal tuberculosis over a 12-year period and proposal of a new management algorithm. World Neurosurg. 2013;80(1-2):190–203. https://doi.org/10.1016/j.wneu.2012.12.019.
Sharma A, Chhabra HS, Chabra T, Mahajan R, Batra S, Sangondimath G. Demographics of tuberculosis of spine and factors affecting neurological improvement in patients suffering from tuberculosis of spine: a retrospective analysis of 312 cases. Spinal Cord. 2017;55(1):59–63. https://doi.org/10.1038/sc.2016.85.
•• Yao Y, Zhang H, Liu M, Liu H, Chu T, Tang Y, et al. Prognostic factors for recovery of patients after surgery for thoracic spinal tuberculosis. World Neurosurg. 2017;105:327–31. https://doi.org/10.1016/j.wneu.2017.05.167 Retrospective review of 237 patients with thoracic spine tuberculosis used the Japanese Orthopedic Association score to assess post-operative recovery. Shorter duration of symptoms (3 months or less), fewer vertebrae involved (2 or less), and the lack of paralysis upon presentation were predictors of positive outcomes following surgery.
Muheremu A, Ma Y, Ma Y, Ma J, Cheng J, Xie J. Halo-pelvic traction for severe kyphotic deformity secondary to spinal tuberculosis. Medicine. 2017;96(28):e7491. https://doi.org/10.1097/MD.0000000000007491.
Jin-tao Q, Yu-quan J, Guo-hua X, Yu T, Zi-tian W, Xiao-jian Y, et al. Clinical characteristics and neurologic recovery of patients with cervical spinal tuberculosis: should conservative treatment be preferred? A retrospective follow-up study of 115 cases. World Neurosurg. 2015;83(5):700–7. https://doi.org/10.1016/j.wneu.2015.01.015.
Zhang P, Shen Y, Ding W-Y, Zhang W, Shang Z. The role of surgical timing in the treatment of thoracic and lumbar spinal tuberculosis. Arch Orthop Trauma Surg. 2014;134(2):167–72. https://doi.org/10.1007/s00402-013-1904-5.
Muheremu A, Niu X, Wu Z, Tian W. Study on anterior and posterior approaches for spinal tuberculosis: a meta-analysis. Eur J Orthop Surg Traumatol. 2015;25(Suppl 1):S69–76. https://doi.org/10.1007/s00590-014-1508-y.
•• Liu J, Wan L, Long X, Huang S, Dai M, Liu Z. Efficacy and Safety of Posterior Versus Combined Posterior and Anterior Approach for the Treatment of Spinal Tuberculosis: A Meta-Analysis. World Neurosurg. 2015;83(6):1157–65. https://doi.org/10.1016/j.wneu.2015.01.041 Meta-analysis of five controlled clinical trials involving 253 patients with spinal TB were analyzed. Posterior only approach was associated with decreased operative time, blood loss, length of stay, and rate of complications. However, there were no difference in correction of angle, loss of correction at final follow-up, time to fusion, and overall neurological improvement. The posterior-only approach has a similar efficacy and better safety profile than a combined-surgical approach for the treatment of spinal TB.
Li J, Li X-L, Zhou X-G, Zhou J, Dong J. Surgical treatment for spinal tuberculosis with bilateral paraspinal abscess or bilateral psoas abscess. J Spinal Disord Tech. 2014;27(8):E309–14. https://doi.org/10.1097/bsd.0000000000000120.
Wang B, Lv G, Liu W, Cheng I. Anterior radical debridement and reconstruction using titanium mesh cage for the surgical treatment of thoracic and thoracolumbar spinal tuberculosis: minimum five-year follow-up. Turk Neurosurg. 2011;21(4):575–81. https://doi.org/10.5137/1019-5149.JTN.4639-11.1.
Yin XH, Liu ZK, He BR, Hao DJ. Single posterior surgical management for lumbosacral tuberculosis: titanium mesh versus iliac bone graft: a retrospective case-control study. Medicine. 2017;96(51):e9449. https://doi.org/10.1097/MD.0000000000009449.
Bhandari A, Garg RK, Malhotra HS, Verma R, Singh MK, Jain A, et al. Outcome assessment in conservatively managed patients with cervical spine tuberculosis. Spinal Cord. 2014;52(6):489–93. https://doi.org/10.1038/sc.2014.49.
He M, Xu H, Zhao J, Wang Z. Anterior debridement, decompression, bone grafting, and instrumentation for lower cervical spine tuberculosis. Spine J. 2014;14(4):619–27. https://doi.org/10.1016/j.spinee.2013.06.076.
Pan Z, Luo J, Yu L, Chen Y, Zhong J, Li Z, et al. Débridement and reconstruction improve postoperative sagittal alignment in kyphotic cervical spinal tuberculosis. Clin Orthop Relat Res. 2017;475(8):2084–91. https://doi.org/10.1007/s11999-017-5306-9.
Li L, Xu J, Ma Y, Tang D, Chen Y, Luo F, et al. Surgical strategy and management outcomes for adjacent multisegmental spinal tuberculosis: a retrospective study of forty-eight patients. Spine. 2014;39(1):E40–8. https://doi.org/10.1097/BRS.0000000000000053.
Zhang P, Peng W, Wang X, Luo C, Xu Z, Zeng H, et al. Minimum 5-year follow-up outcomes for single-stage transpedicular debridement, posterior instrumentation and fusion in the management of thoracic and thoracolumbar spinal tuberculosis in adults. Br J Neurosurg. 2016;30(6):666–71. https://doi.org/10.1080/02688697.2016.1206182.
Boachie-Adjei O, Papadopoulos EC, Pellisé F, Cunningham ME, Perez-Grueso FS, Gupta M, et al. Late treatment of tuberculosis-associated kyphosis: literature review and experience from a SRS-GOP site. Eur Spine J. 2013;22(Suppl 4):641–6. https://doi.org/10.1007/s00586-012-2338-4.
Suk S-I, Kim J-H, Kim W-J, Lee S-M, Chung E-R, Nah K-H. Posterior vertebral column resection for severe spinal deformities. Spine. 2002;27(21):2374–82. https://doi.org/10.1097/00007632-200211010-00012.
Liu C, Lin L, Wang W, Lv G, Deng Y. Long-term outcomes of vertebral column resection for kyphosis in patients with cured spinal tuberculosis: average 8-year follow-up. J Neurosurg Spine. 2016;24(5):777–85. https://doi.org/10.3171/2015.8.SPINE15534.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Boody reports personal fees from Innovative Surgical Designs, outside the submitted work. The other authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Modern Management of TB and Other Chronic Infections
Rights and permissions
About this article
Cite this article
Boody, B.S., Tarazona, D.A. & Vaccaro, A.R. Evaluation and Management of Pyogenic and Tubercular Spine Infections. Curr Rev Musculoskelet Med 11, 643–652 (2018). https://doi.org/10.1007/s12178-018-9523-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-018-9523-y