Abstract
The field of cryosurgery began nearly 40 years ago with the open application of liquid nitrogen to ablate tumors. Recent developments in imaging and cryoprobe technology allow for percutaneous ablation of tumors. Computed tomography (CT)-guided cryoablation has particular use in treating musculoskeletal neoplasms because of the ability to image the lethal ice zone around both bone and soft tissue structures. This manuscript will review the development, indications, and results of cryoablation as applied to musculoskeletal neoplasms. This technique holds promise for the treatment of benign conditions as well as the palliation and durable treatment of musculoskeletal metastases; it is not commonly indicated in the curative treatment of primary malignant bone or soft tissue sarcomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local control and palliation of musculoskeletal neoplasms continues to provide clinical challenges. Patients presenting with aggressive or symptomatic benign bone and soft tissue processes require treatment for durable local cure while minimizing morbidity in the treatment of a benign condition. Similarly, patients with metastatic disease require efficient and safe palliation of sites of disease to improve their quality of life and minimize any morbidity or interruption of adjuvant treatments.
Benign musculoskeletal lesions have traditionally been treated with intralesional resection or conservative excision as a part of open surgery. These procedures necessarily risk leaving tumor cells in the reactive zone around the lesion which may lead to recurrence. Cryosurgery/cryoablation may be considered as an adjunct or stand-alone treatment for these conditions to achieve durable local control and symptom relief.
Similarly, patients with metastatic disease require local control of dangerous lesions and palliation of pain. While surgery and radiation therapy have been the traditional treatment modalities for this population, each have common clinical limitations. Radiation therapy has dose constraints that often prohibit retreatment or overlapping treatments in patients with multifocal disease. Similarly, surgical procedures are associated with elevated risk and morbidity in an often frail patient population. For these reasons, cryoablation is often a valuable treatment option when approaching these patients.
This review will outline the history of cryosurgery and cryoablation as well as the mechanism of action, technique, and contemporary indications and outcomes. For the purpose of this manuscript, cryosurgery will refer to the adjunct use of freezing technologies coupled with open surgery and cryoablation will refer to the percutaneous application of cryotherapy via cryoprobe insertion.
History
Extremes of temperature have long been recognized to cause tissue damage and cell death. Beginning in the late 1960s, Ralph Marcove began initial work using cryosurgery in the treatment of musculoskeletal neoplasms [1]. The initial technique involved open surgical curettage with direct pouring of liquid nitrogen into the tumor cavity and was primarily used to avoid the morbidity of formal en bloc resection in the treatment of aggressive benign bony neoplasms. Very high success rates with modest morbidity (primarily an elevated risk of fracture) were observed. This technique continues to be employed in the treatment of select benign bony neoplasms [2, 3].
Subsequent technological advancements allowed the development of cryoprobes that could be inserted percutaneously with procedures carried out under image guidance. Current generation probes use room temperature argon that provides cooling via rapid expansion (the Joule-Thompson effect) delivered through sealed insulated probes. Modern cryoprobe use has been coupled with image guidance for minimally invasive insertion and monitoring of the cryoablation zone to verify adequate coverage of the tissue to be treated without overlapping toxicity to adjacent structures. While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can all be used with percutaneous cryoablation, CT is most commonly used in the treatment of musculoskeletal neoplasms. This is because ultrasound is unable to adequately penetrate and monitor bone procedures, and MRI-guided procedures are less practical due to special equipment requirements and limitations around the magnetic field.
Mechanism of action and contemporary technique
Freezing temperatures induce coagulation necrosis of tissue. Mechanisms of cell death include direct cell injury due to crystallization of water molecules, the interruption of the local microcirculation in the treated tissue zone, and induction of apoptosis in the periphery of the lesion [4]. A minimum cellular temperature of −20 ° C appears necessary to provide this effect. Treatments use a rapid freeze and slow thaw with repetition of this cycle to insure appropriate tissue ablation.
The formation of lethal ice around a lesion requires monitoring to be certain that the lesion is covered adequately while surrounding normal tissue is not overly damaged. Traditional methods of open cryosurgery involved direct observation of tissue, surgical dissection to mobilize critical structures (e.g., adjacent nerves and vessels), and the insertion of thermosensors around the lesion and adjacent tissue. Current image-guided cryoablation allows monitoring of the extent of the ice ball around a cryoprobe with ultrasound, CT, or MR imaging. While ultrasound allows real time assessment of the cryoablation zone and is commonly used for cryoablation of visceral lesions, it is less useful in musculoskeletal applications with tumor involving or abutting bone. While CT does not provide true live monitoring of the cryoablation zone, its rapid image acquisition is adequate for clinical use and also allows the imaging of bony structures. Frozen tissue displays differential Hounsfield units on CT, allowing direct monitoring of the zone of ablation.
Contemporary cryoablation of musculoskeletal neoplasms involves the use of one or more cryoprobes inserted into the tumor. The cryoablation probes create ice balls of varying sizes depending on the size of the probe and the number of probes used. As the ice ball which is formed is usually oblong, probes are most commonly inserted along the long axis of the tumor although different geometric shapes of ice can be made by arranging the probes in variable orientations. Multiple probes (up to 20 at a time) may be used simultaneously to contour an ablation zone along a large or irregular tumor. Multiple probes are generally placed 1.5–2 cm apart (Fig. 1) to achieve lethal ablation zones between the probes [5•]. The ablation zone may be monitored with CT scans taken every 2 min with lethal temperatures being present 3–5 mm from the edge of the ablation zone. Techniques to preserve the spinal cord and adjacent nerve roots include balloon displacement though a sublaminar approach, sterile CO2 gas in the epidural space, as well as the placement of epidural blood or albumin foams. Techniques to preserve the overlying skin include subdermal installation of saline, placing sterile gloves filled with warm water on the patient’s skin as well as placing the operator’s hand directly on the skin for warming purposes. Injection of saline, sterile CO2 gas, or albumin foams are used to displace away critical structures such as blood vessels, bowel, spinal cord, pharynx, and critical nerves. Procedures are generally performed under conscious sedation or general anesthesia; if spinal cord monitoring is used, a total intravenous anesthetic is necessary.
Thirteen-centimeter paraspinal desmoid. a Sagittal T1WI showing large paraspinal desmoid. Insert: post gadolinium axial T1WI showing relationship to cord. b Cryoablation probes placed 1–2 cm apart for lethal ablation zone between probes. Blacked dashed line demonstrates margin of ice ball. c Two-year follow-up without adjunctive treatment and no recurrence
Current indications for cryoablation of musculoskeletal neoplasms
Primary bone and soft tissue malignancies
Contemporary indications for cryoablation in the musculoskeletal system center on the treatment of benign bone and soft tissue tumors and metastatic disease. While cryoablation has been reported in the treatment of primary bone and soft tissue malignancies, these lesions are rare enough that clinical experience with cryoablation as curative therapies (in lieu of traditional treatment modalities) has not been well established [6]. The exception to this is in the adjuvant treatment of select low-grade cartilaginous lesions (aggressive enchondromas and low-grade intra-osseous chondrosarcomas of the extremities) in which cryosurgery techniques have established good outcomes in conjunction with surgical curettage [7].
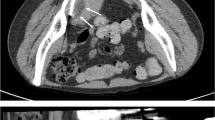
As such, cryoablation for most primary bone and soft tissue malignancies is reserved for unique clinical circumstances (e.g., lesions unresectable by conventional techniques or patients unsuitable for surgery) as experience with these indications accumulates. One example is shown in Fig. 2; an 80-year-old man presented with a neglected leiomyosarcoma of the chest wall invading the spinal canal. The lesion and patient were not suitable for traditional en bloc resection. As such, he underwent a limited surgical decompression and stabilization of the intraspinal portion of the disease with combined adjuvant radiation of the psoas muscle and portion of the tumor that surrounded the lumbosacral nerve roots and cryoablation of the majority of the tumor over four separate treatment cycles. The percutaneous cryoablation in this case had to be done in multiple sessions due to the large volume of tumor necrosis and possibility of tumor lysis syndrome as well as risk for renal failure to size of ablated tissue. Durable local control has been maintained through 19 months.
Use of cryoablation combined with limited surgery and radiotherapy for a neglected, unresectable leiomyosarcoma in a frail 80-year-old. a, b Axial and sagittal contrast-enhanced CT showing large soft tissue mass extending into the spinal canal, psoas muscle, and perispinal musculature. c Plan for needle placement is done for each case. d Sixteen (8 shown) Perc 24 Endocare cryoablation probes placed 1.5 cm apart with hypodense ice seen at 4 min. e Initial ice ball before pull back of probes to get more superficial component
Benign bone and soft tissue tumors
Experience with cryoablation of benign aggressive bony lesions is currently growing. Adjuvant cryosurgery has long been established as a valuable adjuvant to intralesional curettage in the treatment of giant cell tumor of the bone [8]. These patients generally demonstrate a local mechanical insufficiency due to lytic tumor destruction that requires surgical stabilization and bone grafting. As well, fracture has been a common complication following cryosurgery to weight-bearing bones [9]. As such, cryoablation is rarely an adequate stand-alone treatment for these patients because of the need to incorporate bone graft and internal fixation as a part of the treatment but does improve tumor control as part of a conservative surgical procedure. Adjunctive placement of cement within the ablative defect can add structural stability in selected cases where spine instability is less of an issue.
However, experience is increasing with cryoablation as stand-alone treatment for small or axial benign bone tumors. Small series are reported on the use of cryoablation for the treatment of osteoid osteoma [10, 11]. While commonly treated with radiofrequency ablation (RFA), cryoablation may provide a better option for tumors located in the spine or juxta-articular locations where the inability to monitor the lethal tissue zone of RFA is problematic. This benefit is magnified in young children in whom the surgical exposure alone may induce long-term morbidity (Fig. 3). Even the surgical exposure of the spine in a young child may induce local autofusion with subsequent high risk of growth disturbance that may be avoidable with the use of cryoablation techniques.
Osteoblastoma in a 5-year-old girl. a Plain radiographs documenting painful scoliosis. b Axial T2WI demonstrating T2 hypointense mass in the left lamina 2 mm from the conus. c Axial myelogram image from cryoablation. Myelography is often obtained when a critical structure needs to be displaced. d Thirteen-gauge osteosite neeled through the lamina with 15 mm Stryker balloon placed in the epidural space, after a saline window was made. Gently inflated with sterile CO2 due to contrasting densities displacing the conus. e Two 2.5-cm spherical cryoablation probes were placed. At 8 min, you can see hypodense ice (arrows) surrounding the lesion. f Normal spinal growth with resolution of scoliosis is shown 2 years post-procedure
Most benign soft tissue masses are managed surgically as patients are symptomatic from the presence of the mass (and thus surgical removal effectively treats the lesion and removes symptoms). However, surgical removal may be impractical or unreliable in some circumstances. The classic situation for which this applies is the treatment of extra-abdominal desmoid tumors. These tumors are frequently infiltrative and symptomatic; because of a high morbidity and vexingly high local recurrence rate, surgical resection has not proven reliable in the treatment of desmoid tumors [12]. Initial experience with cryoablation as a treatment option is favorable with our current series of 31 patients currently under review for publication [13, 14]. A specific advantage of cryoablation in such a setting is to allow conservative treatment of a benign process, ability to retreat if necessary with decreased chance for significant morbidity, decreased hospital stay (typically an overnight stay), and decreased time away from work (Fig. 4).
Desmoid left triceps in a 60-year-old male with recurrence after surgery. a Initial MRI demonstrating infiltrating enhancing desmoid adjacent to the left humerus. b MRI 1 year later after surgical resection with negative margins demonstrated a larger tumor recurrence. c Axial CT demonstrates several Perc 24 Endocare cryoablation probes angled throughout the lesion. d One-year follow-up demonstrated residual fibrosis at the site without tumor recurrence. e Six months later, a small recurrence seen at the inferior margin. f Recryoablated using four Perc 24 cryoablation probes. Discharged POD 1. No recurrence to date 2 years later. Sterile glove with warm saline is seen on the skin to prevent thermal injury
Metastatic disease
The most common current clinical indication for cryoablation in the musculoskeletal system is the treatment of metastatic bone and soft tissue lesions. Percutaneous ablation has several potential advantages over conventional radiotherapy and surgical management. It is a minimally invasive treatment that is generally completed in a single session with little interruption of adjuvant treatment modalities. Unlike radiation treatments, there are few limitations on retreatment or overlapping treatments. Additionally, tumor ablation is essentially independent of tumor histology (while tumors exhibit varying degrees of radiosensitivity, there are no known cryo-resistant tumor histologies). In comparison to traditional surgical management, cryoablation has a more favorable recovery and complication profile (for example, the risk of post-procedure infection following percutaneous cryoablation is <1 %).
It is important to recognize that traditional surgery and radiation therapy maintain a benefit in several common clinical scenarios. Lytic bone disease with present or impending pathologic fracture generally benefits from surgical stabilization to restore the mechanical integrity of the bone. While cryoablation can achieve local tumor control, it does not augment (and may decrease) mechanical strength. In some axial locations which are subject to primarily compressive forces, this limitation may be alleviated through concurrent vertebroplasty or kyphoplasty procedures. Surgery can more directly deal with neurologic compression (particularly at the spinal cord level). As well, radiation treatment efficiently treats large volumes of disease. For example, radiation can be dosed to cover the entire femur in a patient with metastatic disease following surgical stabilization, a task not readily accomplished with cryoablation. Finally, radiation treatment is less affected by the presence of metallic implants than cryoablation (both as an imaging limitation for probe guidance and monitoring and a thermal conductor that alters the distribution of the freezing zone).
Within these limitations, contemporary percutaneous cryoablation has strong indications and clinical experience to palliate painful metastases and provide durable local control with potential oncologic benefit in patients with oligometastatic disease. Patients with painful metastatic disease have demonstrated rapid and sustained pain relief following percutaneous cryoablation [5•, 15]. In contrast to radiofrequency ablation which is often painful, cryoablation often has a numbing effect on the nerves and appears to have less peri-procedural pain. This technique has been successfully adapted in combination with cementoplasty for patients with pain from impending pathologic fractures [16•]. It is important to recognize that these results are only in patients with metastases subject to primarily an axial load as methylmethacrylate is strongest in compression and relatively weak if loaded in torsion, shear, bending, or tension.
An additional indication for cryoablation is in the treatment of patients with oligometastatic disease. Although controversial, there is likely an oncologic benefit to ablation or surgical removal of tumors in some patients with solitary or oligometastatic disease [17, 18]. The traditional mechanism for rendering patients with limited metastatic burdens disease free is surgical resection, but that is often highly morbid for patients with axial (particularly multiple) lesions. In two series reporting the results of cryoablation in such a setting, local control rates have approximated those expected from traditional surgery with lower morbidity [19•, 20•]. In addition to clinically relevant local control, cryoablation of skeletal mestastases has been shown to have an objective tumor response by [18] F-FDG positron emission tomography, highlighting its potential benefit in this indication [21]. Figure 5 demonstrates the coupled use of cryoablation and cementoplasty procedures for the treatment of oligometastatic disease.
Two separate patients with oligometastatic disease cryoablated followed by kyphoplasty. a Metastatic melanoma to L3 Axial T2WI. b Two Perc 24 Endocare cryoprobes crossed in lesion. c Hypodense ice ball encompassing the lesion. d Kyphoplasty for structural stability. e Recurrent renal cell metastasis previously radiated at T3 with previous pleural reconstruction. f Total of eight cryoablation probes were used. g Twenty-millimeter Stryker balloon used for reconstruction of vertebral body. h Balloon-assisted cementoplasty, T3 and T4
New directions in treatment
Current advances in the field of cryoablation center on expanding experience with the technique and outcomes and improving the safety profile. The primary limitation to cryoablation of tumors (particularly axial lesions) is avoiding thermal damage to adjacent normal structures. The relatively tight anatomic confines of musculoskeletal ablation in the axial skeleton often place risk on the spinal cord or visceral structures. Major blood vessels that are adjacent to a cryoablation zone are at lower risk as the flowing blood acts as a continuous thermal stabilizer.
Investigators have looked at ways to create buffer zones around tumors and at ways to directly monitor local toxicities. Techniques of sterile carbon dioxide or saline dissection around tumors allow greater safety by displacing bowel or other critical structures away from the zone of treatment [22–24]. Given the near real time imaging of CT guidance in these procedures, safe displacement of critical structures and monitoring of the ablation zone is possible. In addition to creating displacement of normal structures away from a planned ablation zone, monitoring techniques developed for open surgery can be applied to axial tumor ablation (for example, spinal cord monitoring of ablations performed near the spinal canal to detect early (and reversible) impairment of spinal cord function) [23, 24].
Contemporary indications and contraindications for cryoablation
With these results and options in mind, what are the indications and contraindications for cryosurgery and cryoablation in 2016? While these will necessarily vary by institutional capabilities and practice patterns, a reasonable current approach would be:
-
(1)
Primary malignant bone and soft tissue sarcomas. Cryoablation is not indicated as primary treatment for these lesions as current clinical experience is inadequate to insure that the oncologic outcome is similar in patients undergoing curative intent treatment of primary sarcomas. Although we have used this treatment on a case by case basis in some patients who are unable to tolerate the primary treatment modality. Adjuvant cryosurgery has been shown to be efficacious when coupled with surgery in the treatment of select low-grade extremity chondrosarcomas and leiomyosarcomas.
-
(2)
Benign bone and soft tissue lesions. Cryoablation is considered as primary treatment for small benign bony lesions which are not accompanied by local structural insufficiency (e.g., osteoid osteoma). For these lesions, cryoablation provides an excellent treatment option. Adjuvant cryosurgery has been shown to be efficacious when coupled with surgery in patients undergoing treatment of benign aggressive bone tumors (e.g., giant cell tumor, aneurysmal bone cyst). Cryotherapy may be considered in the treatment of desmoid tumors and other benign soft tissue processes in which removal of the soft tissue mass is impractical or patients are failing medical treatment.
-
(3)
Painful metastatic bone and soft tissue lesions. Cryotherapy provides an excellent option for palliative treatment of painful metastatic lesions which are not associated with significant neurologic compression or local mechanical insufficiency. Patients with mechanical insufficiency from axial lesions subject primarily to compressive loads (e.g., supra-acetabular region) may benefit from cryoablation combined with cementoplasty with or without balloon assistance procedures. Such procedures are generally not indicated as stand-alone treatments for extremity lesions or other areas subject to torsional, shear, bending, or tensile biomechanical loads but may be coupled with surgical stabilization for select lesions.
-
(4)
Oligometastatic bone and soft tissue lesions. Percutaneous cryoablation provides a good treatment option with excellent local control for patients with limited metastatic disease who may achieve an oncologic benefit from having durable control of all sites of disease.
Conclusions
Technological advances have rapidly improved the capabilities and indications for cryoablation in the last 10 years. While this technology has limitations and continues to evolve, it provides a complementary treatment option for many patients with musculoskeletal tumors that were traditionally managed with open surgery and/or radiotherapy. While long-term results are still maturing, appropriately selected patients appear to achieve results equal to or superior to conventional treatment options. Thus, these are valuable techniques for physicians who treat patients with musculoskeletal tumors to be familiar with.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Marcove RC, Miller TR. The treatment of primary and metastatic localized bone tumors by cryosurgery. Surg Clin N Am. 1969;49:421–30.
Meller I, Weinbroum A, Bickels J, et al. Fiftenn years of bone tumor cryosurgery: a single center experience of 440 procedures and long-term follow-up. Eur J Surg Oncol. 2008;34:921–7.
Veth R, Schreuder B, van Beem H, et al. Cryosurgery in aggressive, benign, and low-grade malignant bone tumors. Lancet Oncol. 2005;6:25–34.
Gage AA, Baust JC. Cryosurgery for tumors. J Am Coll Surg. 2007;205:342–56.
Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases—why cryoablation? Skelet Radiol. 2009;38:835–9. While published in 2009, this article remains current and outlines the rationale and indications for considering cryoablation of musculeoskeletal metastatic disease.
de Vries J, Oldhoff J, Handers HN. Cryosurgical treatment of sacrococcygeal chordoma. Report of 4 cases. Cancer. 1986;58:2348–54.
Meftah M, Schult P, Henshaw RM. Long-term results of intralesional curettage and cryosurgery for treatment of low-grade chondrosarcoma. J Bone Joint Surg Am. 2013;95:1358–64.
van der Heijden L, Dijkstra PD, van de Sande MA, et al. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19:550–61.
Pritsch T, Bickels J, Wu CC, et al. The risk for fractures after curettage and cryosurgery around the knee. Clin Orthop Relat Res. 2007;458:159–67.
Coupal TM, Mallinson PI, Munk PL, et al. CT-guided percutaneous cryoablation for osteoid osteoma: initial experience in adults. AJR Am J Roentgenol. 2014;202:1136–9.
Wu B, Xiao X, Zhang X, Zhao L, Carrino JA. CT-guided percutaneous cryoablation of osteoid osteoma in children: an initial study. Skelet Radiol. 2011;40:1303–10.
Walczak BE, Rose PS. Desmoid: the role of local therapy in an era of systemic options. Curr Treat Options Oncol. 2013;14:465–73.
Havez M, Lippa N, Al-Ammari S, et al. Percutaneous image-guided cryoablation in inoperable extra-abdominal desmoid tumors: a study of tolerability and efficacy. Cardiovasc Intervent Radiol. 2014;37:1500–6.
Kujak JL, Liu PT, Johnson GB, Callstrom MR. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skelet Radiol. 2010;39:175–82.
Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol. 2007;10:120–31.
Castaneda Rodriguez WRC, Callstrom MR. Effective pain palliation and prevention of fracture for axial-loading skeletal metastases using combined cryoablation and cementoplasty. Tech Vasc Interv Radiol. 2011;14:160–9. This recent study provides case examples, indications, and outcomes of patients treated with combined cryoablation and cementation of axial lytic bone lesions.
Alt AL, Boorjian SA, Lohse CM, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–82.
Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006;16:120–30.
Deschamps F, Farouil G, Ternes N, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24:1971–80. This article provides a large cohort of over 100 ablated lesions in 89 patients and provides multivariate modeling of factors associated with success.
McMenomy BP, Kurup AN, Johnson GB, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24:207–13. This recent publication demonstrates excellent local control (87%) at median 21 month follow-up of 52 patients with oligometastatic disease to bone and soft tissues.
Masala S, Schillaci O, Bartolucci AD, et al. Metabolic and clinical assessment of efficacy of cryoablation on skeletal masses by 18F-FDG positron emission tomography/computed tomography (PET/CT) and visual analog scale (VAS): initial experience. Skelet Radiol. 2011;40:159–65.
Filippiadis DK, Tutton S, Mazioti A, Kelekis A. Percutaneous image-guided ablation of bone and sfot tissue tumours: a review of available techniques and protective measures. Insights Imaging. 2014;5:339–46.
Kurup AN, Woodrum DA, Morris JM, et al. Cryoablation of recurrent sacrococcygeal tumors. J Vasc Interv Radiol. 2012;23:1070–5.
Kurup AN, Morris JM, Boon AJ, et al. Motor evoked potentials monitoring during cryoablation of musculoskeletal tumors. J Vasc Interv Radiol. 2014;25:1657–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Orthopedic Oncology: New Concepts and Techniques
Rights and permissions
About this article
Cite this article
Rose, P.S., Morris, J.M. Cryosurgery/cryoablation in musculoskeletal neoplasms: history and state of the art. Curr Rev Musculoskelet Med 8, 353–360 (2015). https://doi.org/10.1007/s12178-015-9307-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-015-9307-6