Abstract
Statins are widely used for primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD), and, under the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol treatment guidelines, more individuals are eligible for statin therapy. In this review, we summarize evidence for a mild increase in serum glucose and increased incidence of diabetes associated with statins, the hypothesized mechanisms by which statins may impair glucose homeostasis, the risk of diabetes associated with particular statins, and the net effect on ASCVD risk. As emphasized by the ACC/AHA guideline group and other experts, the risk-reducing benefits of statin therapy generally outweigh the mild rise in glucose levels or new diagnoses of diabetes. As such, an appropriate balancing of benefits and risks is critical in clinical practice as clinicians engage patients in shared decision making. Moreover, when discussing statins and risk of diabetes, this is a prime opportunity for clinicians to provide further counseling on the central importance of weight loss and adhering to a healthy lifestyle in glucose homeostasis and diabetes prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol treatment guidelines recommend the use of pooled cohort equations to determine the risk of atherosclerotic cardiovascular (CV) disease (ASCVD), and subsequent prescription of statins for primary and secondary prevention after a clinician-patient risk discussion [1••, 2•]. Approximately 56 million individuals may be eligible for statin therapy under the new 2013 ACC/AHA guidelines [3], and statins are the most widely prescribed medication in the USA [4]. The largest contributor to ASCVD risk using the pooled cohort equations is age, leading to higher numbers of statin-eligible patients in older age groups. Even in the younger age group of 40–59 years without CV disease, 30 % would be eligible for statin therapy for primary prevention [3]. However, more than 77 % of individuals in the older age group of 60–75 years would be eligible for statin therapy [3]. Since stroke is now an endpoint of the ASCVD risk score and African Americans are more likely than Caucasians to sustain a future cerebrovascular event, many more African Americans will qualify for a clinician-patient risk discussion than before, even with seemingly good or normal lipid levels.
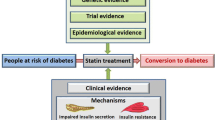
Statins are generally well tolerated, yet they are not void of side effects. There are some concerns about alterations in glucose homeostasis, with a slightly higher incidence of new diagnoses of diabetes mellitus (DM) seen in large epidemiologic studies and placebo controlled trials of patients on statin therapy, especially with evidence of insulin resistance or glucose intolerance at baseline. Also, the Food and Drug Administration (FDA) in February 2012 warned patients and healthcare professionals regarding the potential increased risk of impaired glycemic control or a new diagnosis of type 2 diabetes with the use of statins [5]. Diabetes is a significant risk factor for ASCVD, and it is imperative that the net reduction in risk to the patient be understood since the reduction in major adverse cardiovascular events (MACE) is significantly greater than the increase in new cases of diabetes.
In individuals who have diabetes risk factors (such as BMI ≥30 kg/m2, fasting glucose ≥100 mg/dL, and metabolic syndrome), statins increase the likelihood of developing diabetes and can accelerate the diagnosis of diabetes by approximately 5 weeks due to a small hyperglycemic effect [2•, 6•]. In individuals with pre-existing ASCVD, these risk factors also seem to play a role in predicting a new diagnosis of diabetes [7].
There have been multiple studies and meta-analyses examining the link between statin therapy and glucose homeostasis. In this review, we summarize evidence for a mild increase in serum glucose and increased incidence of diabetes associated with statins, the hypothesized mechanisms by which statins may impair glucose homeostasis, the risk of diabetes associated with particular statins, and the net effect on ASCVD risk.
Association Between Statin Therapy and Impaired Glucose Homeostasis
In 2008, the JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial showed that patients randomized to rosuvastatin 20 mg per day had a slightly higher hemoglobin A1c at trial completion (5.9 versus 5.8 %, p = 0.001) compared to patients randomized to placebo [8]. There was also a mild increase in new diagnoses of diabetes (3.0 versus 2.4 %, p = 0.01) [8]. In a post hoc analysis, Ridker et al. showed that individuals with major risk factors for the development of diabetes had an increase in the incidence of new diagnoses of diabetes with the use of a high-intensity statin compared to those individuals without a major risk factor [6•]. However, the investigators concluded that the benefits of statin therapy outweigh the mild rise in hemoglobin A1c or new diagnoses of diabetes [6•], which is similar to the recommendations by the FDA (Tables 1 and 2).
Other data have suggested that the rise in serum glucose and new diagnoses of diabetes are more common in patients taking high-intensity statins and therefore may be proportional to the relative lowering of low-density lipoprotein cholesterol (LDL-C). Carter et al. conducted a population-based retrospective cohort study to examine the association between various statins and new-onset diabetes [9]. Compared to patients treated with pravastatin, patients treated with the more potent atorvastatin experienced a 22 % increase in the relative risk of new-onset diabetes [9]. Similarly, there was an increased risk of new-onset diabetes with rosuvastatin and simvastatin; however, treatment with fluvastatin or lovastatin did not demonstrate an increased risk [9].
In a pooled analysis of nine trials involving participants who already carried a diagnosis of type 2 diabetes, Erqou et al. showed that hemoglobin A1c was mildly higher in participants on statin therapy (either atorvastatin, pravastatin, or simvastatin) compared to placebo [10]. The pooled hemoglobin A1c was 7.53 % in the statin groups compared to 7.41 % in the control group [10]. Additionally, a retrospective cohort study in Taiwan by Lin et al. involving patients with acute coronary syndrome (ACS) who had undergone percutaneous coronary intervention suggested a 27 % proportional increase in incident diabetes with statin therapy [11].
In contrast, the recent Heart Outcomes Prevention Evaluation (HOPE)-3 trial did not show any significant impact of statin therapy on new-onset diabetes [12]. In this trial, 6361 individuals were assigned to rosuvastatin 10 mg daily and 6344 individuals were assigned to the placebo group [12]. New-onset diabetes occurred in 232 individuals (3.9 %) in the rosuvastatin group versus 226 individuals (3.8 %) in the placebo group (p = 0.82) [12].
Other randomized controlled trial results were acceptable with respect to statins and glucose homeostasis. In the West of Scotland Coronary Prevention Study (WOSCOPS) [12, 13] of pravastatin 40 mg versus placebo, the pravastatin group had a reduced risk of diabetes diagnosis [13]. Moreover, a sub-analysis of the LIVALO Effectiveness and Safety (LIVES) study involving 308 participants with type 2 diabetes demonstrated an absolute decrease in hemoglobin A1c levels of 0.28 % (p < 0.001) [14].
Hypothesized Mechanisms by Which Statins Impair Glucose Homeostasis
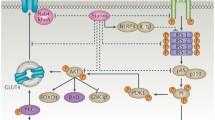
Knowledge gaps exist regarding the mechanisms of impaired glucose homeostasis associated with statin therapy and LDL-C reduction, though several hypotheses have been suggested. Goldfine recently proposed multiple mechanisms including impaired insulin secretion from pancreatic beta cells, decreased activity of insulin-signaling proteins in adipocytes, and reduced expression of glucose transporters (GLUT) [15, 16]. Furthermore, Nowis et al. suggested that the cholesterol activity of statins may also be related to their effects on glucose homeostasis by producing cholesterol-dependent conformational changes in GLUT [17], mainly GLUT type 4, thereby increasing serum glucose levels [18].
Another major hypothesis is that statins increase the risk of diabetes by increasing insulin resistance and altering homeostasis between insulin secretion and insulin sensitivity [19]. Chronic use of lipophilic statins (such as atorvastatin, lovastatin, or simvastatin) may decrease cholesterol in pancreatic beta cells, thereby blocking l-type calcium channels and inhibiting glucose-induced calcium signaling. This may inhibit insulin secretion [20] and reduce synthesis of ubiquinone [18]. Decreased levels of ubiquinone, which is a vital constituent of insulin signaling, may lead to decreased beta cell adenosine triphosphate (ATP) production and delayed insulin release, resulting in elevated postprandial glucose levels [18].
Adiponectin and leptin have received some attention in their role as mediators in the diabetogenic effect of statins; however, no clear conclusion has been made, and there is more ongoing research in this area currently [21–24]. A recent meta-analysis of 43 randomized controlled trial arms suggested that a reduction in adiponectin expression is unlikely to be an explanation for statin-associated new-onset diabetes [25]. Another adipokine, leptin, is vital in the regulation of body weight and is mostly produced in adipose tissue [26]. Leptin inhibits insulin secretion via multiple ways: suppression of preproinsulin messenger ribonucleic acid (mRNA), inhibition of glucagon-like peptide-1 (GLP-1)-induced insulin production, impairment of GLUT 2, regulation of ATP-sensitive potassium channels, and impairment of GLUT 4 translocation, which promotes insulin resistance [27–30]. The clinical importance of leptin with respect to statins and glucose homeostasis remains uncertain.
The rise in serum glucose due to statins may be directly related to their mechanism of action, yet independent from their cholesterol-lowering effects. Kain et al. proposed that by blocking 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, simvastatin leads to accumulation of acetyl CoA, a precursor of fatty acid synthesis [31]. The subsequent intracellular buildup of fatty acids appears to affect the insulin-signaling pathway, leading to increased insulin resistance [31].
Clues to the mechanisms of statin-mediated impaired glucose homeostasis may also lie in genetic studies of families with polymorphisms in the HMG-CoA reductase gene [32]. Swerdlow et al. reported that carriers of HMG-CoA reductase gene single nucleotide polymorphisms (SNP) tended to have lower LDL-C levels, larger waist circumferences, heavier body weights, and a higher prevalence of type 2 diabetes compared to subjects who were carriers of wild type alleles [32].
Another proposed mechanism by Besseling et al. is LDL receptor-mediated transmembrane cholesterol transport [33]. In a cross-sectional study in the Netherlands, the investigators found that the prevalence of diabetes was lower in individuals with familial hypercholesterolemia versus their unaffected relatives (1.75 versus 2.93 %, p < 0.001) [33], with variability by mutation type. It appeared that individuals with mutations leading to more severe familial hypercholesterolemia phenotypes, and thus reduced transmembrane cholesterol transport, were least likely to have diabetes. Importantly, statin inhibition of HMG-CoA reductase increases LDL receptor (LDL-R) expression, thereby promoting transmembrane cholesterol transport [33].
Risk of Diabetes Associated with Particular Statins
There has also been significant discussion regarding the effect a specific dose and type of statin medication on the absolute risk of newly diagnosed diabetes. Erqou et al. showed that across multiple studies, there was moderate heterogeneity observed between the risk of diabetes associated with particular statin medications, with atorvastatin (10 or 20 mg) having a stronger effect on new-onset diabetes compared to pravastatin or simvastatin (p = 0.014) [10]. Preiss et al. showed a relative increase of 12 % in incident diabetes in participants treated with simvastatin 80 mg or atorvastatin 80 mg compared with lower-intensity doses of atorvastatin in five randomized clinical trials [34]. However, there was also a decrease in the number of ASCVD events with the higher-intensity statins compared to the lower-intensity statins [34].
Carter et al. also showed that patients who were treated with higher-intensity statins (such as atorvastatin, rosuvastatin, and simvastatin) had an increased incidence of new-onset diabetes compared to patients treated with fluvastatin or lovastatin over a 14-year study period [9]. A large meta-analysis by Navarese et al. showed that pravastatin had the lowest rate of incident diabetes while rosuvastatin had the highest rate, which correlates with their expected degrees of non-high-density lipoprotein cholesterol (HDL-C) reduction [35]. Higher doses of individual statins were also associated with an increased incidence of new diabetes, suggesting a dose-dependent effect [35].
Net Effect on Risk of ASCVD Due to Statin Therapy Plus a Mild Increase in Serum Glucose
The benefits versus true adverse effects of statin therapy have been recently thoroughly reviewed by Rory Collins and colleagues in Lancet [36]. Based on data from large randomized trials, it was estimated that a reduction in LDL-C by 2 mmol/L (77 mg/dL) would lead to a 10 % absolute benefit in secondary prevention in patients with established ASCVD and a 5 % absolute benefit in patients being treated for primary prevention of ASCVD over the course of 5 years of therapy [36]. Treatment with a high-intensity statin (i.e., atorvastatin 40 mg) is expected to also cause an absolute increase in incidence of diabetes of 0.5–1.0 % [36]. Treatment of 1000 patients with atorvastatin 40 mg daily would lead to prevention of 50–100 strokes, heart attacks, or deaths due to coronary artery disease, and 5–10 cases of new diabetes, most likely in patients with pre-existing risk factors for diabetes [36]. The absolute risk of ASCVD events is also higher in diabetic patients, and they may have even more benefit from statin therapy than patients without diabetes [37]. There have also been no increases in the risk of microvascular disease in kidneys [38, 39] or eyes (EXCEL and 4S, EMPATHY) [40–42] with statin therapy or the associated mild increase in serum glucose.
Conclusion
For the most part, statins are well-tolerated medications, and their relationship with new-onset diabetes or rise in hemoglobin A1c appears to be fairly small, and mostly negligible in individuals without predisposition. In individuals with metabolic syndrome, statin use may increase the hemoglobin A1c mildly, leading to accelerated progression to the threshold used for the diagnosis of diabetes. The underlying incidence of new-onset diabetes in the primary prevention trials has been about 0.5–1 % per year, and the mean increase in hemoglobin A1c in patients on rosuvastatin 20 mg daily in the JUPITER trial was 0.1 % [36]. However, further evaluation of long-term effects of statin-induced diabetes is warranted, including the effect of statin therapy on microvascular complications of diabetes, which thus far have not been increased with statin use. Meanwhile, it is also important to maintain emphasis on lifestyle modifications, such as physical activity and healthy dietary habits, to prevent the development of diabetes.
The current ACC/AHA guidelines do not recommend discontinuing statins in diabetic patients, as the associated rise in average serum glucose is generally mild or modest. Specifically, the panel stressed that the “occurrence of a major ASCVD event represents a much greater harm to health status than does an increase in blood glucose leading to a diagnosis of diabetes” [1••]. In their safety label change in 2012, the FDA also pointed out that the CV benefits of statins usually outweigh the nominal risk of diabetes [5].
As such, an appropriate balancing of benefits and risks is critical in clinical practice as clinicians engage patients in shared decision making, present evidence, exercise clinical judgment, and consider patient preferences to reach thoughtful therapeutic decisions. Finally, when discussing statins and diabetes, this is a prime opportunity for clinicians to provide further counseling on the central importance of weight loss and adhering to a healthy lifestyle, such as avoiding sugar-laden beverages, eating plenty of vegetables, and partaking in physical activity, in glucose homeostasis and diabetes prevention.
References
Papers of particular interest, published recently, are highlighted as: • Of importance •• Of major importance
•• Stone NJ, Robinson JG, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. This is an important reference for up-to-date guidelines regarding the treatment of hyperlipidemia for reducing ASCVD.
• Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65(13):1361–8. doi:10.1016/j.jacc.2015.01.043. Discusses the importance of clinician-patient risk discussion in prevention of ASCVD and when initiating statin therapy in conjunction with 2013 ACC/AHA guidelines.
Pencina MJ, Navar-Boggan AM, D’Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–31.
National Center for Health Statistics. Health, United States 2010: with special feature on death and dying. National Center for Health Statistics, Hyattsville, MD; 2011.
FDA-Administration. FDA Drug Safety Communication: important safety label changes to cholesterol-lowering statin drugs. Secondary FDA Drug Safety Communication: important safety label changes to cholesterol-lowering statin drugs. 2012. http://www.fda.gov/Drugs/DrugSafety/ucm293101.html.
• Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–71. Reviews findings from the JUPITER trial showing that the benefits of statins outweigh the risk of new-onset DM in patients who are at high-risk of developing diabetes.
Waters DD, Ho JE, Boekholdt SM, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–52.
Ridker PM, Danielson E, Fonseca FA, JUPITER Study Group, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi:10.1056/NEJMoa0807646.
Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi:10.1136/bmj.f2610.
Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2014;57:2444–52. doi:10.1007/s00125-014-3374-x.
Lin ZF, Wang CY, Shen LJ, Hsiao FY, Lin Wu FL. Statin use and the risk for incident diabetes mellitus in patients with acute coronary syndrome after percutaneous coronary intervention: a population-based retrospective cohort study in Taiwan. Can J Diabetes. 2016;S1499-2671(15):30026–5. doi:10.1016/j.jcjd.2015.12.006.
Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016.
Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–62.
Teramoto T, Shimano H, Yokote K, Urashima M. New evidence on pitavastatin: efficacy and safety in clinical studies. Expert Opin Pharmacother. 2010;11:817–28. doi:10.1517/14656561003641990.
Goldfine AB. Statins: is it really time to reassess benefits and risks? N Engl J Med. 2012;366:1752–5.
Nakata M, Nagasaka S, Kusaka I, et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–92.
Nowis D, Malenda A, Furs K, et al. Statins impair glucose uptake in human cells. BMJ Open Diabetes Res Care. 2014;2(1):e000017. doi:10.1136/bmjdrc-2014-000017.
Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63:735–45.
Aiman U, Najmi A, Khan RA. Statin induced diabetes and its clinical implications. J Pharmacol Pharmacother. 2014;5(3):181–5. doi:10.4103/0976-500X.136097.
Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2 + signalling and insulin secretion due to blockade of L-type Ca2 + channels in rat islet beta-cells. Br J Pharmacol. 1999;126:1205–13.
Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: a comparative review. Vet J. 2012;191:292–8.
Reddy KJ, Singh M, Bangit JR, Batsell RR. The role of insulin resistance in the pathogenesis of atherosclerotic cardiovascular disease: an updated review. J Cardiovasc Med. 2010;11:633–47.
Jung CH, Rhee EJ, Choi JH, et al. The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy Korean male adults. Korean Diabetes J. 2010;34:237–43.
Kajikawa Y, Ikeda M, Takemoto S, Tomoda J, Ohmaru N, Kusachi S. Association of circulating levels of leptin and adiponectin with metabolic syndrome and coronary heart disease in patients with various coronary risk factors. Int Heart J. 2011;52:17–22.
Chrusciel P, Sahebkar A, Rembek-Wieliczko M, et al. Lipid and blood pressure meta-analysis collaboration (LBPMC) group. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016. doi:10.1016/j.atherosclerosis.2016.07.897.
Dubey L, Hesong Z. Role of leptin in atherogenesis. Exp Clin Cardiol. 2006;11:269–75.
Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53:S152–8. doi:10.2337/diabetes.53.2007.S152.
Marroquí L, Gonzalez A, Ñeco P, et al. Role of leptin in the pancreatic β-cell: effects and signaling pathways. J Mol Endocrinol. 2012;49:R9–R17.
Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51.
Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015:341583.
Kain V, Kapadia B, Misra P, Saxena U. Simvastatin may induce insulin resistance through a novel fatty acid mediated cholesterol independent mechanism. Sci Rep. 2015;5:13823. doi:10.1038/srep13823.
Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–61.
Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313(10):1029–36. doi:10.1001/jama.2015.1206.
Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–64. doi:10.1001/jama.2011.860.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123–30.
Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016. doi:10.1016/S0140-6736(16)31357-5.
Florido R, Elander A, Blumenthal RS, Martin SS. Statins and incident diabetes: can risk outweigh benefit? Curr Cardiovasc Risk Rep. 2015;9:14. doi:10.1007/s12170-015-0444-7.
Su X, Zhang L, Lv J, et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67:881–92.
The SHARP Collaborative Group. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25:1825–33.
Ueshima K, Itoh H, Kanazawa N, et al. Rationale and design of the standard versus intensive statin therapy for hypercholesterolemic patients with diabetic retinopathy (EMPATHY) study: a randomized controlled trial. J Atheroscler Thromb. 2016;23:976–90.
Laties AM, Shear CL, Lippa EA, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results. II. Assessment of the human lens after 48 weeks of treatment with lovastatin. Am J Cardiol. 1991;67:447–53.
Pedersen TR, Berg K, Cook TJ, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156:2085–92.
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–9. doi:10.1016/S0140-6736(94)90566-5.
Shepherd J, Cobbe S, Ford I. West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–8. doi:10.1056/NEJM199511163332001.
Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–22. doi:10.1001/jama.279.20.1615.
The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi:10.1056/NEJM199811053391902.
GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico). Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J. 2000;1(12):810–20.
Collins R, Armitage J, Parish S, Sleigh P, Peto R. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16. doi:10.1016/S0140-6736(03)13636-7.
Shepherd J, Blauw GJ, Murphy MB, PROSPER Study Group, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi:10.1016/S0140-6736(02)11600-X.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998–3007. doi:10.1001/jama.288.23.2998.
Sever PS, Dahlöf B, Poulter NR. et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58. doi:10.1016/S0140-6736(03)12948-0.
Nakamura H, Arakawa K, Itakura H, MEGA Study Group, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–63. doi:10.1016/S0140-6736(06)69472-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs Dahagam, Hahn, Goud, D’Souza, Abdelqader, and Blumenthal have no conflicts of interest.
Dr. Martin has research support from the David and June Trone Family Foundation, PJ Schafer Cardiovascular Research Fund, American Heart Association, Aetna Foundation, CASCADE FH, Google, and Apple. Dr. Martin is listed as a co-inventor on a pending patent filed by Johns Hopkins University for the novel method of low-density lipoprotein cholesterol estimation. Dr. Martin has served as a consultant to Abbott Nutrition, Pressed Juicery, Quest Diagnostics, Sanofi/Regeneron, Amgen, and the Pew Research Center.
Human and Animal Rights and Informed Consent
This article does not contain studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Dahagam, C., Hahn, V.S., Goud, A. et al. Role of Statins in Glucose Homeostasis and Insulin Resistance. Curr Cardiovasc Risk Rep 10, 40 (2016). https://doi.org/10.1007/s12170-016-0523-4
Published:
DOI: https://doi.org/10.1007/s12170-016-0523-4