Abstract
Purpose
Our aim was to study the safety and outcomes of posterior instrumentation and transforaminal lumbar interbody fusion (TLIF) for treating pyogenic lumbar spondylodiscitis.
Methods
Retrospective analysis was performed on prospectively collected data of 27 consecutive cases of lumbar pyogenic spondylodiscitis treated with posterior instrumentation and TLIF between January 2009 and December 2012. Cases were analysed for safety, radiological and clinical outcomes of transforaminal interbody fusion using bone graft ± titanium cages. Interbody metallic cages with bone graft were used in 17 cases and ten cases used only bone graft. Indications for surgical treatment were failed conservative management in 17, neurodeficit in six and significant bony destruction in four.
Results
There were no cases reporting cage migration, loosening, pseudoarthrosis or recurrence of infection at a mean follow-up of 30 months. Clinical outcomes were assessed using Kirkaldy–Willis criteria, which showed 14 excellent, nine good, three fair and one poor result. Mean focal deformity improved with the use of bone graft ± interbody cages, and the deformity correction was maintained at final follow-up. Mean pre-operative focal lordosis for the graft group was 8.5° (2–16.5°), which improved to 10.9 °(3.3–16°); mean pre-operative focal lordosis in the group treated with cages was 6.7 °(0–15°), which improved to 7°(0–15°) .
Conclusion
TLIFs with cages in patients with pyogenic lumbar spondylodiscitis allows for acceptable clearance of infection, satisfactory deformity correction with low incidence of cage migration, loosening and infection recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyogenic vertebral osteomyelitis is an uncommon disorder with a reported incidence between 2–7 % [1–3]. The source of spread of such infections is often haematogenous; however, direct spread may occur during spine surgery or an interventional procedure, such as epidural injections, nerve-root blocks or discography [2–6]. Primary treatment consists of systemic antibiotics, bracing and rest [2, 4, 7, 8]. Surgery is warranted in certain clinical scenarios, such as cases refractory to conservative treatment, presence of neurological deficit, epidural abscess and progressive bony destruction with deformity or instability [2–4, 7–9]. Spondylodiscitis being an anterior lesion has traditionally been treated using anterior debridement with or without supplemented posterior stabilisation [3, 4, 8–10]. However, there has been a recent surge in the literature showing satisfactory results in spondylodiscitis management with a single-stage posterior approach, especially with spinal tuberculosis [11–13]. Metallic implants have been safely used in tuberculosis spondylodiscitis due to poor biofilm formation; however, metallic implants in the setting of pyogenic infections cause concern due to the the risk of biofilm formation and recurrences [14–16]. There are few reports documenting posterior lumbar interbody fusion with instrumentation and its feasibility for treating pyogenic lumbar spondylodiscitis. [1, 9]. We present results of treating pyogenic lumbar spondylodiscitis with a single-stage posterior approach using pedicular screw instrumentation and transforaminal lumbar interbody fusion (TLIF). We assessed the use of posterior instrumentation and metallic interbody spacers with particular focus on safety and outcomes of this technique for treating pyogenic lumbar spondylodiscitis.
Materials and methods
We retrospectively reviewed data collected prospectively from 27 consecutive cases of pyogenic lumbar spondylodiscitis treated using a posterior approach with pedicular screw instrumentation and TLIF between January 2009 and December 2012. All cases had proven spondylodiscitis based on histopathological findings, and 71 cases were treated. The study was approved by the institutional review board, all ethical standards as per the Declaration of Helsinki were followed and informed consent was obtained from all participants. Histopathology confirmed tuberculous aetiology in 29 cases, which were excluded from the study. There were 42 cases of proven pyogenic lumbar spondylodiscitis based on histopathological confirmation. Fifteen patients who did not document significant vertebral body destruction underwent conservative treatment with antibiotics, rest and bracing. The study was conducted on the 27 consecutive cases that underwent transforaminal interbody fusion for lumbar pyogenic spondylodiscitis. Indications for selection into this group were failure of conservative management, significant bony destruction with resulting deformity or instability and neurological deficit. The medical records were assessed for clinical presentation, medical comorbidities, indication for surgery, culture positivity, isolated organism, antibiotic duration, previous surgical procedures and clinical outcome. Baseline erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and total and differential counts prior to surgery and at each follow-up visit were documented.
Radiological parameters for X-rays and magnetic resonance imaging (MRI) were assessed using VEPRO PACS software. Pre-operative MRI scans were assessed to document lesion extent and ascertain the presence of any epidural abscess formation. Pre-operative X-rays were assessed for bony destruction, instability and deformity. These were compared with post-operative X-rays to assess radiological outcome. Follow-up radiographs at six weeks, three months, six months, 12 months and yearly thereafter were analysed from the archives. Final radiological fusion was assessed using plain radiographs by criteria suggested by Lee et. al. (Table 1) [9]. Twenty patients had repeat MRI to document lesion healing. Neurological deficits were assessed using Frankel grading [17]. Clinical outcomes were assessed using Kirkaldy–Willis criteria, defined as:
(a) Excellent. Patient returned to normal work and other activities with little or no complaint
(b) Good. Patient returned to normal work but may have some restriction in other activities and may—on occasion after heavy work—have recurrent back pain requiring a few days’ rest
(c) Fair. Patient work capacity was reduced, necessitating a lighter job or working part time, and may occasionally have pain recurrence requiring absence from work for one or two weeks once or twice a year
(d) Poor. Patient does not return to work [18].
Surgical procedure
All cases were treated with a posterior-only surgery, with no anterior procedure being performed. Fixation was done using pedicular screw instrumentation spanning the lesion. Pedicular screws were used in the involved vertebral body level. Instrumentation was extended cranially and caudally if significant vertebral body destruction precluded short-segment fixation spanning just the index level. Debridement of the spondylodiscitis lesion with fusion was performed using a transforaminal lumbar interbody approach. Debridement was carried out to the opposite side using curettes and rongeurs of a standard TLIF preparation set. End-plate preparation was done with curettes, with meticulous care being taken to avoid breaching the anterior longitudinal ligament. In the setting of a pyogenic infection, the necrotic disc would often separate at the subchondral junction and could be extracted en mass using a transforaminal approach. This would signal debridement adequacy of the infective focus. In cases with epidural-space abscess collection, a posterior decompression was combined with the fusion procedure. Postdebridement interbody fusion was performed with bone graft harvested from the iliac crest. We performed interbody fusion using interbody metallic titanium box cages in conjunction with bone graft in 17/27 cases; in ten cases, iliac graft only was used. Intra-operative debridement samples were sent for cultures and histopathological examination; four patients underwent staged procedures. Cases with large abscess collection and extension to the posterior paraspinal musculature—beyond confines of the epidural space—were selected as candidates requiring staged debridement. First-stage surgery entailed posterior debridement of infective tissue outside the neural canal, and posterior decompression was performed to drain the epidural component of the infective focus. A delayed definitive TLIF was performed as a second-stage procedure over a seven to ten day period.
We performed TLIF with iliac crest bone grafting for initial cases. With satisfactory outcomes noted for these cases, we subsequently used interbody metallic cages as spacers in conjunction with iliac-crest grafts for interbody fusion.
Results
Mean patient age was 48 (22–83) years. There were 19 men and eight women, and mean follow-up was 30 (22—60) months. Patient demographics, microbiological isolates, neurological presentation, medical comorbidities and clinical outcomes are illustrated in Table 2.
Back pain was the most common presenting feature. Surgical indication in 17 (62.9 %) patients was pain refractory to conservative management, six had neurological deficit and four had significant bony destruction requiring surgical stabilisation. Fifteen patients presented with primary pyogenic spondylodiscitis; 12 cases presented with pyogenic spondylodiscitis following surgical intervention in the preceding 12–16 weeks and 11 with spondylodiscitis following lumbar discectomy surgery. One patient developed spondylodiscitis following lumbar decompression for lumbar canal stenosis; two cases with primary spondylodiscitis following previously failed anterior debridement and persistent infection underwent TLIF.
The common organisms isolated were Escherichia coli (three cases), Pseudomonas aeruginosa (three cases) and Staphylococcus aureus (two cases). Mean ESR at the time of diagnosis was 65 (20–115) mm and mean CRP 33 mg/dl (6.9–131). All patients empirically received post-operative ofloxacin and cefoperazone + sulbactam IV until antibiotic sensitivity parameters were available. Patients in whom no organism could be isolated were continued on the same antibiotics for four weeks and then antibiotics orally for a further four weeks. Serial CRP and ESR and clinical improvement were used as guides for antibiotic therapy. Total antibiotic duration was eight weeks.
As per the Kirkaldy–Willis criteria, 14 patients showed excellent results, nine good, three fair and one poor. The five patients with Frankel grade D recovered completely and one with grade C improved to grade D at final follow-up.
Complications
One case of L4-5 spondylodiscitis presented with multiple medical comorbidities, including diabetes mellitus, medical renal disease, hypertension and prolonged steroid intake. The patient underwent TLIF with iliac crest bone grafting. Cultures isolated E. coli, and appropriate antibiotics were initiated; however, the patient failed to respond, developing septicemia and multiorgan dysfunction resulting in death on the third post-operative day.
Radiological outcomes
The patient who died peri-operative was excluded from radiological evaluation. Of the remaining 26 cases evaluated for radiological outcomes, there was evidence of bony end-plate destruction in 15; four had substantial bony destruction resulting in instability. Focal segmental deformity was measured on pre-operative radiographs. Mean pre-operative focal lordosis for those treated with grafts alone (N = 7) was 8.5° (2–16.5°); mean post-operative focal lordosis was 10.9° (3.3–16°) in this group (Table 3). Mean pre-operative focal lordosis in those treated with cages (N = 14) was 6.7° (0–15°) and their mean post-operative focal lordosis was 7° (0–15°) (Table 3). Five patients with segmental kyphosis were assigned a negative prefix; mean kyphosis in these patients was −8.5° (−2 to −14.8°), three of whom were treated with cages and two with iliac crest bone graft for interbody fusion procedures, and their mean post-operative focal kyphosis improved to −1.3° (7.8 to −14.2°) (Table 3). Deformity correction appeared to be maintained at final follow-up; however, statistical significance could be reached for deformity correction in either group. Bridging bone across the fusion site was noted on plain radiographs in 16 patients, showing features of definitive fusion (Figs. 1 and 2). There were ten patients with probable fusion, and no patient had suspected pseudoarthrosis. There were no cases with cage migration or loosening at final follow-up, and 20 patients who underwent follow-up MRI showed satisfactory healing. Financial constraints limited the use of follow-up MRI in all patients.
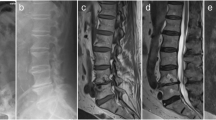
A case of L1-2 spondylodiscitis in a 22-year-old man: a Pre-operative lateral thoracolumbar spine X-ray showing end-plate irregularity with collapsed disc space. b Pre-operative magnetic resonance image (MRI) T2-weighted sagittal section showing hyperintense signal changes in the disc space with marrow signal intensity changes suggestive spondylodiscitis. c Immediate post-operative X-ray following transforaminal lumbar interbody fusion (TLIF) with bone graft. d Final follow-up X-ray at 30 months showing definitive fusion with bridging bone across the disc space. e Computed tomography (CT) scan showing bridging bone across fusion site
A case of L4-5 spondylodiscitis: a Pre-operative magnetic resonance image (MRI) showing collapse of the disc space and retrolisthesis with features of spondylodiscitis. b X-ray showing significant bony destruction. c Final follow-up X-ray showing interbody cage with fusion at 28 months. d Follow-up MRI with resolution of marrow signal changes and satisfactory healing
Discussion
Treatment options for pyogenic lumbar spondylodiscitis include conservative treatment with antibiotics, minimally invasive stabilisation procedures and open surgical debridement and stabilisation using anterior, posterior or combined approaches [1, 9, 10, 19, 20]. Bettini et al., in a retrospective analysis of 56 patients, observed that conservative treatment was satisfactory in treating 87 % of patients, with only 12 % requiring surgical intervention. They advised close monitoring of blood markers, prolonged administration of antibiotics and drug compliance as key factors determining outcome [21]. Zarghooni et al. recommended conservative treatment in patients with minimal bony destruction and a poor general condition [8]. The antibiotic treatment may be based upon blood cultures and computed tomography (CT)-guided tissue culture reports. Antibiotics given empirically to cover the most common organism, including S. aureus and E.coli, are appropriate while culture resultsare awaited [8]. Those authors suggested surgical treatment for patients presenting with neurological deficit, sepsis, significant bony destruction with instability or deformity and in whom conservative treatment failed. In our series, there were 12 cases with postoperative spondylodiscitis and history of previous antibiotic use. In only one of the 12 cases could an organism be isolated, suggesting that widespread antibiotic use may reduce culture positivity.
Spondylodiscitis affects anterior structures of the spinal column and have traditionally been addressed with an anterior approach [3, 4, 8–10]. Anterior debridement is followed by anterior bone grafting and prolonged periods of bed rest and immobilisation until graft consolidation is achieved [9]. Achieving early stability often requires supplementation with posterior instrumented stabilisation. The anterior approach to the lumbar spine also carries the risk of injury to the gastrointestinal tract, genitourinary system, great vessels and hypogastric plexus during lesion debridement.
Many authors have reported satisfactory treatment outcomes of tuberculous spondylodiscitis using a posterior approach [11–13]. Tubercle bacilli have reduced propensity to form biofilm, and thus risk of recurrences with titanium implants is low [11, 14–16]. A posterior approach allows for a single-stage surgical procedure, offering satisfactory lesion debridement and simultaneous placement of instrumentation to provide stability and deformity correction, which is well maintained [11–13]. Such satisfactory results for treating tuberculosis may encourage the use of similar techniques for surgical treatment of pyogenic spondylodiscitis.
Posterior surgery for pyogenic spondylodiscitis with thorough debridement of infective tissue under cover of appropriate antibiotics and coupled with high vascularity of cancellous bone in the vertebral body allows for placement of metal implants, including interbody spacer cages, with reduced chance of infection recurrence and biofilm formation [1, 9]. We believe through debridement of infected disc space is paramount. Quite often in pyogenic spondylodiscitis the infected disc tissue separates from the subchondral bone and can be extracted en mass as a large necrotic fragment, signalling adequacy of the debridement (Fig. 3). Once debridement is complete, metallic cages with bone graft can be used for anterior reconstruction. Pre-operative CT scan in such cases gives an accurate evaluation of bony destruction, which may be underestimated on plain radiographs, as illustrated in Fig. 4. We recommend staged surgery, especially when the infection has extended beyond the posterior elements and into the plane of the paraspinal musculature. In such clinical scenarios, staged debridement and instrumentation are safe and valuable treatment options.
Few studies report having used posterior instrumented stabilisation for treating pyogenic spondylodiscitis. Lee and Suh showed satisfactory results with the use of posterior lumbar interbody fusion using autologous bone graft in 18 patients [9]. An et. al. achieved encouraging results in 15 patients using posterior lumbar interbody fusion and allograft in pyogenic spondylodiscitis and concluded that such a procedure is safe and morbidity associated with autograft harvesting can be avoided [1]. In a retrospective analysis of 48 patients treated with long-segmented fixation with short apical fusion without debridement, Lin and colleagues showed good short-term pain relief and long-term clinical outcome [10]. None of these series, however, used titanium cages for interbody fusion procedures. Sundararaj et al. concluded that titanium cages may be safely used in place of iliac crest graft for treating active spinal infection. Their series included cases of tuberculosis as well as pyogenic spondylodiscitis [14]. Brase et al., in their review of nine prospective cases of purulent spondylodiscitis including cervical and lumbar infections, showed satisfactory clearance of infection with the use of polyetheretherketone (PEEK) cages [22]. Schomacher et al., in a comparative study between the use of titanium and PEEK cages for pyogenic spondylodiscitis, noted satisfactory radiological outcomes with both types and no significant difference in reinfection rates [23]. Though literature reporting the use of PEEK implants in infection is sparse, there is literature supporting use of titanium implants in infections, as it has poor propensity for biofilm formation. Satisfactory results have been documented with the use of mesh cages for treating pyogenic spondylodiscitis; however, those series include patients treated with combined anterior and posterior surgical approaches [15, 16]. It has been noted that stable fitting implants, even in the clinical scenario of infections, may be retained to allow for fusion [24].
Recently, reports on the use of percutaneously placed pedicular screws as a minimally invasive option have been published [25, 26]. However, both those studies used combined surgical procedures with additional anterior debridement in conjunction with percutaneous pedicular screw fixation. Madhavan et al. reported on a direct lateral approach to debride the discitis lesion; they recommended the addition of posterior pedicular screw instrumentation to prevent development of kyphotic deformity [27].
Here, we present the results of a single posterior approach for treating pyogenic lumbar spondylodiscitis with TLIF using metallic interbody spacers. There is only one previous series documenting use of TLIF for lumbar spondylodiscitis: Sheha reported on nine cases of immediate postoperative spondylodiscitis following discectomy surgery treated with TLIF and a short-term follow up of 12 months [28]. Interbody metallic spacer was not used in any of these cases. The use of metallic implants in spinal pyogenic infections has been much debated; however, our series showed satisfactory results with the use of metallic implants and titanium interbody cages in pyogenic spondylodiscitis.
Limitations
This is a case series with a small sample size and varied aetiology, including postoperative and primary pyogenic spondylodiscitis. Larger randomised control trials are needed to analyse the outcomes of TLIF in the setting of lumbar pyogenic spondylodiscitis.
Conclusion
Our results suggest that a posterior approach to surgery for pyogenic spondylodiscitis provides adequate clearance of infected tissue and gives satisfactory functional outcome. Radiological outcomes, including deformity correction, correction maintenance and final fusion, are satisfactory with this approach, thus avoiding the need for anterior surgery. There were no instances of cage migration, cage loosening or infection recurrence over a minimum 2-year follow-up, and these are major concerns limiting the use of metallic interbody spacers in pyogenic spondylodiscitis. We suggest TLIF with titanium cages may be safely used for treating pyogenic lumbar spondylodiscitis provided thorough and adequate debridement of infected tissue can be achieved, appropriate antibiotic coverage is instituted and close follow-up of clinical progress is maintained.
References
An KC, Kim JY, Kim TH, Kim JS, Park DH, Kim JG, Sung TW (2012) Posterior lumbar interbody fusion using compressive bone graft with allograft and autograft in the pyogenic discitis. Asian, Spine J 6(1):15–21
Nasto LA, Colangelo D, Mazzotta V, Di Meco E, Neri V, Nasto RA, Pola E (2013) Is posterior percutaneous screw-rod instrumentation a safe and effective alternative approach to TLSO rigid bracing for single-level pyogenic spondylodiscitis? Results of a retrospective cohort analysis. Spine J 14(7):1139–1146
Cheung WY, Luk KD (2012) Pyogenic spondylitis. Int Orthop 36(2):397–404
Kapsalaki E, Gatselis N, Stefos A, Makaritsis K, Vassiou A, Fezoulidis I, Dalekos GN (2009) Spontaneous spondylodiscitis: presentation, risk factors, diagnosis, management, and outcome. Int J Infect Dis 13(5):564–569
Adam D, Papacocea T, Hornea I, Croitoru R (2014) Postoperative spondylodiscitis. A review of 24 consecutive patients. Chirurgia 109:90–94
Moon M S, Kim S S, Lee B J, Moon J L, Sihn J C, & Moon S I (2012). Pyogenic discitis following discectomy. J Orthop Surg 20(1)
Guerado E, Cerván AM (2012) Surgical treatment of spondylodiscitis. An update. Int Orthop 36(2):413–420
Zarghooni K, Röllinghoff M, Sobottke R, Eysel P (2012) Treatment of spondylodiscitis. Int Orthop 36(2):405–411
Lee JS, Suh KT (2006) Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Joint Surg Bri Vol 88(6):765–770
Lin CP, Ma HL, Wang ST, Liu CL, Yu WK, Chang MC (2012) Surgical results of long posterior fixation with short fusion for treating pyogenic spondylodiscitis of the thoracic and lumbar spine: a retrospective study. Spine 37(25):E1572–E1579
Jain AK, Aggarwal A, Dhammi IK, Aggarwal PK, Singh S (2004) Extrapleural anterolateral decompression in tuberculosis of the dorsal spine. J Bone Joint Surg (Br) 86-B:1027–1031
Moon MS, Woo YK, Lee KS et al (1995) Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine 20:1910–1916
Garg B, Kandwal P, Upendra BN, Goswami A, Jayswal A (2012) Anterior versus posterior procedure for surgical treatment of Thoracolumbar tuberculosis: a retrospective analysis. Ind J Orthop 46:165–170
Sundararaj GD, Amritanand R, Venkatesh K, Arockiaraj J (2011) The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: can we rest the crest? Asian Spine J 5(3):155–161
Hee HT, Majd ME, Holt RT, Pienkowski D (2002) Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech 15:149–156
Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W (2003) Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J 12:606–612
Frankel HL, Hancock DO, Hyslop G et al (1969) The value of postural reduction in the initial management of the closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 7:179–192
Kirkaldy-Willis WH, Paine KW, Cauchoix J et al (1974) Lumbar spinal stenosis. Clin Orthop 99:30–50
Deininger MH, Unfried MI, Vougioukas VI, Hubbe U (2009) Minimally invasive dorsal percutaneous spondylodesis for the treatment of adult pyogenic spondylodiscitis. Acta Neurochir 151(11):1451–1457
Hadjipavlou AG, Katonis PK, Gaitanis IN, Muffoletto AJ, Tzermiadianos MN, Crow W (2004) Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J 13(8):707–713
Bettini N, Girardo M, Dema E, Cervellati S (2009) Evaluation of conservative treatment of non specific spondylodiscitis. Eur Spine J 18(1):143–150
Brase A, Ringel F, Stüer C, Meyer B, Stoffel M (2010) Debridement and fusion with polyetheretherketone implants in purulent spondylodiscitis: a clinical experience with nine patients. Acta Neurochir 152(11):2001–2004
Schomacher M, Finger T, Koeppen D, Süss O, Vajkoczy P, Kroppenstedt S, Cabraja M (2014) Application of titanium and polyetheretherketone cages for treating pyogenic spondylodiscitis. Clin Neurol Neurosurg 127:65–70
Quaile A (2012) Infections associated with spinal implants. Int Orthop 36(2):451–456
Lin Y, Li F, Chen W, Zeng H, Chen A, & Xiong W (2015). Single-level lumbar pyogenic spondylodiscitis treated with mini-open anterior debridement and fusion in combination with posterior percutaneous fixation via a modified anterior lumbar interbody fusion approach. J Neurosurg: Spine 1–7
Lin TY, Tsai TT, Lu ML, Niu CC, Hsieh MK, Fu TS et al (2014) Comparison of two-stage open versus percutaneous pedicle screw fixation in treating pyogenic spondylodiscitis. BMC Musculoskelet Disord 15(1):443
Madhavan K, Vanni S, Williams SK (2014) Direct lateral retroperitoneal approach for the surgical treatment of lumbar discitis and osteomyelitis. Neurosurg Focus 37(2), E5
Sheha AF (2011) Surgical management of post-discectomy spondylodiscitis with transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. Life Sci J 8(4)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
None
Funding
Ganga Orthopaedic Research & Education Foundation (GOREF), Coimbatore.
Rights and permissions
About this article
Cite this article
Shetty, A.P., Aiyer, S.N., Kanna, R. et al. Pyogenic lumbar spondylodiscitis treated with transforaminal lumbar interbody fusion: safety and outcomes. International Orthopaedics (SICOT) 40, 1163–1170 (2016). https://doi.org/10.1007/s00264-015-3063-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3063-5