Abstract
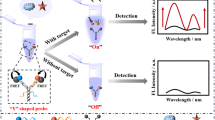
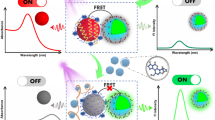
The development of detection method for aflatoxin gene cluster in Aspergillus flavus and Aspergillus parasiticusis is an effective approach to prevent the widespread of aflatoxins, which are considered as extremely, toxic substances. Herein, a hairpin DNA-assisted fluorescence probe switch based on Förster resonant energy transfer (FRET) was designed. In this work, the two terminals of hairpin DNA probe were combined with CdTe/CdS quantum dots as donor and gold nanoparticles (AuNPs) as acceptor respectively to fabricate the FRET hairpin probe switch, which were utilized to detect the aflatoxigenic fragment of aflD genes for the first time. In the absence of target DNA, the hairpin probe formed the double stem loops state to trigger the FRET to induce the fluorescence-quenching (turning off). With the addition of target DNA, the specific interaction between target and hairpin probe switch brought about the separation of fluorescent energy donor–acceptor pairs to create an increasing fluorescence response (turning on). Consequently, the fluorescence intensity recovers can be employed to detect aflD genes in homogenous phase through optical responses. This proposed FRET hairpin probe switch performed a great limit of detection of 0.02 nM with a linear range of 0.05–200 nM under optimal conditions. Furthermore, the feasibility of the fabricated strategy was demonstrated in fermented soybean paste with the advantages of simplicity, rapidity, and sensitivity, which indicated that this method exhibited the potential and practicability in foodborne biological samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are highly toxic metabolites, which frequently contaminate food to provoke the major concerning of food safety in the world (Dalie et al. 2010; Kollia et al. 2017; Sardinas et al. 2011). Especially the aflatoxins, which are kinds of mycotoxins, are considered as the most violent in toxicity due to that they can cause potent chronic toxicity in humans and recognized as the carcinogens causing mortality (Donner et al. 2010; Khaleghipour et al. 2020). Therefore, various determination methods were fabricated to detect the aflatoxins in food including chromatography, (Madurangika Jayasinghe et al. 2020; Thomas et al. 2016), enzyme-linked immunosorbent assay (ELISA) (Peng et al. 2020; Shephard 2008), and biosensor (Geleta et al. 2018; Guo et al. 2018). Additionally, biosensors based on signal-labeled can effectively improve the detection sensitivity and apply for target gene identification (Li et al. 2020a; Wu et al. 2021). Aflatoxins were mutagenic secondary metabolites produced by the Aspergillus genus and the potent hepatotoxin of aflatoxins is associated with the aflD genes of aflatoxin gene cluster from Aspergillus flavus and Aspergillus parasiticus which both are the main aflatoxin producers (Scherm et al. 2005). Therefore, the existence of aflD structural genes can be employed, as the marker of aflatoxin producers and the development of a simple, rapid, and sensitive method for aflatoxigenic species detection, which can play an important role to ensure the safety of food (Abdel-Hadi et al. 2010; Sedighi-Khavidak et al. 2017).
The most common method for identifying aflD genes is polymerase chain reaction (PCR) method, including real-time quantitative PCR and multiplex PCR which generally require sample enrichments, expensive reagents, and trained personnel (Cruz and Buttner 2008; Yunes et al. 2020). It is necessary to develop the effective, simple, and sensitive analysis strategy to improve aflatoxigenic identification, control the greater widespread pollution of aflatoxin, and reduce the possibility of threatening human health.
Förster resonant energy transfer (FRET) was nominated on its discoverer’s name Theodor Förster, who was a German scientist (Förster. 1948). There are three key factors to fabricate a FRET system, including energy donor and acceptor FRET pairs, micro-distances, and degree of spectral overlap. FRET was triggered once the energy was transferred from one excited energy donor to another accepter molecule, via intermolecular dipole–dipole interaction in close proximity (Xu et al. 2014; Zamborini et al. 2012). The FRET biosensor employing the quantum dots (QDs) as the donor has attracted great interest due to its excellent features such as broad excitation spectral, merit simplicity, tunable spectral, and high fluorescent yields (Algar et al. 2011; Dezhurov et al. 2011; Li et al. 2019; Mo et al. 2020). Meanwhile, various QDs including but not limited to magnetic QDs (Wang et al. 2019), ZnS QDs (Liu et al. 2019), CdSe QDs (Hao et al. 2019), carbon QDs (Jalili et al. 2020), and CdTe QDs (Yang et al. 2021; Zhai et al. 2016) were investigated gradually to fabricate kinds of biosensor for poisonous substance detection in food safety field. In order to obtain high sensitivity, it is critical to improve the fluorescent stability and quantum yield which are strongly dependent on the properties of their surface. As a result, the poor surface passivation and surface defects can lead to enhanced trap state emission or non-radiative recombination and generate a low fluorescence quantum yield and photo-bleaching (Lee and Osborne 2009; Medintz et al. 2005). To overcome these issues, QDs were designed and applied to allow large improvements in photoluminescence and increased thermal and chemical stability (Deng and Guyot-Sionnest 2016; Guo et al. 2003). The benefit of using heterostructured QDs as a FRET donor in biosensors has the potential to extent significantly the candidate pool to enlarge the overlapping in donor–acceptor pair, which could efficiently enhance the effect of energy transfer and obtain favorable stability (Cirillo et al. 2014).
Inspired by the merit fluorescent property of CdTe and the advantageuos stability of the heterostructured nanomaterials in our previous studies (Li et al. 2020b; Li et al. 2017), the homogeneous FRET hairpin probe switch based on CdTe/CdS-AuNPs donor–acceptor pair and hairpin DNA was developed to detect aflD genes, which is an aflatoxin gene clustered in Aspergillus flavus and Aspergillus parasiticus, for the first time. The novel fluorescent biosensor was constructed successfully on account of DNA-specific recognition between target DNA and hairpin probe. It was credited to the hairpin DNA, which was an optimal candidate for making the opportunity to bring donor–acceptor pair together (Farjami et al. 2011; Jockusch et al. 2006). In this developed strategy, an obvious FRET occurred to induce the fluorescent signal “turn off.” With the addition of a series of target aflD gene, the fluorescent signal was “turned on” in varying degrees accordingly to achieve the goal of quantitative determination aflD gene under optimal conditions. This proposed homogeneous FRET hairpin probe switch performed excellent selectivity, repeatability, and reliability. Furthermore, this fabricated FRET hairpin probe switch was utilized in aflD gene PCR product detection and achieved a good result, which indicated this method had the potential capability to be the feasible tool for the aflD gene determination in the real fermented soybean paste samples.
Experimental
Agents and Apparatus
The main chemical agents for CdTe/CdS QDs synthesis were as follows: Tellurium powder (99%), CdCl2·2.5H2O (99%), NaBH4 (99%), 3-mercaptopropionic acid (MPA, 99%), thioglycolic acid (TGA, 98%), thioacetamide (TAA, 98%), and sodium hydroxide (NaOH, 96%). The main chemical agents for AuNP synthesis were as follows: HAuCl4·4H2O (47.8%) and sodium citrate solution (98%). All of the above-mentioned agents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). The main chemical agents for construction fluorescent probe were as follows: Tris (2-carboxyethyl) phosphine hydrochloride (TCEP), 1-Ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS). They were purchased from Sigma-Aldrich (Shanghai, China). All reagents in this study belonged to analytical grade and were used without any further purification. All DNA sequences were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The sequences of DNA used in this work are listed in Table 1. The distilled water was used in the whole experiments. The main apparatus for this work were as follows: JEOL 2100 TEM (JEOL, Japan), UV-2450 spectrophotometer (Shimadzu,Japan), Hitachi F-4500 fluorescence spectrophotometer (Tokyo, Japan).
Synthesis of CdTe/CdSQDs
The synthesis of CdTe/CdS QDs was prepared according to the method reported before with brief modification (Li et al. 2017; Zhu et al. 2018). The synthesis procedure of CdTe/CdS was as follows: First, 0.0449 g NaBH4 and 0.0638 g tellurium powder were added to 5 mL of water, under nitrogenous atmosphere and stored in fridge for 12 h. In this step, tellurium powder was reduced by the NaBH4 and the fresh NaHTe was generated continuously. Meanwhile, 100 μL MPA and 0.1142 g CdCl2·2.5H2O were dissolved in 50 mL water in three necked bottles and 1.0 M NaOH was used to adjust the pH value of the solution to pH 9.0. Secondly, 2.5 mL NaHTe solution was injected rapidly into the above mixture solution under 2000 rpm vigorous stirring and protected by nitrogen atmosphere. And the mixture solution was heated at 100 °C for 30 min to gain light yellow CdTe QDs. And then, the CdTe QDs were precipitated with excess absolute ethyl alcohol (volume ratio = 1:4) at 10,000 rpm for 5 min to remove unreacted substance. The lower CdTe QDs sediments were redispersed in the same volume of water and stored less than 4 °C in fridge for subsequent use. Thirdly, 190 mg CdCl2·2.5H2O and 64.15 mg TAA were used for cadmium and sulfur source respectively and resolved simultaneously in 100 mL water to fabricate S and Cd stock solution. Followed by adding 100 μL 10 M TGA into the latter solution to be stabilizer. The pH value of the solution was adjusted to pH 12.0 by 1.0 M NaOH. Finally, 5 mL as-prepared pure CdTe QDs was added into 50 mL water which was heated to 100C. Subsequently, 10 mL prepared S and Cd stock solution were added into the above hot solution and continued to be stirred to obtain epitaxial growth of CdS QDs. The mixture solution was refluxed 40 min at 100 °C to form CdTe/CdS QDs. The purification procedure was similar to CdTe QDs and the purified CdTe/CdS QDs were redispersed in the same volume of water and stored under4 °C in fridge for further use.
Preparation of CdTe/CdS-HP Probe
Two milliliters as-prepared CdTe/CdS QDs was dispersed in 5.0 mL 25 mM PBS buffer (pH7.4). Followed by adding 38.0 mg NHS and 76.0 mg EDC to activate the surface carboxylic group for 40 min under even stirs at 25 ℃ temperature. Subsequently, 10.0 μL 100.0 μM hairpin DNA was added into the above mixture solution and continued to whisk gentler for further 24 h. The resulting mixture was centrifuged at 10,000 rpm for 8 min and washed 3 times with 25 mM PBS buffer (pH 7.4) to wipe excessive NHS and EDC to obtain CdTe/CdS-HP probe. The eventual precipitate of CdTe/CdS-HP probes was dispersed in the same volume water and stored at 4 °C for later use.
Synthesis of AuNPs
AuNPs were prepared according to the previous reported reduction method (Ye et al. 2014). Briefly, a mixture of 2 mL HAuCl4 (2.0 wt%) and water (98 mL) was added and boiled at 2500 rpm vigorous stirring in a clean beaker which was washed thoroughly with aqua regia (the volume ratio of HNO3:HCl at 1:3 ratio) and rinsed with water. Subsequently, 1 mL sodium citrate solution (2.0 wt%) was added into the boiled mixture solution within 2 s under the same stirring rate. The color of the solution changed from light fuchsia to deep wine red in a few minutes. The solution was percolated by the 0.8-µm membrane filter after it was boiled for around 15 min and cooled it to room temperature. Finally, the prepared AuNPs were dispersed in 50 mL water and stored at 4 °C for the later use.
Construction of Homogeneous FRET Hairpin Probe Switch
The homogeneous FRET hairpin probe switch was constructed by linking the prepared thiol-modified CdTe/CdS-HP probe and AuNPs. The detailed operation process was following the pre-literature described elsewhere (Wang et al. 2017). The thiol-modified CdTe/CdS-HP probe was incubated with 2.0μL TCEP (15 mM) for 1 h to cleave disulfide linkage under gentle vibration. Then 100 μL activated CdTe/CdS-HP probe and 900 μL AuNPs were mixed and incubated for 24 h at 25 ℃ temperature with gentle stirring. The final solution was centrifuged at 10,000 rpm for 8 min 3 times with deionized water and the homogeneous FRET hairpin probe switch was obtained by re-dispersing the precipitation in the same volume of water.
The Detection of aflD and Application in Real Fermented Soybean Paste
The fabricated homogeneous FRET hairpin probe switch was employed to detect aflD by the specific recognition between hairpin probe and target aflD gene. Briefly, a series of 200 μL target aflD genes with different concentrations were added to 1.0 mL prepared FRET hairpin probe switch solution to incubate for 40 min to trigger the fluorescence signal “turn on.” The final solutions were diluted to 2.0 mL respectively and measured the fluorescence intensity by fluorescent spectrophotometer under 360-nm excitation wavelength. The various mismatched gene sequences were used to assess the selectivity of the FRET hairpin probe switch. Additionally, the real DNA samples were extracted from fermented soybean paste by the Plant Genomic DNA Kit, (Sangon Biotech Co., Ltd. Shanghai, China) and amplified by PCR method. Subsequently, 200-μL PCR amplification products were employed to estimate the utility of the fabricated homogeneous FRET hairpin probe switch.
Results and Discussion
Mechanism of FRET Hairpin Probe Switch for aflD Detection
Donor and acceptor coexisting in the homogeneous solution with in adjacent distance could trigger the FRET. In this study, the hairpin DNA whose two end sequence was conjugated with the amino and sulfhydryl group respectively was chosen to be the bridge for FRET. Meanwhile, the AuNPs were used to be an acceptor and CdTe/CdS QDs were employed as a donor contributed to its superior and stabilized fluorescent characters. The heterostructured CdTe/CdS QDs were immobilized with the amino terminus of hairpin DNA by the reaction of amides to form CdTe/CdS-HP and gain the strong fluorescent signals to turn fluorescent signal “turn on.” Simultaneously, the AuNPs was linked with other terminal of CdTe/CdS-HP by the strong affinity of Au–S bonding, which approximated the distance between CdTe/CdS and AuNPs; it provided the chance for FRET occurrence from the CdTe/CdS to AuNPs, leading to the FRET hairpin probe switch showing the fluorescent signal “turn off” state. The principle of the FRET hairpin probe switch is shown in Scheme 1. The presences of the target aflD gene could hybrid with hairpin probe and extended the distance between donor and acceptor as far as possible, which could reduce energy transfer to form the “open” beacon state and turned fluorescent signal “turn on.”
Characterization of CdTe/CdSQDs and AuNPs
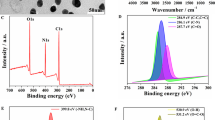
To estimate the properties of CdTe/CdS QDs and AuNPs, the TEM images of CdTe/CdS and AuNPs were taken to study the morphology and size. It was shown in Fig. 1A, AuNPs were in a great-dispersed state with the approximate round and 13-nm average diameter, which was beneficial for FRET occurrence. TEM image of CdTe/CdS QDs and high-resolution (HR) TEM image of CdTe/CdS QDs are shown in Fig. 1B and Fig. 1B (a), the crystal structure of CdTe/CdS QDs was clearly visible, and the formation of the CdS QDs on the CdTe surface is consistent with previous reports (Yue et al.2016; Jing et al.2015). The high-angle annular dark-field scanning TEM (HAADF-STEM) image and EDX were obtained and as shown in Fig. 1C and D. The elemental mapping (Fig. 1C (a–d) and profile analyses by energy-dispersive spectroscopy (EDS) of CdTe/CdS indicated containing Cd, Te, and S, which illustrated the successful preparation of CdTe/CdS quantum dots. The spectral overlapping between CdTe/CdS QDs and AuNPs was researched by normalized analysis method and it was performed in Fig. 1E. The result illustrated the ultraviolet absorption wavelength of AuNPs was 530 nm and the fluorescent emission wavelength was 539 nm under the exciting wavelength of 360 nm. The diameter of synthesized CdTe/CdS QDs can be calculated as previous report and followed (Zhu et al. 2018):
Characterization of AuNPs and CdTe/CdS QDs, the TEM image of AuNPs (A); TEM image of CdTe/CdS QDs (B) and HR-TEM image of CdTe/CdS QDs (B (a)); HAADF-STEM image and corresponding elemental mapping analysis of CdTe/CdS from top view (C-HAADF). Green, yellow, orange, and red colors indicated Te-L (C(a)), Cd-L (C(b)), Cd-K (C(c)), and S-K (C(d)) respectively; EDS of CdTe/CdS (D); Spectra overlapping between normalized emission spectra of CdTe/CdS and normalized absorption spectra of AuNPs (E); Fluorescence spectra of CdTe, CdTe/CdS, CdTe/CdS-HP, and CdTe/CdS-HP-AuNPs (F)
In this equation, D (nm) is the diameter size of the given nanoparticles, and the λ is the first excitonic peak of the CdTe/CdS QDs. According to the above equation, the corresponding diameter size of CdTe/CdS QDs was around 3.1 nm. Moreover, the overlapping of the two spectral obviously exhibited overwhelming extent, which provided the advantageous condition for the homogeneous FRET hairpin probe switch.
Spectral Properties of CdTe/CdS, CdTe/CdS-HP, and FRET Hairpin Probe Switch
The fluorescent properties of CdTe/CdS, CdTe/CdS-HP, and FRET hairpin probe switch were the critical indicators for this proposed strategy; therefore, it was necessary to study the fluorescent properties of various steps. As shown in Fig. 1F, the CdTe performed certain fluorescent intensity; meanwhile, the fluorescent intensity increased after the CdTe was capped by CdS to form CdTe/CdS QDs, which was contributed to the heterostructured structure enhancing stability of QDs. While the hairpin DNA probe was combined with CdTe/CdS QDs to form CdTe/CdS-HP, there was no obvious change of the fluorescent intensity; it could be observed a slight red shift in emission spectra, which demonstrated the successful conjugation. Additionally, the fluorescent intensity had greatly reduced when the AuNPs was linked with CdTe/CdS-HP, and it attributed to the triumphant constitution of FRET hairpin probe switch and the energy transfer from the donor of CdTe/CdS QDs to the acceptor of AuNPs. The successful FRET hairpin probe switch placed the foundation and opportunity for the target DNA detection.
Optimization of Assay Conditions for aflD Detection
To achieve the excellent test condition, the important experimental parameters of concentration of CdTe/CdS QDs, pH values, reaction temperature, and hybridization time of DNA were optimized respectively. First of all, the CdTe/CdS QDs was employed as the fluorescent signal as it was significant to optimize its concentration. Therefore, the concentration of CdTe/CdS QDs was studied by in the range of 1.5–6.8 ng/ mL and shown in Fig. 2A. The result illustrated that the fluorescent intensity increased with the CdTe/CdS QD addition in the initial stage and began to decrease from the 4.2 ng/mL; therefore, the 4.2 ng/mL was chosen to be the best concentration of QD donor. Secondly, the pH value of the detection system was closely related to the stability of the FRET hairpin probe switch, which was necessary to study pH environment. The proposed method was carried out in different pH values such as 6.6, 7, 7.4, 7.8, and 8, and the experimental result is shown in Fig. 2B; an increasing tendency of fluorescent intensity was observed until pH 7.4 and the curve went down. Hence, the pH value of 7.4 was used as the buffer with the optimum pH for the later detection. Furthermore, the reaction temperature and hybridization time were the most important elements to reduce the detection speed, hence, then need to investigate them. Consequently, the effect of reaction temperature was also investigated in various temperatures between 33 and 45℃. As illustrated in Fig. 2C, the fluorescence intensity increased with the extension of reaction temperature and achieved a maximum level at 37 ℃. In consequence, the 37 ℃ was employed to be the optimal reaction temperature. Finally, the hybridization time of the target DNA and FRET hairpin probe switch had a direct bearing on the fluorescence signal turning “on.” To improve the detection efficiency, the hybridization time of 20 min, 30 min, 40 min, 50 min, and 60 min was estimated respectively and presented in Fig. 2D. The intensity of fluorescence signal turning “on” advanced gradually with the extension of hybridization time and there was no obvious change from 40 min. Hence, 40 min was selected as the high efficiency in subsequent experiments.
Parameter optimization for the aflD gene of production aflatoxin FRET detection platform. (A) Evaluation of the effect of CdTe/CdS concentration; (B) Evaluation of the effect of pH value; (C) Influence of temperature to the proposed aflD gene detection strategy; (D) Evaluation of the hybridization time
Construction of Homogeneous FRET Hairpin Probe Switch for aflD Detection
The proposed homogeneous FRET hairpin probe switch was employed for aflD detection under the optimal conditions by adding different concentrations of 0 to 200 nM. When the target aflD gene was added into this detection system, the FRET hairpin probe switch was turned to signal “on” state. As illustrated in Fig. 3A, fluorescence intensity was increasing gradually with the addition of target aflD gene. Meanwhile, the linear regression analysis of response value from various concentrations was performed to verify the reliability and feasibility of this FRET hairpin probe switch. It was presented in Fig. 3B, the linear range for target aflD gene was obtained in the concentration range of 0.05–200 nM and established the standard regression equations:y = 105.0789x + 195.45 with the correlation coefficient R2 = 0.9948. The low limit of detection (LOD) for aflD gene was 0.02 nM. This result demonstrated this strategy exhibited the excellent linear relation.
This work was compared with previous reported results of other recent literatures (Sedighi-Khavidak et al. 2017; Tombelli et al. 2009), and the result of comparison illustrated our work displayed a lower LODs and broader linear ranges, which is good for the practical application of this homogeneous FRET hairpin probe switch.
Specificity and Repeatability of the Proposed FRET Hairpin Probe Switch
In order to verify the specificity of this FRET hairpin probe switch, mismatched DNA sequence was added into the fabricated FRET hairpin probe switch solution to measure the fluorescent intensity. As shown in Fig. 4, the detection system of perfectly complementary DNA for the hairpin probe exhibited maximum fluorescence recovery, which attributed to DNA base pairing to enlarge the further distance between CdTe/CdS and AuNPs. With the number of mismatched bases increasing, the degree of fluorescence recovery accordingly reduced as expected. What’s more, the fluorescence recover from response of random DNA sequence was not observed clearly and the fluorescent intensity was approximate with blank group. It was demonstrated this proposed method performed the excellent specificity. To evaluate the repeatability, this proposed FRET hairpin probe switch was employed for 100 nM target aflD gene detection in five independent experiments, and the relative standard deviation (RSD) was obtained at 4.36%, illustrating the proposed method possessing great reproducibility. This was good for the utilization in complex sample with the advantaged selectivity and repeatability.
Feasibility of the Develop Strategy in Fermented Soybean Paste
To demonstrate to the feasibility of the fabricated strategy in actual detection, this method was applied to determine aflD gene fermented soybean paste samples. The extracted DNA sample by the Plant Genomic DNA Kit was amplified with the traditional PCR, and this developed FRET hairpin probe switch displayed effective correspondence for the PCR product in the range of 0.087–6.25 ng/μL. The accuracy of aflD gene detection in fermented soybean paste samples was also evaluated by determining the recovery of aflD gene by a standard addition method and the recovery values were calculated as 89.6%. Therefore, the results validated this developed FRET hairpin probe switch revealed the outstanding reliability in real fermented soybean paste samples.
Conclusion
In conclusion, a new strategy based on heterostructured CdTe/CdS and AuNP donor–acceptor pairs was developed for aflD gene detection and applied in the real fermented soybean paste samples. In this strategy, the FRET hairpin probe switch was fabricated through donor–acceptor pairs combined with hairpin DNA probe. Meanwhile, the satisfactory spectra overlapping of donor–acceptor pairs provided guarantee for formation of the high efficiency FRET to measure the target aflD gene. When the aflD gene was presented, the remarkable fluorescent recovery was performed with the wide linearity range of 0.05–200 nM and LOD of 0.02 nM. Moreover, various numbers of mismatched base DNA sequence were selected to demonstrate the specificity and five independent experiments were used to evaluate the repeatability; the results displayed the good selectivity and reproducibility. Additionally, the reliability and the feasibility of this developed strategy were demonstrated in PCR product of fermented soybean paste samples. Therefore, all this mentioned above accounted for this method had the potency to apply in the gene segment detection from fungal strains and pathogenic bacterium.
References
Abdel-Hadi A, Carter D, Magan N (2010) Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B-1 production during storage of peanuts under different water activity levels. J Appl Microbiol 109:1914–1922
Algar WR, Susumu K, Delehanty JB, Medintz IL (2011) Semiconductor quantum dots in bioanalysis: crossing the valley of death. Anal Chem 83:8826–8837
Cirillo M, Aubert T, Gomes R, Van Deun R, Emplit P, Biermann A, Lange H, Thomsen C, Brainis E, Hens Z (2014) “Flash” synthesis of CdSe/CdS core-shell quantum dots. Chem Mat 26:1154–1160
Cruz P, Buttner MP (2008) Development and evaluation of a real-time quantitative PCR assay for Aspergillus flavus. Mycologia 100:683–690
Dalie DKD, Deschamps AM, Richard-Forget F (2010) Lactic acid bacteria - potential for control of mould growth and mycotoxins: a review. Food Control 21:370–380
Deng Z, Guyot-Sionnest P (2016) Intraband luminescence from HgSe/CdS core/shell quantum dots. ACS Nano 10:2121–2127
Dezhurov SV, Volkova IY, Wakstein MS (2011) FRET-based biosensor for oleic acid in nanomolar range with quantum dots as an energy donor. Bioconjug Chem 22:338–345
Donner M, Atehnkeng J, Sikora RA, Bandyopadhyay R, Cotty PJ (2010) Molecular characterization of atoxigenic strains for biological control of aflatoxins in Nigeria. Food Addit Contam Part A-Chem 27:576–590
Farjami E, Clima L, Gothelf K, Ferapontova EE (2011) “Off on” electrochemical hairpin-DNA-based genosensor for cancer diagnostics. Anal Chem 83:1594–1602
Förster T (1948) Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann Phys 437:55–75
Geleta GS, Zhao Z, Wang Z (2018) A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B-1. Analyst 143:1644–1649
Guo W, Li JJ, Wang YA, Peng X (2003) Luminescent CdSe/CdS core/shell nanocrystals in dendron boxes: superior chemical, photochemical and thermal stability. J Am Chem Soc 125:3901–3909
Guo T, Lin X, Liu Y, Deng J, Qian P, Lyu Y, Zhang Z, Wang S (2018) Target-induced DNA machine amplification strategy for high sensitive and selective detection of biotoxin. Sensors and Actuators B-Chemical 262:619–624
Hao H, Hao S, Hou H, Zhang G, Hou Y, Zhang Z, Bi J, Yan S (2019) A novel label-free photoelectrochemical immunosensor based on CdSe quantum dots sensitized Ho3+/Yb3+-TiO2 for the detection of Vibrio parahaemolyticus. Methods 168:94–101
Jalili R, Khataee A, Rashidi M-R, Razmjou A (2020) Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem 314(126172):1–7
Jing L, Kershaw SV, Kipp T, Kalytchuk S, Ding K, Zeng J, Jiao M, Sun X, Mews A, Rogach AL, Gao M (2015) Insight into strain effects on band alignment shifts, carrier localization and recombination kinetics in CdTe/CdS core/shell quantum dots. J Am Chem Soc 137:2073–2084
Jockusch S, Marti AA, Turro NJ, Li Z, Li X, Ju J, Stevens N, Akins DL (2006) Spectroscopic investigation of a FRET molecular beacon containing two fluorophores for probing DNA/RNA sequences. Photochem Photobiol Sci 5:493–498
Khaleghipour B, Khosravinia H, Toghiyani M, Azarfar A (2020) Efficacy of silymarin-nanohydrogle complex in attenuation of aflatoxins toxicity in Japanese quails. Ital J Anim Sci 19:351–359
Kollia E, Proestos C, Zoumpoulakis P, Markaki P (2017) Inhibitory effect of Cynara cardunculus L. extract on aflatoxin B1 production by Aspergillus parasiticus in sesame (Sesamum indicum L.). Int J Food Prop 20:1270–1279
Lee SF, Osborne MA (2009) Brightening, blinking, bluing and bleaching in the life of a quantum dot: friend or foe? ChemPhysChem 10:2174–2191
Li Y, Sun L, Qian J, Long L, Li H, Liu Q, Cai J, Wang K (2017) Fluorescent “on-off-on” switching sensor based on CdTe quantum dots coupled with multiwalled carbon nanotubes@graphene oxide nanoribbons for simultaneous monitoring of dual foreign DNAs in transgenic soybean. Biosens Bioelectron 92:26–32
Li Y, Luo S, Sun L, Kong D, Sheng J, Wang K, Dong C (2019) A green, simple, and rapid detection for amaranth in candy samples based on the fluorescence quenching of nitrogen-doped graphene quantum dots. Food Anal Methods 12:1658–1665
Li H, Lin H, Lv W, Gai P, Li F (2020a) Equipment-free and visual detection of multiple biomarkers via an aggregation induced emission luminogen-based paper biosensor. Biosens Bioelectron 165:1–8
Li Y, Hao N, Luo S, Liu Q, Sun L, Qian J, Cai J, Wang K (2020b) Simultaneous detection of TNOS and P35S in transgenic soybean based on magnetic bicolor fluorescent probes. Talanta 212(120764):1–7
Liu Z, Wang X, Hou J, Huo D, Hou C (2019) A simple and rapid phosphorescence probe based on Mn-doped ZnS quantum dots for chloramphenicol detection. NANO 14:1–10
Madurangika Jayasinghe GDT, Dominguez-Gonzalez R, Bermejo-Barrera P, Moreda-Pineiro A (2020) Ultrasound assisted combined molecularly imprinted polymer for the selective micro-solid phase extraction and determination of aflatoxins in fish feed using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1609:1–11
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Mo GCH, Posner C, Rodriguez EA, Sun T, Zhang J (2020) A rationally enhanced red fluorescent protein expands the utility of FRET biosensors. Nat Commun 11:1848–1848
Peng H, Chang Y, Baker RC, Zhang G (2020) Interference of mycotoxin binders with ELISA, HPLC and LC-MS/MS analysis of aflatoxins in maize and maize gluten. Food Addit Contam Part A-Chem 37:496–506
Sardinas N, Vazquez C, Gil-Serna J, Teresa Gonzalez-Jaen M, Patino B (2011) Specific detection and quantification of Aspergillus flavus and Aspergillus parasiticus in wheat flour by SYBR (R) Green quantitative PCR. Int J Food Microbiol 145:121–125
Scherm B, Palomba M, Serra D, Marcello A, Migheli Q (2005) Detection of transcripts of the aflatoxin genes aflD, aflO, and aflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus parasiticus. Int J Food Microbiol 98:201–210
Sedighi-Khavidak S, Mazloum-Ardakani M, Khorasgani MR, Emtiazi G, Hosseinzadeh L (2017) Detection of aflD gene in contaminated pistachio with Aspergillus flavus by DNA based electrochemical biosensor. Int J Food Prop 20:119–130
Shephard GS (2008) Determination of mycotoxins in human foods. Chem Soc Rev 37:2468–2477
Thomas K, Wechsler D, Chen Y-M, Crain S, Quilliam MA (2016) Analysis of natural toxins by liquid chromatography-chemiluminescence nitrogen detection and application to the preparation of certified reference materials. J AOAC Int 99:1173–1184
Tombelli S, Mascini M, Scherm B, Battacone G, Migheli Q (2009) DNA biosensors for the detection of aflatoxin producing Aspergillus flavus and A-parasiticus. Monatshefte Fur Chemie 140:901–907
Wang Y, Gan N, Zhou Y, Li T, Cao Y, Chen Y (2017) Novel single-stranded DNA binding protein-assisted fluorescence aptamer switch based on FRET for homogeneous detection of antibiotics. Biosens Bioelectron 87:508–513
Wang C, Xiao R, Wang S, Yang X, Bai Z, Li X, Rong Z, Shen B, Wang S (2019) Magnetic quantum dot based lateral flow assay biosensor for multiplex and sensitive detection of protein toxins in food samples. Biosens Bioelectron 146:1–8
Wu J, Lv W, Yang Q, Li H, Li F (2021) Label-free homogeneous electrochemical detection of MicroRNA based on target-induced anti-shielding against the catalytic activity of two-dimension nanozyme. Biosens Bioelectron 171:1–8
Xu W, Xiong Y, Lai W, Xu Y, Li C, Xie M (2014) A homogeneous immunosensor for AFB1 detection based on FRET between different-sized quantum dots. Biosens Bioelectron 56:144–150
Yang Q, Li Q, Li H, Li F (2021) pH-response quantum dots with orange−red emission for monitoring the residue, distribution, and variation of an organophosphorus pesticide in an agricultural crop. J Agric Food Chem 69:2689–2696
Ye WW, Shi JY, Chan CY, Zhang Y, Yang M (2014) A nanoporous membrane based impedance sensing platform for DNA sensing with gold nanoparticle amplification. Sensors Actuators B Chem 193:877–882
Yue D, Qian X, Zhang Z, Kan M, Ren M, Zhao Y (2016) CdTe/CdS core/shell quantum dots cocatalyzed by sulfur tolerant [Mo3S13]2− nanoclusters for efficient visible-light-driven hydrogen evolution. ACS Sustainable Chem Eng 4, 6653–6658
Yunes NBS, Oliveira RC, Reis TA, Baquiao AC, Rocha LO, Correa B (2020) Effect of temperature on growth, gene expression, and aflatoxin production by Aspergillus nomius isolated from Brazil nuts. Mycotoxin Res 36:173–180
Zamborini FP, Bao LL, Dasari R (2012) Nanoparticles in measurement science. Anal Chem 84(2):541–576
Zhai H, Feng T, Dong L, Wang L, Wang X, Liu H, Liu Y, Chen L, Xie M (2016) Development of dual-emission ratiometric probe-based on fluorescent silica nanoparticle and CdTe quantum dots for determination of glucose in beverages and human body fluids. Food Chem 204:444–452
Zhu J, Wang SN, Li JJ, Zhao JW (2018) The effect of core size on the fluorescence emission properties of CdTe@CdS core@shell quantum dots. J Lumines 199:216–224
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31901799), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Nos. 19KJB550006, 18KJB550003), the Natural Science Foundation of Jiangsu Province (No. BK20180979), the Research Foundation of Zhenjiang Science and Technology Bureau (No. FZ2017068), the Central Public-interest Scientific Institution Basal Research Fund (No. 1610212018005), and the Research start-up fund of Jiangsu University of science and technology (Nos. 1182931701, 1182931801, 1182932001, 1182931804).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Yaqi Li declares that there is no conflict of interest. Tao Yu declares that there is no conflict of interest. Jiyang Li declares that there is no conflict of interest. Dezhao Kong declares that there is no conflict of interest. Qiaoqiao Shi declares that there is no conflict of interest. Chang Liu declares that there is no conflict of interest. Chunwang Dong declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Yu, T., Li, J. et al. A Novel Fluorescent FRET Hairpin Probe Switch for aflD Gene Detection in Real Fermented Soybean Paste. Food Anal. Methods 14, 2469–2477 (2021). https://doi.org/10.1007/s12161-021-02080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02080-7