Abstract
Germination is an excellent green food development process for improving the nutritional content of grains. Baking with sprouted barley flour is highly recommended to get the most out of barley nutrition. Soaking and sprouting whole grains helps to release their nutrients, and then the body can absorb and use the various vitamins and minerals contained in the grain, making the grains more health-beneficial. This study was conducted to investigate the effect of germination of different barley cultivars on the improved health-benefit quality of flours. Germination of different barley cultivars resulted in a significant increase in ash, protein and fiber content, antioxidant properties (total flavonoids content, total phenolic content, antioxidant activity, metal chelating activity, and ABTS+ scavenging activity), and a*, b*, and redness intensity values related to color parameters, compared to the L* value of germinated flours which decreased. Several studies have shown that digestibility and nutrient absorption improve when grains are processed, soaked, and sprouted and that the levels of vitamins, minerals, proteins, and antioxidants also increase. The present study results are in line with highlighting the benefit of germination in helping for a better valorization and increasing potential applications of barley.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
People recognized and appreciated the fact that germinated grain is something special and extremely healthy from an early age. The germ is the grain’s heart. The grain begins to germinate after being exposed to light, water, and warmth (Benincasa et al. 2019; Lemmens et al. 2019). Many enzymes are now being formed. The metabolically active enzymes improve the grain by breaking down and converting nutrients and forming new ones. Not only are many vitamins produced during germination, but large macromolecules such as gluten and other proteins are enzymatically broken down (Canlı et al. 2021). This significantly improves digestibility. Therefore, sprouted grain is something special, and the sprout contains the maximum amount of vital substances (Pagand et al. 2017). The germinated grain is a treasure trove of vitamins, whole grain energy, trace elements, secondary plant substances, and high-quality protein packed into the tiniest space (Cho and Lim 2016; Dietrich et al. 2016).
Barley (Hordeum vulgare), considered the oldest cereal cultivated by humans, is one of the most enzyme-rich plants known, and even in its resting state, it is “a bundle full of enzymes” (FAO 2017). Barley contains less gluten than wheat and is therefore not as suitable for baking. Germinated barley is naturally rich in maltose, a type of sugar that is used for various purposes (Andriotis et al. 2016). Barley malt is barley that has been briefly germinated and dried again. Therefore, barley malt syrup made from barley maltose can be used as a natural sweetener (Neylon et al. 2020). Barley flour (or barley meal) has also been used for centuries to make traditional “porridge,” representing its basic ingredient of it. Barley bread is brown bread made from barley flour, which dates back to the Iron Age (Revilla et al. 2022).
Sprouted barley flour for baking is highly recommended to get the most benefit from barley nutrition (Rico et al. 2020). Sprouting whole grains helps to release their nutrients, and then the body can absorb and use the various vitamins and minerals contained in the grain. Whole grain products contain certain anti-nutrients, such as phytic acid and oxalates, which bind to nutrients and make them very difficult to absorb (Călinoiu and Vodnar 2018). Germination is an excellent green food development process for improving the nutritional content of grains and seeds (Ikram et al. 2021). Soaking and sprouting grains, such as uncooked barley in the husk, can reduce anti-nutrient levels, making the grains more nutritious and easier to digest (Sinha and Khare 2017). In this way can be also reduced the amount of gluten present in barley to some extent. Several studies have shown that digestibility and nutrient absorption improve when grains are processed, soaked, and sprouted and that the levels of vitamins, minerals, proteins, and antioxidants also increase (Pradeep and Guha 2011; Chinma et al. 2015). Barley flour has been demonstrated to possess different antioxidant capacities playing an important role in health benefits (Bangar et al. 2022; Zanoaga et al. 2019; Punia Bangar et al. 2022).
The present research has been driving to find out how germination affects different characteristics and physical qualities of six different barley cultivars’ flours. Therefore, the present study aimed to formulate germinated barley flours and analyze the effect of germination on ash, protein and fiber content, antioxidant properties, and related color parameters of barley flour obtained after germination.
Materials and Methods
Materials
Six barley cultivars (BH-393, BH-932, BH-902, BH-885, DWR-52, and PL-172) were procured from Haryana Agriculture University, Hisar, India, as described in the previous studies (Punia Bangar et al. 2022; Bangar et al. 2022).
Germination of Barley
According to the method described by Sharma and Gujral (2010), grains were germinated. Samples of barley grains were steeped for 24 h at 25 °C, with water changes every 2 h. After soaking, barley seeds were placed in a BOD incubator (Calton, Delhi) and allowed to germinate for 24 and 48 h at 25 °C and 100% relative humidity (RH). The germinated barley was dried to a moisture content (mc) of 10% in a dryer at 40 °C. The dried samples were then sealed in airtight bags and kept for further examination.
Dehusking and Milling of Barley Grains
Dehusking of barley samples was carried out using a rice miller (Khera, Delhi). Barley cultivars were conditioned to 10% moisture content before de-husking. 150 g of conditioned barley was de-husked with the rice polisher (Khera, Delhi). The de-husked grains were then debranned and further ground.
Flours Analysis
Proximate Composition Analysis
The flour samples from different barley cultivars, non-germinated and germinated, were tested using standard analysis methods for their ash, fat, protein, and fiber content (AOAC 1990). The carbohydrate content was calculated by difference. All the measurements were replicated thrice and reported on a dry weight basis (dwb).
Color Characteristics of Flours
A Hunter Colorimeter with an optical sensor (Hunter Associates Laboratory Inc., Reston, VA, USA) was used to measure the color of flours using the L*, a*, and b* color system. The color difference (ΔE) was calculated as:
The lightness is indicated by the L* value, which ranges from 0 to 100. The degree of red-green color is determined by the a* value, with a more positive a* value suggesting more red. The degree of yellow-blue color is indicated by the b* value, with a more positive b* value suggesting more yellow. Redness intensity (RI) was calculated for each sample as: RI = a*/b*.
Total Phenolic Content (TPC) of Flours
TPC of flours from different barley cultivars non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated (at 24 h and 48 h) was determined by following the Folin-Ciocalteau method described by Gao et al. (2002). Briefly, 200 mg of ground barley samples were extracted with acidified methanol (HCl/methanol/water, 1:80:10, v/v) (4 mL) at room temperature for 2 h. The extracts obtained were oxidized with a Folin-Ciocalteau reagent, and the reaction mixture was neutralized with sodium carbonate. The mixture was incubated at room temperature for 90 min, and its absorbance using a spectrophotometer (Systronics-106, Ahmedabad) was measured at 725 nm. Acidified methanol was used as a blank. Gallic acid was used as the standard, and the results are expressed as μg gallic acid equivalents (GAE)/g of flour.
Total Flavonoids Content (TFC) of Flours
TFC of flours from different barley cultivars non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated (at 24 h and 48 h) was determined by following the method described by Jia et al. (1998). 1.25 mL distilled water was used to dilute the barley extract (250 μL). The sodium nitrite (75 μL of a 5% solution) was added, and the mixture was allowed to stand for 6 min. In addition, 150 μL of a 10% aluminum chloride solution was added, and the combination was allowed to react for 5 min. After that, the solution was then stirred well with 0.5 mL of 1 M sodium hydroxide. A spectrophotometer was used to detect the absorbance at 510 nm. The standard was catechin, and the results were expressed as g of catechin equivalents (CE)/g of flour.
Antioxidant Activity (AOA) of Flours
Antioxidant Potential Radical 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
The activity of DPPH radical scavenging was determined using a modified version of the Brand-Williams et al.’s method described (1995). Ground non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated (at 24 h and 48 h) barley cultivars samples were extracted with 100% methanol for 2 h at ambient temperature. The extracts were mixed with DPPH solution (6 × 10−5 mol/L of methanol). The absorbance (A) of the mixture at 515 nm was determined at 0 and 30 min. Methanol was used as a blank, and AOA was calculated as percent discoloration.
Metal Chelating Activity (MCA)
MCA of non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated (at 24 h and 48 h) barley cultivars extract was measured by the method described by Dinis et al. (1994). The extract (0.5 mL) was mixed with 50 μL of ferrous chloride (2 mM/L) and added 1.6 mL of 80% methanol. The reaction was initiated after 5 min by adding 5 mM/L ferrozine (100 μL), and the mixture was shaken on a vortex. The mixture was incubated at room temperature (25 °C) for 10 min. The absorbance of the solution was recorded at 562 nm on a spectrophotometer (Systronics, Ahmedabad). The chelating activity of the extract for Fe2+was calculated as follows:
ABTS+ Scavenging Capacity
ABTS+ scavenging activity was measured following the method described by Re et al. (1999). This involved a diammonium salt of free radical ABTS, 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid). ABTS+ was generated by the oxidation of ABTS with potassium persulfate. 3 mL of ABTS cation solution was mixed with 30 μL extract in a disposable microcuvette, and the decrease of absorption was measured after 1 min of incubation. A standard curve was prepared using different vitamin C concentrations similar to the DPPH assay. ABTS+ scavenging property was expressed as vitamin C in μmol/g of non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated (at 24 h and 48 h) barley.
Results and Discussion
Physicochemical Composition
The chemical composition of flours from germinated barley cultivars varied significantly (P < 0.05) (Table 1). Germination increased ash, protein, and fiber content with values ranging from 1.48 to 1.95%, 12.5 to 14.3%, and 1.3 to 1.8%, respectively. Germinated flour has a higher ash concentration than non-germinated flour, which can be attributed to lower total soluble solids and likely dry matter loss (Chinma et al. 2015). Researchers reported that the increase of protein content by germination could be attributed to the net synthesis of enzyme protein which might have resulted in the production of some amino acids during protein synthesis (Ali and Elozeiri 2017; Nkhata et al. 2018). The higher soluble dietary fiber of germinated flour than non-germinated counterparts could be attributed to new primary cell walls (Benincasa et al. 2019; Budhwar et al. 2020). However, fat and carbohydrate content among cultivars decreased after germination and ranged from 1.9 to 3.2% and 71.3 to 76.3%, respectively. Amylose content of barley flour decreased after germination and ranged between 13.1 and 19.3%, respectively, among cultivars. Chinma et al. (2015) reported that decreased amylose contents of flours from germinated grains could be due to amylase activity in respiration metabolism during germination.
Hunter Color Characteristics of Flours
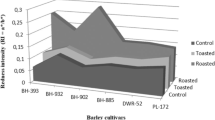
Color parameters of flour from different non-germinated (published by Punia Bangar et al. 2022; Bangar et al. 2022) and germinated barley cultivars were evaluated using a hunter color lab (Table 2). The L* value of germinated flours decreased, and the values ranged from 77.6 to 80.6; the highest for cv.BH-902 and the lowest for cv.PL-172 were observed. The higher L* value of flours from cv.BH-902 and cv.BH-393 indicates their light color. The a* value increased after germination and ranged from 1.23 to 2.33; the highest and the lowest values were observed for cv.BH-885 and cv.BH-393, respectively. The b* value also increased during germination, ranging from 9.95 to 13.6 among cultivars. ΔE ranged from 78.3 to 82.4 among germinated cultivars. A decrease in L* and an increase in a* and b* values of oats after germination have been reported by other studies as well (Tian et al. 2010; Li et al. 2020). An increase in a* and b* values could be due to more starch and protein hydrolysates formed, and the Maillard reaction occurs during drying treatment (Kleekayai et al. 2022). Overall, RI increased from non-germinated (range: 0.06–0.12) to germinated (range: 0.12–0.21) (Table 2 and Fig. 1).
TPC of Flour
TPC significantly (P < 0.05) increased (up to 35.3%) among non-germinated barley cultivars upon germination for 24 h. The highest TPC of 4548 μg GAE/g was observed for cv.BH-885, while cv.BH-393 showed the lowest value (3814 μg GAE/g) (Table 3). Sharma and Gujral (2010) reported the increase in TPC of barley flours when germinated for 24 h with values ranging between 2477 and 2723 μg GAE/g. Further, TPC increased among all cultivars when germination was allowed for 48 h, with values ranging between 3898 and 4604 μg GAE/g. The % increase was from 13.6 to 42.3%. The rise in phenolic compounds in germinated seeds could be attributed to the breakdown of the cell wall during germination, which leads to an increase in free phenolic forms (Van Hung 2016; Šimić et al. 2017).
TFC of Flour
The changes in TFC during barley germination are shown in Table 4. Upon germination for 24 and 48 h, a significant (P < 0.05) increase (up to 61.2% and 68.8%) in TFC was observed among flours from different cultivars. TFC significantly (P < 0.05) varied among cultivars, and the values ranged from 2778 to 3545 μg CE/g and 2937 to 3711 μg CE/g when germinated for 24 h and 48 h, respectively. The highest TFC was observed for cv.BH-885. Pradeep and Guha (2011) reported that the flavonoids content of the germinated millet extract showed an increase of up to 4.6% compared to native millet. This may be attributed to the biochemical changes of millet during germination, which leads to the production of these secondary plant metabolites (Sharma et al. 2018; Gowda et al. 2022).
AOA of Flours
Antioxidant Potential Radical DPPH Radical Scavenging Activity
DPPH radical scavenging activity of flours from germinated (up to 24 h and 48 h) barley differed significantly (P < 0.05) among cultivars, and values ranged from 27.5 to 34.6% (Table 5). The highest DPPH was observed for cv.BH-885, and the lowest was for cv.PL-172. An increase in AOA by 21.2 to 50.2% was observed. An increase in the duration of germination to 48 h increased the DPPH by 47.4 to77.2%, and the values ranged from 31.1 to 39.4%. Sharma and Gujral (2010) reported an increase in the AOA of barley cultivars after germination, with values ranging from 26.84 to 35.90%. Yang et al. (2001) studied the increase in DPPH for sprouted barleys and reported that the antioxidant compounds such as vitamin C and tocopherols also increased with the length of germination.
Metal Chelating Activity
MCA of flours from germinated barley differed significantly (P < 0.05) among cultivars (Table 6). Germination for 24 h resulted in an increase of 11.7 to 30.9%, with values ranging between 39 and 57% compared to non-germinated. Upon germination for 48 h, the MCA of flours increased (by 17 to 35.4%), and the values varied significantly (P < 0.05) among cultivars. Tian et al. (2004) reported that an increase in MCA might be due to increased alanine and amino butyrate and synthesis of vitamins C and E.
ABTS+ Scavenging Activity
The antioxidant activity of flours from germinated barley (using ABTS+) differed significantly (P < 0.05) among germinated cultivars (Table 7) compared to non-germinated. The germination of barley for both 24 and 48 h increased the ABTS+ scavenging activity among all cultivars. Duenas et al. (2009) reported that germination is attributed to the biochemical metabolism of seeds during germination resulting in increased scavenging activity. Hydrolytic enzymes change the endosperm during germination and may free some of the bound components that have a role in antioxidant activity (Singh et al. 2019; Tarasevičienė et al. 2019, Doblado et al. 2007).
Conclusion
The present study has been shown that germination of different barley cultivars increased ash, protein, and fiber content, antioxidant properties (total flavonoids content, total phenolic content, antioxidant activity, metal chelating activity, and ABTS+ scavenging activity), and a*, b*, and redness intensity values related to color parameters, whereas the L* value of germinated flours decreased. Several studies have shown that when grains are processed, soaked, and sprouted, digestibility and nutrient absorption improve, as well as the levels of vitamins, minerals, proteins, and antioxidants, and the findings of this study highlight the benefit of germination in helping to improve barley valorization and other application perspectives. Germination seems to represent an excellent green food developing process for improving the nutritional content of grains.
Data Availability
All the data used in the manuscript are available in the tables and figures.
Code Availability
Not applicable.
References
Ali AS, Elozeiri AA (2017) Metabolic processes during seed germination. In (ed) Advances in seed biology. IntechOpen
Andriotis VM, Saalbach G, Waugh R, Field RA, Smith AM (2016) The maltase involved in starch metabolism in barley endosperm is encoded by a single gene. PLoS ONE 11(3):e0151642
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Bangar SP, Sandhu KS, Trif M, Lorenzo JM (2022) The effect of mild and strong heat treatments on in vitro antioxidant properties of barley (Hordeum vulgare) cultivars. Food Anal. Methods (in press)
Benincasa P, Falcinelli B, Lutts S, Stagnari F, Galieni A (2019) Sprouted grains: a comprehensive review. Nutrients 11:421
Călinoiu LF, Vodnar DC (2018) Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability. Nutrients 10(11):1615
Canlı M, Çelik EE, Kocadağlı T, Kanmaz EÖ, Gökmen V (2021) Formation of bioactive tyrosine derivatives during sprouting and fermenting of selected whole grains. J Agric Food Chem 69(42):12517–12526
Chinma CE, Anuonye JC, Simon OC, Ohiare RO, Danbaba N (2015) Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chem. 185:454–458
Cho DH, Lim ST (2016) Germinated brown rice and its bio-functional compounds. Food Chem. 196:259–271
Dietrich T, Velasco MV, Echeverría P, Pop B, Rusu A (2016) Crop and plant biomass as valuable material for BBB. Alternatives for valorization of green wastes. In: Biotransformation of agricultural waste and by-products: the food, feed, fiber, fuel (4F) economy. Elsevier, San Diego, CA
Dinis TCP, Madeira VMC, Almeidam LM (1994) Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and peroxylradicals scavengers. Arch Biochem Biophys 315:161–169
Duenas M, Hernandez T, Estrella I, Farnandez D (2009) Germination as process to increase the polyphenol content and antioxidant activity of lupin seeds. Food Chem. 117:599–607
FAO-Food and Agriculture Organization of the United Nations (2017) FAOSTAT Statistics Database-Agriculture. FAO, Rome
Gao L, Wang S, Oomah BD, Mazza G (2002) Wheat quality: antioxidant activity of wheat millstreams. In: Wrigley W (ed) Wheat quality elucidation, P. Ng, and C. American Association of Cereal Chemists, St. Paul, MN, pp 219–233
Gowda NAN, Siliveru K, Prasad PVV, Bhatt Y, Netravati BP, Gurikar C (2022) Modern processing of Indian millets: a perspective on changes in nutritional properties. Foods. 11(4):499
Ikram A, Saeed F, Afzaal M, Imran A, Niaz B, Tufail T, Hussain M, Anjum FM (2021) Nutritional and end-use perspectives of sprouted grains: a comprehensive review. Food Sci Nutr 9(8):4617–4628
Kleekayai T, O’Neill A, Clarke S, Holmes N, O’Sullivan B, FitzGerald RJ (2022) Contribution of hydrolysis and drying conditions to whey protein hydrolysate characteristics and in vitro antioxidative properties. Antioxidants. 11(2):399
Lemmens E, Moroni AV, Pagand J, Heirbaut P, Ritala A et al (2019) Impact of cereal seed sprouting on its nutritional and technological properties: a critical review. Compr Rev Food Sci Food Safety 18:305
Li C, Jeong D, Lee JH, Chung HJ (2020) Influence of germination on physicochemical properties of flours from brown rice, oat, sorghum, and millet. Food Sci Biotechnol. 29(9):1223–1231
Neylon E, Arendt EK, Lynch KM, Zannini E, Bazzoli P, Monin T, Sahin AW (2020) Rootlets, a malting by-product with great potential. Fermentation 6:117
Nkhata SG, Ayua E, Kamau EH, Shingiro JB (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 6(8):2446–2458
Pagand J, Heirbaut P, Pierre A, Pareyt B (2017) The magic and challenges of sprouted grains. Cereal Foods World 62(5):221–226
Pradeep S, Guha M (2011) Effect of processing methods on the nutraceutical and antioxidant properties of little millet (Panicum sumatrense) extracts. Food Chem 126(4):1643–1647
Punia Bangar S, Singh Sandhu K, Trif M, Rusu A, Pop ID, Kumar M (2022) Enrichment in different health components of barley flour using twin-screw extrusion technology to support nutritionally balanced diets. Front Nutr 8:823148
Punia S, Sandhu KS (2015) Functional and antioxidant properties of different milling fractions of indian barley cultivars. Carpathian J Food Sci Technol 7(4):19–27
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237
Revilla P, Alves ML, Andelković V, Balconi C, Dinis I, Mendes-Moreira P, Redaelli R, Ruiz de Galarreta JI, Vaz Patto MC, Žilić S, Malvar RA (2022) Traditional foods from maize (Zea mays L.) in Europe. Front Nutr 8:683399
Rico D, Peñas E, García MC, Martínez-Villaluenga C, Rai DK, Birsan RI, Frias J, Martín-Diana AB (2020) Sprouted barley flour as a nutritious and functional ingredient. Foods 9:296
Sharma P, Gujral HS (2010) Antioxidant and polyphenol oxidase activity of germinated barley and its milling fractions. Food Chem 120(3):673–678
Sharma N, Goyal SK, Alam T, Fatma S, Chaoruangrit A, Niranjan K (2018) Effect of high pressure soaking on water absorption, gelatinization, and biochemical properties of germinated and non-germinated Foxtail millet grains. J Cereal Sci 83:162–170
Šimić G, Horvat D, Dvojković K, Abičić I, Viljevac Vuletić M, Tucak M, Lalić A (2017) Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J Food Sci 35:73–78
Singh A, Sharma S, Singh B, Kaur G (2019) In vitro nutrient digestibility and antioxidative properties of flour prepared from sorghum germinated at different conditions. J Food Sci Technol. 56(6):3077–3089
Sinha K, Khare V (2017) Review on: antinutritional factors in vegetable crops. Pharma Innov J 12:353–358
Tarasevičienė Ž, Viršilė A, Danilčenko H, Duchovskis P, Paulauskienė A, Gajewski M (2019) Effects of germination time on the antioxidant properties of edible seeds. CyTA J Food 17(1):447–454
Tian S, Nakamura K, Kayahara H (2004) Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem 52(15):4808–4813
Tian B, Xie B, Shi J, Wu J, Cai Y, Xu T, Xue S, Deng Q (2010) Physicochemical changes of oat seeds during germination. Food Chem 119(3):1195–1200
Van Hung P (2016) Phenolic Compounds of cereals and their antioxidant capacity. Crit Rev Food Sci Nutr 56:25–35
Yang TK, Basu B, Ooraikul F (2001) Studies on germination conditions and antioxidant contents of wheat grain. Int J Food Sci Nutr. 52:319–330
Zanoaga O, Braicu C, Jurj A, Rusu A, Buiga R, Berindan-Neagoe I (2019) Progress in research on the role of flavonoids in lung cancer. Int J Mol Sci. 20(17):4291
Acknowledgements
Thanks to GAIN (Axencia Galega de Innovación) for supporting this review (grant number IN607A2019/01).
Author information
Authors and Affiliations
Contributions
SPB: Methodology, data curation, formal analysis, writing-original draft preparation; KSS: project administration, supervision, writing—review and editing; MT: writing—original draft preparation, writing—review and editing; VM: writing—original draft preparation; JML: resources, writing—review and editing.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors have given their full consent to participate.
Consent for Publication
All authors have given their full consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bangar, S.P., Sandhu, K.S., Trif, M. et al. Germinated Barley Cultivars: Effect on Physicochemical and Bioactive Properties. Food Anal. Methods 15, 2505–2512 (2022). https://doi.org/10.1007/s12161-022-02311-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-022-02311-5