Abstract
The neomycin residues in milk could pose potential hazard for human health, and now, immunoassays are widely used for on-site screening of neomycin and other food contaminate residues. In this paper, the neomycin B was conjugated with carrier protein as immunogens, and a highly specific antibody was produced. Based on the produced antibody, an enzyme-linked immunosorbent assay (ELISA) and a new flow-through immunoaffinity chromatography test (FTIACT) were developed for rapid analysis of neomycin B residues in milk. The developed ELISA has a detection limit of 162.5 pg/mL in milk, which is suitable for detecting neomycin B residues at picogram levels, while the optimized FTIACT exhibited satisfactory compatibility with the ELISA, with limits of detection of 326.8 fg/mL in milk, which is more suitable for on-site detecting neomycin B residues at femtogram levels. For the quantitative FTIACT, the recoveries from spiked milk ranged from 69 to 131%, with a relative standard deviation of less than 15%. Compared to traditional ELISA formats, the developed FTIACT provided lower detection limits for neomycin B residue analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk is one of the most important foods for human beings; however, the dairy products are often contaminated by some antibiotics or mycotoxins (Han et al. 2017). These antibiotics and mycotoxins residues in milk could pose potential hazard for human health (Barreto et al. 2019). Of all these contaminates, neomycin is a common antibiotic residue found in milk (Siljanoski et al. 2018). Neomycin is a complex of water-soluble aminoglycoside antibiotics purified from the fermentation of the actinomycete Streptomyces fradiae, and it contains two or more amino sugars connected by glycosidic bonds. The widely used neomycin in veterinary medicine is composed of a number of related compounds, including neomycin A (also known as neamine, inactive degradation product of neomycin B and C), neomycin B (main component with the highest antibiotic activity) and neomycin C (neomycin B’s isomer) (Fig. 1), and the quantities of these components vary from lot-to-lot depending on the manufacturer and manufacturing process (Burkin and Galvidis 2011). The United States Pharmacopeia and European Pharmacopoeia measure neomycin B as the primary antibiotics for neomycin sulfate, with neomycin A and B as impurities (accounting for 5–15%) (EU Pharmacopia 2005; US Pharmacopeia 2006).
Similar to other aminoglycosides, neomycin B posed excellent activity against Gram-Negative bacteria, and also, it is partially effective against some Gram-Positive bacteria, which makes it has a variety of pharmaceutical applications in animal husbandry (Chen et al. 2008; Jin et al. 2006). However, the neomycin residues in foods of animal origin could cause some toxic effects on human health, such as potential ototoxicity and nephrotoxicity, which could cause the damage to the inner ear (Tian et al. 2015). Besides, the neomycin residues in the environment could cause adverse effects on the ecological environment. Furthermore, the unsuitable use of neomycin also has a relationship with the emergence of drug-resistant bacteria. Thus, the Ministry of Agriculture of China promulgated “maximum residue limits for veterinary drugs in foods” (GB 31,650–2019), and set the maximum residue limits (MRLs) for neomycin B to be 500 μg/kg in milk and goat milk (Chen et al. 2007).
Microbiological assays could qualitatively or semi-quantitatively determine the antibiotic residues based on the inhibitory effect of antibiotics on specific microorganisms (Wu et al. 2019). However, these methods could only determine the bioactive residues, and also it was time-consuming and could not distinguish neomycin from other related antibiotics (Gaudin et al. 2017). Due to the high polarity of aminoglycosides, hydrophilic interaction liquid chromatography had been adopted for neomycin residue analysis (Asakawa et al. 2018). The aminoglycoside antibiotics have weak ultraviolet absorption characteristics; thus, liquid chromatography with tandem mass spectrometry (LC–MS/MS) methods are widely used to quantify several aminoglycoside antibiotics without derivatization (Tölgyesi et al. 2018; Wang et al. 2018; Zhu et al. 2016). However, these applications are usable only on a laboratory scale, because the chromatographic methods require extensive sample preparation procedures and expensive equipment (Delatour et al. 2018). In contrast, immunological methods were widely used in the field of antibiotics residue analysis due to their simplicity, sensitivity, and high sample throughput (He et al. 2016; Xu et al. 2011). Recently, some researchers have reported the development of ELISA, lateral flow assays, and other immunoassay formats for the detection of the neomycin residues (Loomans et al. 2003; Shi et al. 2018; Wan et al. 2018; Wang et al. 2009; Zhu et al. 2010). In our previous research, anti-neomycin antibody was produced, and a chemiluminescent enzyme immunoassay was developed for rapid screening neomycin residues in milk (Luo et al. 2016). All these reports contribute greatly for the on-site screening purpose; however, it is more important to make some efforts on improving assay sensitivity of these immunoassays (Wang et al. 2016a).
In this study, the neomycin B was coupled with carrier protein to synthesize immunogens for monoclonal antibody production. Based on an established enzyme-linked immunosorbent assay (ELISA), a sensitive and reliable flow-through immunoaffinity chromatography test (FTIACT) was developed for detecting neomycin B residues in milk. In addition, a simple sample pre-treatment procedure was applied to eliminate the complicated food matrix effects, allowing the entire extraction procedure to be completed within 10 min. In contrast to ELISA, the developed FTIACT has a much lower detection limit, and it could be a feasible tool for detecting neomycin B residues in milk at femtogram levels.
Material and Methods
Chemicals and Apparatus
Neomycin B (96%), neomycin A (95%), and neomycin C (95%) were obtained from China Food and Drug Administration (Beijing, China). Bovine serum albumin (BSA), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, N-hydroxy succinimide, and ProClin™ 300 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-mouse IgG and peroxidase-conjugated goat-anti-mouse IgG (IgG-HRP) were purchased from Jackson Immuno-Research Laboratories Inc. (West Grove, PA, USA). CNBr-activated sepharose 4B was purchased from GE Healthcare (Pittsburgh, PA, USA). The 96-well polystyrene plates were obtained from Costar Co. (Milpitas, CA, USA). Bond Elut SPE cartridges (1 mL) and polyethylene frits were purchased from Agilent Technologies (Santa Clara, CA, USA). The biotech multi-mode microplate reader (Winooski, VT, USA) was used for absorbance measurement.
Artificial Antigen Preparation
The immunogens and HRP tracers were prepared by conjugating neomycin B with BSA and HRP through the carbodiimide method according to our previous reports (Jiang et al. 2017; Luo et al. 2016). Briefly, neomycin B (18 mg) was dissolved in 800 μL of N,N-dimethylformamide, and then, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (13 mg) and N-hydroxysuccinimide (8 mg) were added to the mixture. Afterwards, the reaction mixture was added to a BSA or HRP solution (10 mg dissolved in 4 mL of borate buffer) and reacted at room temperature for another 12 h. The synthesized immunogens or HRP tracers were dialyzed against 0.01 M phosphate-buffered saline (PBS) for 3 days, and then, the supernatant was dispensed and stored at − 20 °C after centrifugation.
Antibody Production
In this paper, female BALB/c mice were immunized using the synthesized immunogens for monoclonal antibody production. The first immunization was carried out by the immunogens emulsified with same volume of freund’s complete adjuvant at a dosage of 0.1 mg/mouse. The boost immunizations were administered with the same dosage of immunogens emulsified in freund’s incomplete adjuvant every 2 weeks. After the third immunization, the blood was collected, and the antibody titer was monitored by indirect ELISA. The mouse with the highest antibody titer was used for monoclonal antibody production according to our previous research (Jiang et al. 2016).
ELISA Development
The anti-neomycin antibody was diluted with an appropriate amount of the coating buffer. Then, 100 μL of the diluted antibody was added to each well of the microtiter plate, and the plates were incubated overnight at 4 °C. After discarding the remaining liquid in the well, the plates were blocked using 1% skim milk solutions for 1 h at room temperature. After another washing, 50 μL of neomycin standard solutions and 50 μL of diluted neomycin-HRP tracer solutions were pipetting into each well, and the microplate was incubated at 37 °C for 30 min. After washing, 100 μL of TMB substrates were pipetting into each well and the plates were incubated in the dark at 37 °C for 10 min. The absorbance of each well was measured at 450 nm after adding 50 μL of 2 M H2SO4 to each well to stop the enzymatic reaction.
ELISA Characterization and Real Sample Analysis
The calibration standard solutions were run in duplicate wells, and the average absorbance values of these standards were plotted against the analyte concentrations on a logarithmic scale. The calibration curves were fitted by a four-parameter logistic equation, from which IC50 values were calculated for sensitivity evaluation. In order to determine the specificity, a cross-reactivity study was carried out under optimum conditions. The cross-reactivity of the antibody against neomycin B and other cross-reactants was calculated using the following equation: cross-reactivity (%) = (IC50 of neomycin B)/(IC50 of analogs) × 100%
The milk samples were purchased from local supermarkets and stored at 4 °C until use. All samples were verified as free of neomycin B residues using a well-established LC–MS/MS method before the final validation (Arsand et al. 2016; Wang et al. 2016b). Then, 2.0 mL of milk sample was extracted using 0.04 mL of 0.36 M sodium nitroprusside and 0.04 mL of 1 M zinc sulfate. After centrifugation, the supernatant was diluted 10 times, and then tested by the developed ELISA.
FTIACT Development
The sepharose 4B gels were coupled with anti-neomycin and anti-HRP antibodies according to the instruction of the manufacturers and our previous research (Wang et al. 2016a). The anti-neomycin antibody was coupled onto the sepharose 4B gels to prepare the neomycin B detection gels, while the anti-HRP IgG was coupled with sepharose 4B gels to generate the control gels. The prepared gels were suspended in PBS containing 0.03% ProClin™ 300 as preservatives, and stored at 4 °C before use.
The FTIACT columns were setup by placing prepared gels on the solid phage extraction columns. Briefly, the first frit was placed on the bottom at the 1 mL Bond Elut reservoir, and 200 μL of anti-HRP gels were added to the column. The second frit was used to cover the control layers, and the third frit was added to the column to produce an air gap layer (about 0.3 cm). Then, 200 μL of anti-neomycin gels were added to the column above the third frit, and the fourth frit was used to cover the neomycin B detection layers. The prepared columns were stored at 4 °C until use.
FTIACT Characterization and Real Sample Analysis
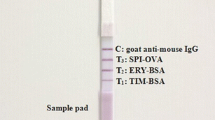
The milk sample was diluted with assay buffer, and then directly loaded onto the FTIACT columns for neomycin B residue analysis. In this study, 10 mL of the diluted milk samples were added to the FTIACT columns from the inlet at the speed of 1 drop/second. Then, 200 μL of diluted HRP tracers were loaded onto the FTIACT columns at the same speed. After that, the columns were washed by 6 mL of PBST solution in order to remove the unbound tracers. Finally, 200 μL of the chromogenic substrate were added, and the visual color was observed after incubation for 4 min. The result illustrations of the FTIACT were shown in Fig. 2 for the visual inspection of neomycin B residue. Furthermore, the images of the FTIACT were record, and the blue-color saturation values of test layers were evaluated with Adobe Photoshop CS6. Each detection layer was evaluated three times at different spots, and the saturation ratio (blue-color saturation of the detection layer with spiked samples/blue-color saturation of detection layer with blank samples, T/T0) was calculated for quantitative analysis.
Results and Discussion
Artificial Antigen Synthesis and Antibody Characterization
Neomycin is a small molecule hapten (molecule weight: 614.65 Da) without immunogenicity. It will acquire immunogenicity and cause a specific immune response in animals when neomycin was coupled to carrier proteins. In veterinary practice, a complex of neomycin A, neomycin B, and neomycin C was widely used as veterinary drugs for disease prevention and treatment. However, neomycin B was retained as the marker residue for surveillance purpose. The ratio of marker residue to total residues varied from lot-to-lot depending on the manufacturer and manufacturing process. Thus, most countries focus on detecting neomycin B, the most effective antibacterial component, residues in foods of animal origin. In this paper, we want to detect ultratrace neomycin B residues, and thus, the neomycin B was selected as target analyte for the conjugation with carrier proteins in order to improve assay performance.
The design of immunogens is one critical procedure on the production of highly sensitive antibody. Previous reports utilized carbodiimide method, N-hydroxy succinimide active ester method, mixed anhydride method and glutaraldehyde method to synthesize artificial antigen between the hapten and carrier proteins. In this paper, we utilized carbodiimide method to synthesize artificial antigens in this experiment, without introducing a spacer arm between the hapten and the carrier protein. The synthesized immunogens were determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, and the results showed qualitative differences between the carrier protein and artificial antigen, which indicated the successful coupling of the haptens with the carrier protein.
Different mice immunized with different immunogens could produce antibodies with different characters. In this paper, we focus on highly specific anti-neomycin B antibody production and ultra-sensitive immunoassay development. Thus, pure neomycin B was coupled with carrier proteins to synthesize immunogens for highly specific antibody production. The results in Table 1 indicated that the antibody produced in this paper indeed have different recognization rates with neomycin B and other neomycin isomers. In previous research , Loomans et al. utilized neamin (neomycin A) as haptens to synthesize immunogens for antibody production, and the produced antibody showed broad specificity with many aminoglycoside antibiotics because neamin share a common structure with neomycin, gentamicin, and kanamycin (Loomans et al. 2003). In most previous reports, the neomycin mixtures (5–15% neomycin A and C impurities exist in the mixtures) were utilized to synthesize immunogens for antibody production. Few researches focused on the relationship between the impurity of the neomycin complexes and immunoassay specificity. To overcome this disadvantage, we utilized pure neomycin B as standard to assess the sensitivity and specificity of the produced antibody. The results in Table 1 indicated that the produced antibody indeed showed different recognization rates with neamine, neomycin B, and its isomer. The produced antibody from the same coupling method has varied sensitivity and specificity due to the purities of neomycin B in the mixtures.
ELISA Development and Real Sample Analysis
After the third immunization, the serums were collected from immunized mice, and then tested by direct competitive ELISA. The mouse with high antibody titer was used for monoclonal antibody production and posterior immunoassay development. When the dilution of antibody and neomycin-HRP tracers were 1:2000 and 1:1000, the absorbance of the assay buffer was in the range of 1.5–2.0 at 450 nm. Furthermore, the inhibition rate was > 50% when using 1.0 ng/mL neomycin B as standard solutions.
The micro-environments of the assay buffer have predominant effects on the assay sensitivity. Thus, we optimized the pH and ionic strength of the assay buffer, and organic solvent tolerance based on our previous research (Jiang et al. 2016). Detail optimization information was given in the supplementary information. The calibration curves were then constructed by plotting absorbance (y axis) against neomycin B concentrations (x axis). As shown in Fig. 3, the IC50 was calculated to be 56.1 pg/mL, which indicated that the ELISA can measure extremely small amount of neomycin B residues. Furthermore, its working range was calculated to be from 19.4 pg/mL (IC20) to 155.5 pg/mL (IC80). In our previous report , we established a chemiluminescent immunoassay with the working range from 0.52 to 9.8 ng/mL (Luo et al. 2016), while Chen et al. and Wang et al. developed ELISAs with linear ranges from 2.5 to 20 ng/mL in milk, and from 25 to 500 ng/g in in food of animal origin, respectively (Chen et al. 2007; Wang et al. 2009). Burkin and Galvidis reported an indirect competitive enzyme immunoassay with a linear range from 0.1 to 100 ng/mL (Burkin and Galvidis 2011). In contrast, the ELISA developed in this study was more sensitive than these reports, and allowed detection of ultratrace neomycin B residues with a dynamic range of 19.4–155.5 pg/mL.
The specificity of the ELISA was assessed by the cross-reactivity against the analogs compared with neomycin under the same experimental conditions. Table 1 gives the IC50 values of other structurally related analytes and their cross-reactivity values. When the cross-reactivity with neomycin B was set as 100%, the cross-reactivity values with neomycin C and neomycin A were calculated to be 95.6% and 3.9%, respectively. The developed ELISA showed negligible cross-reactivity (< 0.1%) with other aminoglycoside antibiotics, and the results suggested that these analogs should not interfere with neomycin detection.
In this paper, the analytical performance of the ELISA was evaluated by investigating the limit of detection (LOD), the repeatability, and the reproducibility according to our previous researches (Li et al. 2021; Luo et al. 2016). Based on the average values measured from blank samples and their standard deviations, the LOD was calculated to be 162.5 pg/mL for neomycin B residues in milk samples. The Ministry of Agriculture of China set the MRLs to be 500 μg/kg, while the European Commission and Food and Agriculture Organization of the United Nations also set the MRLs to be 1500 μg/kg for cattle milk. The LOD obtained in this study was far below the MRLs set by these authorities.
A spike-and-recovery experiment was carried out to evaluate the accuracy, repeatability, and within-laboratory reproducibility. The blank samples were spiked at three concentration levels (200, 400, and 800 pg/mL), and the milk extracts were tested by the developed ELISA. As shown in Table 2, the average recoveries of the ELISA were in the range of 81–94%, and the coefficient of variances (CVs) ranged from 6.8 to 10.2%. These results indicate that ELISA has good effectiveness and applicability for neomycin B residue analysis.
FTIACT Development and Real Sample Analysis
The non-specific adsorption effects could greatly affect assay sensitivity of the FTIACT. In this study, the working concentration of the antibodies and neomycin-HRP tracers was optimized first, and dilutions of immune-reagents ranging from 1:1000 to 1:20,000 were tested for optimization. In consideration of color saturation development and non-specific adsorption effects, the anti-neomycin antibody’s and neomycin-HRP tracer’s dilution at 1:10,000 and 1:4000 was chosen, respectively. If the working concentrations of the antibodies and HRP tracers are higher than the selective level, the non-specific adsorption’s interference would affect the visual color development results, while the lower concentrations of antibodies and HRP tracers may lead to weak color development on the detection layers. In this paper, the sample addition, washing, and incubation process were optimized according to our previous research (Wang et al. 2016a), and detail information could be obtained in the supplementary information. After the addition of chromogenic substrate, we especially optimized the reaction time, which has great influences on the standard calibration curve generation. As shown in Fig. 4, the blue color saturation values of the detection layers and control layers increased when prolonged the colorimetric time, while the non-specific adsorption would affect the interpretation of the visual inspections when the colorimetric time was over 6 min. The colorimetric reaction at 4 min generated satisfying differences between the blank and spiked samples, and was chosen as optimal colorimetric time.
The food matrix interference is a common challenge for food contaminate residue analysis by immunoassays. These matrix interferences could affect the specific binding reaction between neomycin and anti-neomycin antibody, and thus maybe cause false positives by lowering color development. For milk samples, removal of interferences by addition of heavy metal salts for precipitation of macromolecular substances is an effective way to reduce the matrix effects (Jiang et al. 2015). In this paper, the raw milk, diluted milk, and milk extract were directly loaded onto the FTIACT columns for on-site screening of neomycin B residues in milk samples. The results in Fig. 5 indicated that the original milk sample could be directly analyzed by the developed FTIACT; however, some milk ingredients could supress the colorimetric reaction. The diluted milk and milk extract were also tested by the FTIACT, and the results in Fig. 5 indicated that they also obtained satisfying results. Considering the milk extract need utilizing the sodium nitroprusside and zinc sulphate to remove macromolecular substances with precipitation, we utilized diluted milk samples for neomycin B residue analysis, and the results in Fig. 5 also indicated that the matrix interference was reduced to an insignificant level.
Under above optimization, a series of spiked milk samples were diluted for calibration curves development to estimate the neomycin B residue in milk. As shown in Fig. 6, the color saturation of the detection layers fades when increasing analyte concentration in spiked milk samples. When the neomycin B concentration in milk samples was higher than the cut-off level (defined as qualitative LOD), the neomycin B residues could occupy enough binding sites of antibodies on the detection layers and lead to the color disappearance. From the results of Fig. 6, the qualitative LOD was 800 fg/mL for neomycin B. Furthermore, the probabilities of positive and negative results were calculated to evaluate the analytical performance of the FTIACT method. In this paper, 100 blank samples and 100 spiked samples (spiked with neomycin B at 800 fg/mL) were tested by the developed FTIACT, and the false-positive rate was calculated according to previous research (Trullols et al. 2004). As summarized in Table 3, the false-positive and false-negative rates were 2.5% and 1.5%, respectively. All these results fulfilled the requirements of a screening method (Community Reference Laboratories Residues 2010).
The above described FTIACT method could only provide a rough result through the visual observation with naked eyes, which could result in a relative low detection limit. In order to improve assay sensitivity, the color saturation levels of the test layers were obtained from the images according to previous research (Beloglazova et al. 2010). The standard curves were constructed using the standard concentrations as the abscissae and the T/T0 values as the ordinates, where T0 and T were the color intensity obtained from neomycin B detection layers with blank and spiked samples. A series of tests with spiked milk samples were conducted by the developed FTIACT method, and the color saturation level values are inversely proportional to the increasing analyte concentrations on the calibration curves in milk. A four-parameter logistic equation was used to fit the immunoassay data, and the calibration curves were shown in Fig. 6. In this paper, the quantitative LOD was defined as the concentrations that provided 80% T/T0 values, which was calculated to be 326.8 fg/mL. The quantitative LOD is far below the LOD of the developed ELISA (162.5 pg/mL) and the MRLs of the EU, the USA, and China. Comparing with observation by naked eyes, the quantitative FTIACT has higher resolution to screen ultratrace neomycin B contaminated samples. The spiking and recovery tests were used to evaluate the accuracy and precision, and the results showed good agreements between the spiking levels and the concentrations detected. As shown in Table 4, the average recoveries ranged from 69 to 131%, with CV values lower than 14.1%. In addition, two naturally contaminated milk samples were tested by the FTIACT and LC-MS/MS method for further validation. The neomycin B concentrations presented in the naturally contaminated milk samples were 18.6 and 26.8 ng/mL, while the developed FTIACT gave positive results by visual inspection, and the neomycin B concentrations were measured to be 20.5 and 32.6 ng/mL. These results indicated that the FTIACT was suitable for on-site screening of ultratrace neomycin B residues in milk.
Comparison of ELISA and FTIACT
Recently, some researches reported the anti-neomycin antibody production and ELISA development for neomycin residue analysis (Chen et al. 2007; Xu et al. 2011). Moreover, chemiluminescent enzyme immunoassay (Luo et al. 2016), lateral flow assay (Shi et al. 2018), and other immunoassay formats (Wan et al. 2018) also have been established for detecting neomycin residues in milk and other food matrices. In contrast to these reports, the FTIACT utilized sepharose 4B gels as immuno-reaction carriers to concentrate ultratrace amount of neomycin B residues in milk samples. The sepharose 4B gels were immobilized with anti-neomycin antibody and anti-HRP antibody in order to prepare the neomycin detection layer and control layer, respectively. Then, the neomycin B residues in milk samples could be captured by the highly specific antibody on the detection layer, while the neomycin-HRP tracers would be captured by the unbounded anti-neomycin antibody, and the excess neomycin-HRP tracers would be captured by the anti-HRP antibody on the control layer. After chromogenic substrate addition, the results can be observed by naked eyes from the color development on the detection layers. The negative results would present a visible blue color on the detection layer, while the positive results presented no or negligible color development.
Compared with traditional ELISA, the FTIACT had a larger sample-loading volume (10 mL in this study), which could greatly improve the assay sensitivity (form pg/mL to fg/mL). Although it could detect ultratrace amount of neomycin B residues, the developed FTIACT has a relative narrow working range. When the neomycin B concentration was higher than 1326.6 fg/mL or lower than 265.3 fg/mL in the milk sample, the FTIACT would not give an accurate result. In contrast to previous reports, we developed a much more sensitive FTIACT, and its analytical performances satisfied the requirements and regulations of the European Union, the USA, and China.
Conclusions
In this paper, neomycin B was selected as target analyte, and a high-sensitive antibody was produced by immunizing BALB/c mice. Based on the produced antibody, a sensitive ELISA and FTIACT method were developed for detecting ultratrace neomycin B residues in milk. The FTIACT had a much lower limit detection (326.8 fg/mL) than ELISA (162.5 pg/mL). In the real sample analysis, the results of the FTIACT were in good agreement with the well-established ELISA and LC–MS/MS method. In conclusion, the FTIACT method could inspect ultratrace neomycin B contamination at femtogram levels in milk, and it can be performed in any routine laboratory without the needs of special equipment.
References
Arsand JB, Jank L, Martins MT, Hoff RB, Barreto F, Pizzolato TM, Sirtori C (2016) Determination of aminoglycoside residues in milk and muscle based on a simple and fast extraction procedure followed by liquid chromatography coupled to tandem mass spectrometry and time of flight mass spectrometry. Talanta 154:38–45. https://doi.org/10.1016/j.talanta.2016.03.045
Asakawa D, Uemura M, Sakiyama T, Yamano T (2018) Sensitivity enhancement of aminoglycosides in hydrophilic interaction liquid chromatography with tandem mass spectrometry by post-column addition of trace sodium acetate in methanol. Food Addit Contam A 35:1116–1126. https://doi.org/10.1080/19440049.2017.1388543
Barreto F, Jank L, Castilhos T, Rau RB, Tomaszewski CA, Ribeiro C, Hillesheim DR (2019) Chemical residues and mycotoxins in raw milk. Raw Milk. Elsevier, Amsterdam, pp 273–293
Beloglazova NV, Goryacheva IY, Rusanova TY, Yurasov NA, Galve R, Marco MP, De Saeger S (2010) Gel-based immunotest for simultaneous detection of 2,4,6-trichlorophenol and ochratoxin A in red wine. Anal Chim Acta 672:3–8. https://doi.org/10.1016/j.aca.2010.05.024
Burkin M, Galvidis I (2011) Development and application of indirect competitive enzyme immunoassay for detection of neomycin in milk. Appl Biochem Micro. 47:321–326. https://doi.org/10.1134/S0003683811030045
Chen Y-Q, Shang Y-H, Wu X-P, Qi Y-T, Xiao X-L (2007) Enzyme-linked immunosorbent assay for the detection of neomycin in milk: effect of hapten heterology on assay sensitivity. Food Agr Immunol 18:117–128. https://doi.org/10.1080/09540100701579829
Chen Y, Shang Y, Li X, Wu X, Xiao X (2008) Development of an enzyme-linked immunoassay for the detection of gentamicin in swine tissues. Food Chem 108:304–309. https://doi.org/10.1016/j.foodchem.2007.10.022
Community Reference Laboratories Residues (2010) Guidelines for the validation of screening methods for residues of veterinary medicines (Initial validation and transfer). http://ec.europa.eu/food/food/chemicalsafety/residues/Guideline_Validation_Screening_en.pdf. Accessed 1 May 2021
Delatour T, Racault L, Bessaire T, Desmarchelier A (2018) Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit Contam A 35:633–646. https://doi.org/10.1080/19440049.2018.1426890
EU Pharmacopia (2005) Fifth Edition, Neomycin Sulphate. Section 0197. The Council of Europe; Strasbourg, France, 2005; www.pheur.org
Gaudin V, Rault A, Hedou C, Soumet C, Verdon E (2017) Strategies for the screening of antibiotic residues in eggs: comparison of the validation of the classical microbiological method with an immunobiosensor method. Food Addit Contam A 34:1510–1527. https://doi.org/10.1080/19440049.2017.1339331
Han R-W, Yu Z-N, Zhen T-Y, Wang J (2017) Survey of veterinary drug residues in raw milk in Hebei Province, China. J Food Protect 80:1890–1896. https://doi.org/10.4315/0362-028X.JFP-17-105
He J, Wang Y, Zhang X (2016) Preparation of artificial antigen and development of IgY-based indirect competitive ELISA for the detection of kanamycin residues. Food Anal Method 9:744–751. https://doi.org/10.1007/s12161-015-0248-x
Jiang W, Beloglazova NV, Wang Z, Jiang H, Wen K, de Saeger S, Luo P, Wu Y, Shen J (2015) Development of a multiplex flow-through immunoaffinity chromatography test for the on-site screening of 14 sulfonamide and 13 quinolone residues in milk. Biosens Bioelectron 66:124–128. https://doi.org/10.1016/j.bios.2014.11.004
Jiang W, Beier RC, Luo P, Zhai P, Wu N, Lin G, Wang X, Xu G (2016) Analysis of pirlimycin residues in beef muscle, milk, and honey by a biotin–streptavidin-amplified enzyme-linked immunosorbent assay. J Agr Food Chem 64:364–370. https://doi.org/10.1021/acs.jafc.5b05711
Jiang W, Beloglazova NV, Luo P, Guo P, Lin G, Wang X (2017) A dual-color quantum dots encoded frit-based immunoassay for visual detection of aflatoxin M1 and pirlimycin residues in milk. J Agr Food Chem 65:1822–1828. https://doi.org/10.1021/acs.jafc.6b05337
Jin Y, Jang J-W, Lee M-H, Han C-H (2006) Development of ELISA and immunochromatographic assay for the detection of neomycin. Clin Chim Acta 364:260–266. https://doi.org/10.1016/j.cca.2005.07.024
Li G, Liu C, Zhang X, Luo P, Lin G, Jiang W (2021) Highly photoluminescent carbon dots-based immunosensors for ultrasensitive detection of aflatoxin M1 residues in milk. Food Chem 355:129443. https://doi.org/10.1016/j.foodchem.2021.129443
Loomans EEMG, Van Wiltenburg J, Koets M, Van Amerongen A (2003) Neamin as an immunogen for the development of a generic ELISA detecting gentamicin, kanamycin, and neomycin in milk. J Agr Food Chem 51:587–593. https://doi.org/10.1021/jf020829s
Luo PJ, Zhang JB, Wang HL, Chen X, Wu N, Zhao YF, Wang XM, Zhang H, Zhang JY, Zhu L, Jiang WX (2016) Rapid and sensitive chemiluminescent enzyme immunoassay for the determination of neomycin residues in milk. Biomed Environ Sci 29:374–378. https://doi.org/10.3967/bes2016.048
Shi Q, Huang J, Sun Y, Deng R, Teng M, Li Q, Yang Y, Hu X, Zhang Z, Zhang G (2018) A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim Acta 185:84. https://doi.org/10.1007/s00604-017-2556-x
Siljanoski A, Ciglarič R, Pezdir T, Lainšček PR, Dolenc J, Starič J, Šinigoj-Gačnik K (2018) Detection of tetracycline and other antimicrobial residues in milk from cows with clinical mastitis treated by combination therapy. J Dairy Res 85:321–326. https://doi.org/10.1017/S0022029918000389
Tian Y-F, Chen G-H, Guo L-H, Guo X, Mei X-Y (2015) Methodology studies on detection of aminoglycoside residues. Food Anal Method 8:1842–1857. https://doi.org/10.1007/s12161-014-0067-5
Tölgyesi Á, Barta E, Sohn M, Sharma VK (2018) Determination of antimicrobial residues in honey by liquid chromatography tandem mass spectrometry. Food Anal Method 11:2043–2055. https://doi.org/10.1007/s12161-018-1166-5
Trullols E, Ruisánchez I, Rius FX (2004) Validation of qualitative analytical methods. TrAC Trends Anal Chem 23:137–145. https://doi.org/10.1016/S0165-9936(04)00201-8
US Pharmacopeia (2006) The National Formulary. Neomycin Sulfate. USP 29, NF 24, 2006, p. 1491
Wan Y-C, Liu Y-J, Liu C, Ma H-T, Yu H-F, Kang J-W, Gao C-L, Wu Z-Q, Zheng D, Lu B (2018) Rapid determination of neomycin in biological samples using fluorescent sensor based on quantum dots with doubly selective binding sites. J Pharmaceut Biomed 154:75–84. https://doi.org/10.1016/j.jpba.2018.02.028
Wang S, Xu B, Zhang Y, He J (2009) Development of enzyme-linked immunosorbent assay (ELISA) for the detection of neomycin residues in pig muscle, chicken muscle, egg, fish, milk and kidney. Meat Sci 82:53–58. https://doi.org/10.1016/j.meatsci.2008.12.003
Wang X, Luo P, Chen J, Huang Y, Jiang W (2016a) Development of a quantitative immuno-affinity test column assay for on-site screening of clindamycin residues in milk. Int Dairy J 55:59–63. https://doi.org/10.1016/j.idairyj.2015.12.002
Wang Y, Li S, Zhang F, Lu Y, Yang B, Zhang F, Liang X (2016b) Study of matrix effects for liquid chromatography–electrospray ionization tandem mass spectrometric analysis of 4 aminoglycosides residues in milk. J Chromatogr A 1437:8–14. https://doi.org/10.1016/j.chroma.2016.02.003
Wang X, Yang S, Li Y, Zhang J, Jin Y, Zhao W, Zhang Y, Huang J, Wang P, Wu C, Zhou J (2018) Optimization and application of parallel solid-phase extraction coupled with ultra-high performance liquid chromatography–tandem mass spectrometry for the determination of 11 aminoglycoside residues in honey and royal jelly. J Chromatogr A 1542:28–36. https://doi.org/10.1016/j.chroma.2018.02.029
Wu Q, Peng D, Liu Q, Shabbir MAB, Sajid A, Liu Z, Wang Y, Yuan Z (2019) A novel microbiological method in microtiter plates for screening seven kinds of widely used antibiotics residues in milk, chicken egg and honey. Front Microbiol 10:436. https://doi.org/10.3389/fmicb.2019.00436
Xu N, Qu C, Ma W, Xu L, Xu L, Liu L, Kuang H, Xu C (2011) Development and application of one-step ELISA for the detection of neomycin in milk. Food Agr Immunol 22:259–269. https://doi.org/10.1080/09540105.2011.569882
Zhu Y, Son JI, Shim Y-B (2010) Amplification strategy based on gold nanoparticle-decorated carbon nanotubes for neomycin immunosensors. Biosens Bioelectron 26:1002–1008. https://doi.org/10.1016/j.bios.2010.08.023
Zhu Z, Liu G, Wang F, Sasanya JJ, Cannavan A (2016) Development of a liquid chromatography tandem mass spectrometric method for simultaneous determination of 15 aminoglycoside residues in porcine tissues. Food Anal Method 9:2587–2599. https://doi.org/10.1007/s12161-016-0446-1
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31972763 and No. 31602103), the Natural Science Foundation of Guangdong Province, China (No. 2019A1515011834), the Science and Technology Planning Project of Guangdong Province, China (No. 2017B020207009), the Shenzhen Basic Research Projects (No. JCYJ20190808160003705), the Sanming Project of Medicine in Shenzhen (No. SZSM201611068), and the medical young scientists program in Shenzhen University.
Author information
Authors and Affiliations
Contributions
Chen Liu: project administration and formal analysis; Yanliang Jiang: project administration and writing—original draft; Linyun Xiu: project administration; Ruijun Qian: formal analysis; Mengxi Zhao: project administration; Pengjie Luo: validation; Yuebin Ke: methodology; Guangming Li: conceptualization; Wenxiao Jiang: funding acquisition, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Conflict of Interest
Chen Liu declares that she has no conflict of interest. Yanliang Jiang declares that he has no conflict of interest. Linyun Xiu declares that she has no conflict of interest. Ruijun Qian declares that she has no conflict of interest. Mengxi Zhao declares that she has no conflict of interest. Pengjie Luo declares that he has no conflict of interest. Yuebin Ke declares that he has no conflict of interest. Guangming Li declares that he has no conflict of interest. Wenxiao Jiang declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 320 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Jiang, Y., Xiu, L. et al. Ultratrace Analysis of Neomycin Residues in Milk at Femtogram Levels by Flow-Through Immunoaffinity Chromatography Test. Food Anal. Methods 14, 2298–2307 (2021). https://doi.org/10.1007/s12161-021-02058-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02058-5