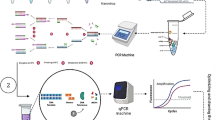
Abstract
Quantification of viable lactic acid bacteria is an important quality index of fermented dairy products, but the current detection method, which is plate counting, is time-consuming. This study describes a modified PMA-qPCR assay that allows the detection and enumeration of total viable Lactobacillus spp. in commercial fermented milk products. Here, a new pair of genus-specific primers and minor groove binder probes was designed based on the 16S rRNA gene sequences of 24 reference strains, 13 of which were from different Lactobacillus species commonly used in commercial fermented dairy products. Subsequently, compared with the heat killing method, the 20-cycle homogenization method was chosen for initial PMA-qPCR optimization. Standard curves were prepared, and the efficiency obtained was 97% (R2 = 0.992), demonstrating that this PMA-qPCR method was feasible and effective. Additionally, the viable Lactobacillus spp. detection range was 103–109 CFU/mL, which meets the detection requirements in fermented dairy products. Furthermore, total number of viable Lactobacillus spp. in six different commercial fermented dairy products was estimated by three individual methods (plate count in MRS agar, qPCR, and PMA-qPCR). Comparison of results obtained by PMA-qPCR and plate counts was similar, and both decreased accordingly, showing a good correlation with the decline of strain vitality, 30 days after the expiration date. The PMA-qPCR procedure established in this paper allows the quantification of viable Lactobacillus spp. in approximately 3 h, greatly improving detection efficiency, and has broad application prospects in the testing of fermented dairy products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fermented dairy products are produced by lactic acid bacteria fermentation and are known to have health effects on the human body which are widely welcomed by consumers worldwide (Gao et al. 2019; Casas and Dobrogosz 2000; Jones and Versalovic 2009). Studies have shown that lactic acid bacteria can play a probiotic role only when they reach a certain number [≥1×106 CFU/g (mL)] (R. Tabasco et al. 2007). In addition, the quantity of viable bacteria in fermented dairy products directly affects the quality of the products. Therefore, fermented dairy products have defined product standards for the quantity of lactic acid bacteria. The International Dairy Foods Association (IDFA) clearly stipulates that the number of live lactic acid bacteria should be more than 1×107 CFU/mL (CODEX STAN 243-2003, Codex Standard For Fermented Milks). The current QB1554 Lactobacillus Beverage Industry Standard and GB16321 Lactobacillus Beverage Hygiene Standard both stipulate that the number of live lactic acid bacteria should be more than 1 × 106 CFU/mL at the time of leaving the factory, and live bacteria are detected at the time of sale. At present, the quantification of lactic acid bacteria is mainly by plate isolation and culture method. Although this method is simple to perform, it is laborious and time-consuming (48–72 h) (García-Cayuela et al. 2009). Therefore, a fast and effective detection method for lactic acid bacteria in fermented milk is conducive to speeding up the detection time, prolonging the shelf life of products, and thus improving the profit of enterprises. Likewise, rapid and effective detection methods also provide more favorable conditions for product quality supervision.
Recently, in the fields of biology, environmental science, and food science, real-time quantitative PCR (qPCR) estimation assays of microorganisms have been reported (Ahmed et al. 2018; Akyol 2018; Frentzel et al. 2018; Portilho et al. 2018; Wan et al. 2018). However, the application of qPCR for the quantitative detection of lactic acid bacteria in fermented dairy products is limited due to its inability to distinguish between viable and non-viable cells (Fittipaldi et al. 2012; Millette et al. 2007). The novel nucleic acid–intercalating dye named propidium monoazide (PMA) can covalently cross-link with DNA under strong visible light, thus inhibiting the PCR amplification of the DNA (Scariot et al. 2018). Consequently, DNA from membrane-intact cells is selectively detected by the real-time quantitative PCR coupled with propidium monoazide (PMA-qPCR) method. Coincidentally, PMA-qPCR is not only used to detect living microorganisms in the samples (Banihashemi et al. 2017; Fan et al. 2017; Liu et al. 2017; Truchado et al. 2015), but also has been utilized in the detection of lactic acid bacteria in fermented dairy products (García-Cayuela et al. 2009; Padilha et al. 2016; Scariot et al. 2018; Shao et al. 2016). However, at present, the use of PMA-qPCR technology for the detection of lactic acid bacteria in fermented dairy products is mainly focused on the quantitative determination of a single strain.
There are many kinds of lactic acid bacteria in fermented dairy products. The current detection standard for lactic acid bacteria in fermented milk is for the quantification of Lactobacillus spp., Streptococcus thermophilus, and Bifidobacterium spp. In this paper, we focused on the quantification of total viable Lactobacillus spp. in fermented dairy products. Different Lactobacillus spp. are often used as a starter strain for yogurt, cheese, and lactobacillus beverages (Morales Villarreal et al. 2013), such as L. delbrueckii subsp. bulgaricus, L. casei, and L. paracasei. On the other hand, with the extensive research on the probiotics, many lactobacilli have also been added to fermented dairy products as probiotics, such as L. acidophilus, L. rhamnosus, and L. plantarum, which are research and development hotspots for fermented dairy products. In addition, compared with Streptococcus thermophilus and Bifidobacterium spp., the quantification of Lactobacillus spp. is more difficult due to the variety of lactobacilli. Therefore, the method established in this study can provide more favorable support for the quantification of lactic acid bacteria in fermented dairy products.
Here, we circumvent the above problem by designing a new pair of universal primers with probes for all Lactobacillus spp. present in fermented dairy products. In order to obtain dead cells of Lactobacillus spp. for the working curve of PMA-qPCR, two different killing methods, heating and homogenization treatment, were compared in this study. Results of the presented study confirm that the optimized PMA-qPCR method can be used for the estimation of total viable Lactobacillus spp. in fermented milk products. These results pave the way for further improvement of rapid and efficient determination of total viable lactic acid bacteria in fermented milk products.
Materials and Methods
Microorganisms and Culture Conditions
Bacterial strains and culture media used in this study are listed in Table 1. Bacteria requiring anaerobiosis for growth were cultured at 37 °C in an anaerobic chamber (the AnaeroPack system, Mitsubishi Gas Chemical, H2: 5%, CO2: 10%, N2: 85%). Man-Rogosa-Sharpe (MRS) broth was purchased from BIOKAR Diagnostics, and Luria broth was purchased from Difco (BD DIFCO, Kansas, USA).
Species-Specific General Primers and Probes Designed for Lactobacillus spp.
The primers (PMA-F: 5′-CGAGGAACTGCATCGGAAAC-3′; PMA-R: 5′-CTTCGCCACTGGTGTTCTTC-3′, 140 bp) and probes (CTTGAGTGCAGAAGAG) used to detect Lactobacillus spp. were based on 16S rRNA gene sequences retrieved from the National Center for Biotechnology Information databases (http://www.ncbi.nim.nih.gov). The Lactobacillus spp. sequences were aligned with sequences from 24 reference strains (13 of them were different species of Lactobacillus, which are commonly used in fermented dairy products) listed in Table 1 by means of the ClustalW program from the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw.htm). The pair of primers and probes designed in this study was applicable for these species used in the fermented dairy products, not for the entire Lactobacillus genera. The specificity of these oligonucleotide sequences was checked by the NCBI BLAST database and the ClustalW program. Primers and the TaqMan MGB probes in this study were manufactured by TAKARA Biotech (TaKaRa Ltd., Dalian, China).
DNA Extraction
In this study, total genomic DNA were extracted using the phenol-chloroform method from the cultures of lactobacilli or fermented dairy products samples (Yanlin et al. 2017). The samples were pre-mixed with glass bead (1:1) and disrupted by Bioprep-24 for 30 s at a speed setting of 6.0 m/s. These pre-treated samples, mixed with equal volume of water saturated phenol solution, were centrifuged at 12,000×g at room temperature for 10 min, respectively. These supernatants, also mixed with equal volume of chloroform/isoamyl alcohol (24: 1) solution, were centrifuged at 12,000×g for 10 min, respectively. The supernatant was added with 0.6 volume of isopropanol for DNA precipitation, and the precipitate was washed twice with 1 mL of 75% ethanol and dried at room temperature. The DNA precipitate was finally dissolved in 50 μL of ultra-pure water with added 0.5 μL RNAse (10 mg/mL).
Quantitative qPCR Analysis of Lactobacillus spp.
Purified DNA were serially diluted to a final concentrate 60 ng/μL, as described by Jean-Marc et al. (2006). One microliter of each dilution was subjected to the qPCR assay on an ABI 7500 Fast real-time PCR system with 2.0.1 version software. The reaction mix contained 1.0 μL of DNA, 0.3 μM each of primers, 0.2 μM TaqMan MGB probe, and 10 μL of 1×TaqMan Universal PCR Master Mix (TaKaRa Ltd., Dalian, China). The thermal cycle program was as follows: 95 °C for 30 s, 95 °C for 5 s, followed by 40 cycles of 58 °C for 30 s and 72 °C for 30 s. The Lactobacillus spp. fluorescent probe was labeled at its 5′ end with the reporter dye 6-carboxyfluorescein (FAM), and the MGB fluorescent probes with nonfluorescent quencher dyes were used (TaKaRa Ltd., Dalian, China). Each assay was performed in duplicate in the same run. The cycle threshold (Ct) was calculated as the cycle number at which the reaction became exponential. The cycle threshold of each sample was then compared to a standard curve made by diluting genomic DNA (10-fold serial dilution) from cultures of pure or mixture lactobacilli. Meanwhile, cell counts before DNA extraction were determined, as control.
Specificity and Sensitivity of qPCR
The specificity of the PCR assays was confirmed by using other lactic acid bacteria (five species) or contaminated microorganisms (six species) that may appear in fermented dairy products as controls, listed in Table 1. To determine the sensitivity of our qPCR assays, 10-fold serial dilutions (103–109 CFU/mL) of target DNA extracted from pure cultures of five different Lactobacillus species (L. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) were performed. Subsequently, the DNA of these five different lactobacilli were also mixed in equal proportion and used for qPCR verification in order to quantify the lactobacilli in a more complex environment.
Killing Method for Obtaining the Dead Cells of Lactobacillus spp.
In order to obtain dead cells of Lactobacillus spp. to produce the standard curve for PMA-qPCR, two different killing methods, heating and homogenization treatment, respectively, were compared in this study. Totally, lactobacilli from five different Lactobacillus species (L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) in Table 1 were selected for heating treatment, homogenization treatment, and subsequent PMA treatment. Each of the pure strains was cultured in MRS broth at 37 °C until the OD600 value reaches up to 1.0, respectively.
Three kinds of heating conditions, 80 °C for 10 min (Fujimoto et al. 2011), 100 °C for 30 min (Lai et al. 2016), and 121 °C for 20 min (García-Cayuela et al. 2009), were used for heat killing (Vondrakova et al. 2017), respectively. Untreated bacterial suspension was used as control group. In addition, after centrifugation of each bacterial solution, each bacterial solution was also suspended in an equal amount of 10% reconstituted skim milk containing 0.8% casein acid hydrolysate, respectively, and the bacterial suspension was homogenized with glass bead by Bioprep-24 (Allsheng Instruments Co., Ltd, Hangzhou, China). The condition of homogenization was as follows: speed 6.0 m/s, working time 30 s, and interval 30 s. The number of homogenization cycles per each sample is 0, 5, 10, and 20 times, respectively. Subsequently, each treated sample and the control group were divided into two parts for subsequent verification of PMA-qPCR and MRS agar plate counting, respectively.
PMA Treatment for PMA-qPCR
Lactobacillus culture samples and the commercial fermented dairy products samples were adjusted to pH 6.5 with 1 M NaOH, and then casein micelles were dispersed by addition of 1 M trisodium citrate (García-Cayuela et al. 2009). Cells were resuspended in 500 μL PBS, after the sample was centrifuged at 12,000×g for 10 min and washed with PBS. The PMA (Biotium Inc., Hayward, CA, USA) was dissolved in 20% dimethyl sulfoxide (DMSO) and added to 500 μL of cell suspensions to achieve final concentrations of 40 μg /mL (García-Cayuela et al. 2009; Millette et al. 2007; Scariot et al. 2018). The mixed samples were then placed in the dark for 5 min to allow PMA to penetrate into dead cells and bind to the DNA (Zhou et al. 2019). The treated samples were placed on ice and exposed to a 650 W halogen light source at a 15 cm distance for 15 min (Millette et al. 2007; Scariot et al. 2018). After PMA treatment, cell suspensions of lactobacilli or fermented dairy products samples were subjected to DNA extraction and qPCR assay described above.
Construction of PMA-qPCR Standard Curve
Lactobacilli from five different Lactobacillus species (L. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) were mixed proportionally to obtain the viable lactobacilli cell samples, and the dead lactobacilli cell samples were obtained after the homogenization (30 s at 6.0 m/s, stop 30 s, 20 cycles) and PMA pretreatment (PMA final concentration 40 μg/mL, light exposure for 15 min) (Brescia et al. 2009). Subsequently, the DNA samples from the dead lactobacilli were used to dilute the DNA samples of viable lactobacilli, so that the DNA samples of viable lactobacilli with a 10-fold gradient (103-109 CFU /mL) were finally obtained. All these treated samples of lactobacilli were determined by PMA-qPCR, and cell counts before homogenization were determined, as control. The standard curve was obtained by plotting the cycle threshold (Ct) value against the corresponding logarithm of the concentration of the viable lactobacilli.
Lactobacillus spp. Enumeration by PMA-qPCR, qPCR, and Plate Counting
To verify that PMA method can be used to detect the number of viable Lactobacillus spp. in actual samples, the total number of the lactobacilli in six different commercial fermented dairy product samples (containing different species of lactic acid bacteria, more details in Table 2) was estimated by plate count in MRS agar, qPCR, and PMA-qPCR, respectively. The quantity of viable Lactobacillus spp. was determined in these six different commercial product samples stored at 4 °C, after 0 day, and 30 days of the shelf life of these products, respectively.
Statistical Analysis
The data in this study were analyzed using IBM SPSS Statistics version 21.0. T-test was used to determine statistically significant differences between the counts derived from PMA-qPCR, qPCR, and plate counts. All analyses were performed at least in triplicate, values were presented as means ± SD, and P values < 0.05 were considered statistically significant.
Results
Specificity and Sensitivity of the Novel General Primers and Probes for Lactobacillus spp.
Lactic acid bacteria in fermented dairy products mainly include Lactobacillus spp., Streptococcus thermophilus, Bifidobacterium spp., and some Lactococcus spp. Among them, the Lactobacillus species are the most diverse. To establish a method for the detection of the total viable Lactobacillus spp. in fermented milk products, pairs of general primers and probes were designed. Twenty-four bacterial strains were selected as references for primer design: 13 of them were different species of Lactobacillus, which are commonly used in fermented dairy products; five of them were other lactic acid bacteria in fermented milk, such as Streptococcus thermophilus, Bifidobacterium spp., and Lactococcus spp.; and the other six strains are microorganisms commonly found in contaminated foods. The latter two groups used as reference strains were identified to be non-target microorganism controls. Primer specificity was first evaluated to be accurate through in silico assay by a primer-BLAST search against the NCBI nr database. Subsequently, qPCR was performed to analyze the 24 reference strains (Table 1). The results indicated that the Ct value of all the reference Lactobacillus spp. ranged between 17.59 and 18.92. Moreover, no cross reaction was observed with any of the non-target microorganisms (Table 1). Taken together, these findings indicate that the newly designed qPCR general primers and probes for Lactobacillus spp. were positive and specific for the corresponding target species.
The sensitivities of the novel general primers and probes were calculated from Ct value—DNA concentration standard curves derived from serially diluted DNA samples (Ct values of 10-fold serial DNA dilutions) from five Lactobacillus species most commonly used in commercial fermented dairy products. The slope of the linear equation between the Ct values of individual strains and the logarithm of the number of viable bacteria is −3.11 to −3.52, and R2 is 0.9915–0.9998 (Table 3), which is consistent with the amplification efficiency of existing primers for Lactobacillus spp. in fermented milk. In addition, a standard curve was also constructed based on the average Ct values of the 10-fold serial dilutions of the target DNA extracted from an equal proportion mixture of the five lactobacilli. Results thus show that the qPCR assay is exponential over a broad dynamic range, from 103 to at least 109 CFU. Meanwhile, the generated standard curves presented a suitable linear correlation coefficient (R2 = 0.997) and mean efficiency of 101% (Fig. 1), suggesting that the new general primers and probes also have good sensitivity and can be used for the detection of Lactobacillus spp. in fermented dairy products.
Sensitivity of the new general primer and probe for quantification of viable Lactobacillus spp. by qPCR. The standard curve was constructed based on the average Ct values of the 10-fold serial dilutions of target DNA extracted from an equal proportion mixture of these five lactobacilli (L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) and the logarithm of the concentration of these five viable lactobacilli. Each bar represents the average Ct value from triplicate assay
Homogenization Is More Suitable for PMA-qPCR in Detection of Lactobacillus spp.
When samples of bacterial culture were treated with PMA, this intercalating dye can covalently cross-link with DNA under strong visible light, thus inhibiting the PCR amplification. Efficacy of this treatment, however, depends on bacterial membrane permeability. The standard curve for PMA-qPCR was done by mixing dead and viable bacterial cells proportionally and then performing PCR after PMA treatment. Therefore, a killing assay was performed, evaluating the impact of two cell inactivation techniques (heating and homogenization) on PMA-qPCR results.
Five tested lactobacilli were subjected to two inactivation procedures where all cells were killed, as confirmed by suspension spreading on agar plates. As shown in Fig. 2a, heating at 100 °C for 30 min and at 121 °C for 20 min effectively killed all the cells of the five tested lactobacilli; however, some L. plantarum and L. casei were able to survive under heating at 80 °C for 10 min. In parallel, 15–20 homogenization cycles (Fig. 2c) were enough to bring about total death of these five tested lactobacilli. Compared with the high temperature heating method, homogenization is easier to perform.
Two quantification methods (plate counting and PMA-qPCR) were used to detect the number of viable Lactobacillus spp. treated by two different killing methods (heating and homogenization treatment). The inactivation effects of three different heating conditions (80 °C for 10 min, 100 °C for 30 min, and 121 °C for 20 min) on five lactobacilli (L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) were detected by plate counting (a) and PMA-qPCR (b). The inactivation effects of different homogenization times (5, 10, 15, and 20 cycles) on these five lactobacilli were also detected by plate counting (c) and PMA-qPCR (d). Results represent the mean value of three repeats ± SD
The effect of inactivation methods on PMA-qPCR efficacy, particularly on qPCR signal reduction, and the reduction power of PMA treatment were evaluated. To assess the effect of killing methods on PMA-qPCR efficacy, the difference between the Ct values of untreated and PMA-treated samples containing dead cells (ΔCt) was determined. Although all the cells of the five tested lactobacilli were killed, which was confirmed by spreading the suspension on agar plates, the ΔCt values of the different procedures was different. The 20-cycle homogenization process generated the highest ΔCt value (> 9, Fig. 2d) for each Lactobacillus. The qPCR signal was reduced significantly for killing methods by 100 °C for 30 min and 121 °C for 20 min (Fig. 2b), suggesting that DNA may be damaged by prolonged treatment at high temperatures, which is not conducive for subsequent PCR amplification. Moreover, the ΔCt values of each Lactobacillus killed by the same heating procedure were also different from each other, indicating that various species have different membrane permeability, which may be related to individual surface structure. Taken together, the 20-cycle homogenization was chosen for initial PMA-qPCR optimization.
Quantification of Viable Lactobacillus spp. by PMA-qPCR
To establish a standard curve for the quantitative estimation of viable Lactobacillus spp. using PMA-qPCR, graph plots of Ct vs. the logarithm value of the number of viable cells were generated using solutions with known DNA concentrations from viable Lactobacillus cells. This was carried out in parallel with plate counting and qPCR-based estimations of viable cells. The results revealed that the PMA-qPCR method generates a standard curve with a linear correlation coefficient (R2 = 0.992) and has a mean efficiency of 97 % (Fig. 3), which are consistent with the results of the previous studies (Tong and Fang 2006). Furthermore, the sensitivity of viable cell detection of the novel PMA-qPCR method lies in the currently well-accepted range of 103–109 CFU /mL (Fig. 3). This novel PMA-qPCR method was further used for detection and enumeration of the total viable Lactobacillus spp. in fermented dairy products.
Standard curves of PMA-qPCR assay for detection of viable Lactobacillus spp. (L. delbrueckii subsp. bulgaricus, L. casei, L. acidophilus, L. plantarum, and L. paracasei) in a certain proportion of viable/dead cell mixed samples. Combined with the plate counts and qPCR detection, the standard curve was obtained by plotting the Ct value against the logarithm of the number of the viable bacteria. Each bar represents the average Ct value from triplicate assay.
Quantification of Viable Lactobacillus spp. in Fermented Dairy Products
The effectiveness of this novel PMA-qPCR method for the quantification of viable Lactobacillus spp. in actual commercial fermented dairy products was tested. In order to do so, the total viable Lactobacillus spp. in six different fermented dairy products were estimated by three individual methods (plate count in MRS agar, qPCR, and PMA-qPCR). When tested well within the shelf life, the total number of viable Lactobacillus spp. of each product detected by the three individual methods showed no significant difference (P > 0.05, Fig. 4). However, the viability of Lactobacillus spp. in all fermented dairy products tested was affected 30 days after the expiration date (while being preserved at 4 °C). Comparison of results obtained by PMA-qPCR and plate counts was similar, and both decreased after the expiration date, showing good correlation with the decline of strain viability. When compared, the difference between the Ct values obtained by qPCR and PMA-qPCR increased with post-expiration date storage time. The significant difference (P < 0.05) between the qPCR and PMA-qPCR methods in enumerating viable bacteria can be explained by the fact that PMA treatment can distinguish between live and dead bacteria based on membrane integrity, leading to inaccurate detection results for the former. It is worth noting that the PMA-qPCR procedure established in this paper allows for the specific detection and enumeration of viable Lactobacillus spp. in fermented dairy products in about 3 h, while the plate counting method takes 72 h, which greatly improves the detection efficiency and has broad application prospects in the testing of fermented dairy products.
Three individual methods (plate count in MRS agar, qPCR, and PMA-qPCR) were used to detect the number of viable Lactobacillus spp. in six different commercial fermented milk products with two different storage periods (4°C, shelf life, 30 days after the expiration date). Abbreviations of the different commercial fermented milk products (A, B, C, D, E, and F) refer to Table 2. Results represent the mean value of three repeats ± SD. Values with different superscripts for the same group are significantly different (P < 0.05)
Discussion
At present, PMA-qPCR method is used for the individual detection of viable lactic acid bacteria, such as S. thermophilus, L. paracasei, L. delbrueckii subsp. bulgaricus, and Bifidobacterium Bb-12 in fermented dairy products (García-Cayuela et al. 2009). To overcome this limitation, in this study, we have designed a new pair of sensitive and specific universal primers and probes for the detection of total Lactobacillus spp. present in commercial fermented dairy products. We show that the new general primers and probes designed for Lactobacillus spp. can effectively amplify Lactobacillus spp. sequences specifically (Table 1). Compared with the primers specific to individual lactobacilli, our new universal primers show better applicability in terms of primer specificity and sensitivity. Moreover, they can detect various commercially available Lactobacillus spp. and matches the accuracy of the qPCR method, which is a method of choice in detecting Lactobacillus spp. present in a wide variety of commercial fermented dairy products.
PMA-qPCR can effectively distinguish viable and dead cells. To evaluate the feasibility and practicability of the assay, it is important to first establish a standard curve. Here, to quantify the number of viable Lactobacillus spp. in different samples, the standard curve of Ct value vs. logarithm of the concentration of the viable lactobacilli was plotted using DNA from known numbers of viable cells. The preparation of membrane-damaged bacteria is a key factor for obtaining the standard curve using PMA-qPCR method, since it directly affects the binding efficiency of PMA to DNA. At present, heat killing method is a commonly used (Cattani et al. 2016; García-Cayuela et al. 2009; Scariot et al. 2018; Shao et al. 2016; Vondrakova et al. 2017; Zhang et al. 2015; Zhou et al. 2019). However, considering that Lactobacillus spp. is a gram-positive bacterium with a dense peptidoglycan layer with a variety in surface structure among species (Bottari et al. 2017; Lai et al. 2016), the method of heat killing may not be suitable for preparing the dead lactobacilli cells. Expectedly, heating at 80 °C for 10 min was not enough to completely kill two strains of Lactobacillus spp., viz., L. plantarum and L. casei (Fig. 2a). This, in agreement with previous studies (Bottari et al. 2017), indicates that different species of the Lactobacillus genera have varying sensitivity to heat. Additionally, although the effect of different heating conditions on subsequent DNA amplification is not obvious, the effect of PMA treatment is significant. The ΔCt values of five lactobacilli under three different heat killing conditions after PMA treatment were inconsistent. Except for L. plantarum and L. casei, post-PMA treatment ΔCt values of the lactobacilli heat-killed at 121 °C was significantly reduced compared with those heat-killed at 100 °C (Fig. 2b). Thus, high temperature treatment may affect the binding of DNA and PMA and in turn may affect the accuracy of estimation (Lai et al. 2016). This factor has been overlooked in the previous studies. Contrary to heat lethality, the killing method by homogenization (> 15 cycles, Fig. 2c) established in this study could completely kill the five tested lactobacilli. These results suggest that homogenization is a very effective method. Moreover, with the homogenization killing method, the post-PMA treatment ΔCT values decreased significantly. Furthermore, there is no significant difference between the post-PMA treatment ΔCT values of different species of Lactobacillus spp. (P > 0.05, Fig. 2d). Thus, estimation by PMA-qPCR method was more accurate and reliable in samples treated with 20-cycle homogenization. Taken together, unlike the heat killing method, which is the method of choice for the disruption of gram-positive cells and affects estimation accuracy due to negative effects of high temperature on the binding of PMA and DNA (Nocker et al. 2006; Rudi et al. 2005; Tabasco et al. 2007), homogenization showed better reliability and lethality, helping in the precise estimation of Lactobacillus spp.
It is well known that an accurate standard curve can aid in the accurate estimation of viable cells using PMA-qPCR. In acquiring the standard curve for PMA-qPCR detection method, samples with known proportions of viable and dead cells are needed (Nocker et al. 2006). This was accomplished in this study by standardizing the homogenization killing method for Lactobacillus spp., PMA concentration, and the light exposure time for PMA-DNA binding. From literature, it is known that the R2 value of a suitable standard curve should be higher than 0.95, the slopes of the linear regression equation should be between −3.9 and −3.0, and the corresponding amplification efficiency should be between 80 and 115% (Tong and Fang 2006). The results obtained here indicate clearly that the standard curves of our PMA-qPCR method present suitable linear correlation coefficient (R2 = 0.992) and mean efficiency of 97% (Fig. 3), which is consistent with the previous studies (Tong and Fang 2006). Moreover, the number of viable Lactobacillus spp. detected by our PMA-qPCR method ranges from 103 to 109 CFU mL−1 (Fig. 3), which also meets the current estimation requirements in the EU, USA, and China. Additionally, the standard curve acquired in this studied shows that PCR amplification of DNA is effectively inhibited when DNA is obtained from PMA-treated dead bacteria.
As the number of viable lactic acid bacteria is an important indicator of the quality of fermented dairy products, we tested whether our PMA-qPCR method can be used to accurately detect the number of viable Lactobacillus spp. in different commercially available fermented diary samples. As expected, the results show that the modified PMA-qPCR method put forth in this study can be used to quickly and accurately estimate the total number of viable Lactobacillus spp. in the samples. When carried out with respect to the shelf life of the product, the number of total viable lactobacilli estimated by three different methods (plate count in MRS agar, qPCR, and PMA-qPCR) is similar (P > 0.05, Fig. 4). However, 30 days beyond the expiration date, due to the death of some lactobacilli in the sample, the number of viable Lactobacillus spp. detected by qPCR was higher than that by plate count (P < 0.05, Fig. 4), while the number of viable Lactobacillus spp. detected by PMA-qPCR was similar to that obtained by plate count (P > 0.05, Fig. 4). Meanwhile, it was observed that the number of lactobacilli in plate count was relatively lower than that in PMA-qPCR. Earlier studies suggest the existence of viable but not culturable (VBNC) cells in samples (Junyan et al. 2018; Xu 1982), which cannot grow normally in the medium but still maintain metabolic activity and a prebiotic effect. Hence, compared with the more time-consuming and labor-intensive plate counting method, our PMA-qPCR method is not only convenient and timesaving but also more accurate in detecting the number of viable bacteria in the commercial fermented dairy products.
According to the current national standard in China, the number of viable lactic acid bacteria in fermented dairy products is the sum total of the quantitatively detected Lactobacillus spp., S. thermophilus, and Bifidobacterium spp. based on the plate count method. However, in this study, we only optimize the detection of Lactobacillus spp. in commercially available fermented dairy products. Once S. thermophilus and Bifidobacterium spp. detection methods have been established, it is likely that the PMA-qPCR viable lactic acid bacteria estimation method put forth here will prove to be highly convenient, time-saving, and accurate and can be used for the simultaneous detection of the number of viable lactic acid bacteria in the products.
Conclusion
In this study, a new combined PMA-qPCR method with novel general primers with probes was designed for rapid determination of the total number of viable Lactobacillus spp. in fermented dairy products. The PMA-qPCR method established in this study can accurately determine the total number of viable Lactobacillus spp. cells in fermented milk with a minimum detection limit of 1 × 103 CFU mL−1. Moreover, when applied on commercially available fermented dairy products, there was no significant difference in the total number of viable Lactobacillus spp. estimated by PMA-qPCR and plate counting methods. Thus, the PMA-qPCR method established here, with its good specificity and high amplification efficiency, can provide a theoretical basis for quantifying Lactobacillus spp. in the commercial fermented dairy products in the future.
References
Ahmed W, Zhang Q, Ishii S, Hamilton K, Haas C (2018) Microfluidic quantification of multiple enteric and opportunistic bacterial pathogens in roof-harvested rainwater tank samples. Environ Monit Assess 190:105
Akyol I (2018) Development and application of RTi-PCR method for common food pathogen presence and quantity in beef, sheep and chicken meat. Meat Sci 137:9–15
Banihashemi A, Van Dyke MI, Huck PM (2017) Application of long amplicon propidium monoazide-PCR to assess the effects of temperature and background microbiota on pathogens in river water. J Water Health 15(3-4):418–428
Bottari B, Felis GE, Salvetti E, Castioni A, Gatti M (2017) Effective identification of Lactobacillus casei group species: genome-based selection of the gene mutL as the target of a novel multiplex PCR assay. Microbiology 163(7):950–960
Brescia CC, Griffin SM, Ware MW, Varughese EA, Villegas EN (2009) Cryptosporidium propidium monoazide-PCR, a molecular biology-based technique for genotyping of viable Cryptosporidium oocysts. Appl Environ Microbiol 75(21):6856–6863
Casas IA, Dobrogosz WJ (2000) Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 12(4):247–285
Cattani F, Barth VC Jr, Nasario JSR, Ferreira CAS, Oliveira SD (2016) Detection and quantification of viable Bacillus cereus group species in milk by propidium monoazide quantitative real-time PCR. J Dairy Sci 99(4):2617–2624
Fan L, Bo L, Hui D, Quanmin K, Liu Y, Yuanxin W, Zoraida PA, Weihua L, Hengyi X (2017) Viable pathogens detection in fresh vegetables by quadruplex PCR. LWT Food Sci Technol 81:306–313
Fittipaldi M, Nocker A, Codony F (2012) Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J Microbiol Methods 91(2):276–289
Frentzel H, Thanh MD, Krause G, Appel B, Mader A (2018) Quantification and differentiation of Bacillus cereus group species in spices and herbs by real-time PCR. Food Control 83:99–108
Fujimoto J, Tanigawa K, Kudo Y, Makino H, Watanabe K (2011) Identification and quantification of viable Bifidobacterium breve strain Yakult in human faeces by using strain-specific primers and propidium monoazide. J Appl Microbiol 110(1):209–217
Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, Gong Z, Zeng Q, Wei Y, Yang W (2019) A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol 10:477
García-Cayuela T, Tabasco R, Peláez C, Requena T (2009) Simultaneous detection and enumeration of viable lactic acid bacteria and bifidobacteria in fermented milk by using propidium monoazide and real-time PCR. Int Dairy J 19(6-7):405–409
Hühne K, Axelsson L, Holck A, Kröckel L (1996) Analysis of the sakacin P gene cluster from Lactobacillus sake Lb674 and its expression in sakacin-negative Lb. Sake Strains. Microbiology 142(6):1437–1448
Jean-Marc D, Anne-Lise B, Isabelle P, Robin DD, Micheline V, Daniel P (2006) Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res 163:663–670
Jones SE, Versalovic J (2009) Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9(1):35–30
Junyan L, Yang D, Lin L, Bing L, Yanyan L, Shishui Z, Mark ES, Zhenbo X, Brian MP (2018) Discovery and control of culturable and viable but non-culturable cells of a distinctive Lactobacillus harbinensis strain from spoiled beer. Sci Rep 8(1):11446
Lai CH, Wu SR, Pang JC, Ramireddy L, Chiang YC, Lin CK, Tsen HY (2016) Designing primers and evaluation of the efficiency of propidium monoazide – quantitative polymerase chain reaction for counting the viable cells of Lactobacillus gasseri and Lactobacillus salivarius. J Food Drug Anal 25(3):533–542
Liu Y, Fang X, Liao Z, Wang L, Zhong Q (2017) Quantitative detection of viable but non-culturable (VBNC) Vibrio parahaemolyticus cells induced by different conditions using PMA-qPCR and respiratory activity analysis. Food Sci 38(20):215–221
Millette M, Luquet FM, Lacroix M (2007) In vitro growth control of selected pathogens by Lactobacillus acidophilus And Lactobacillus casei-fermented milk. Lett Appl Microbiol 44(3):314–319
Nocker A, Cheung CY, Camper AK (2006) Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods 67(2):310–320
Padilha M, Morales MLV, Vieira ADS, Costa MGM, Saad SMI (2016) Prebiotic mixture improved Lactobacillus acidophilus and Bifidobacterium animalis gastrointestinal in vitro resistance in petit-suisse. Food Funct 7(5):2312–2319
Portilho MM, Mendonça ACF, Bezerra CS, do Espirito-Santo MP, de Paula VS, Nabuco LC, Villela-Nogueira CA, Lewis-Ximenez LL, Lampe E, Villar LM (2018) Usefulness of in-house real time PCR for HBV DNA quantification in serum and oral fluid samples. J Virol Methods 256(1):100–106
Rudi K, Naterstad K, Dromtorp SM, Holo H (2005) Detection of viable and dead Listeria monocytogenes on Gouda-like cheeses by real-time PCR. Lett Appl Microbiol 40(4):301–306
Scariot MC, Venturelli GL, Prudêncio ES, Arisi ACM (2018) Quantification of Lactobacillus paracasei viable cells in probiotic yoghurt by propidium monoazide combined with quantitative PCR. Int J Food Microbiol 264:1–7
Shao Y, Wang Z, Bao Q, Zhang H (2016) Application of propidium monoazide quantitative real-time PCR to quantify the viability of Lactobacillus delbrueckii ssp. bulgaricus. J Dairy Sci 99(12):9570–9580
Tabasco R, Paarup T, Janer C, Peláez C, Requena T (2007) Selective enumeration and identification of mixed cultures of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. paracasei subsp. paracasei and Bifidobacterium lactis in fermented milk. Int Dairy J 17(9):1107–1114
Tong Z, Fang HHP (2006) Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol 70(3):281–289
Truchado P, Gil MI, Kostic T, Allende A (2015) Optimization and validation of a PMA qPCR method for Escherichia coli quantification in primary production. Food Control 62:150–156
Villarreal MLM, Padilha M, Vieira ADS, Franco BDGDM, Martinez RCR, Saad SMI, Zhou D (2013) Advantageous direct quantification of viable closely related probiotics in Petit-Suisse cheeses under in vitro gastrointestinal conditions by propidium monoazide - qPCR. PLoS ONE 8(12):e82102
Vondrakova L, Turonova H, Scholtz V, Pazlarova J, Demnerova K (2017) Impact of various killing methods on EMA/PMA-qPCR efficacy. Food Control 85:23–28
Wan C, Cheng L, Fu G, Chen C, Liu R, Shi S, Chen H, Fu Q, Huang Y (2018) Rapid detection of goose hemorrhagic polyoma virus using TaqMan quantitative real-time PCR. Mol Cell Probes 39:61–64
Xu HS (1982) Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol 8(4):313–323
Yanlin M, Yang D, Zhenbo X, Junyan L, Jianjun D, Hua Y, Junhong Y, Zongming C, Dongfeng W (2017) Development of a propidium monoazide-polymerase chain reaction assay for detection of viable Lactobacillus brevis in beer. Braz J Microbiol 48(4):740–746
Yao Y, Yanrong L, Sisi Z, Lu H, Ying C, Hailin H (2019) Bile salt hydrolase can improve Lactobacillus plantarum survival in gastrointestinal tract by enhancing their adhesion ability. FEMS Microbiol Lett 366:fnz100
Zhang Z, Liu W, Xu H, Aguilar ZP, Shah NP, Wei H (2015) Propidium monoazide combined with real-time PCR for selective detection of viable Staphylococcus aureus in milk powder and meat products. J Dairy Sci 98(3):1625–1633
Zhou P, Xie G, Liang T, Yu B, Xu H (2019) Rapid and quantitative detection of viable emetic Bacillus cereus by PMA-qPCR assay in milk. Mol Cell Probes 47:101437
Funding
This work was supported by the National Natural Science Foundation of China [Grant number 31501507]; State Administration for Market Regulation Foundation of China [Grant number S2020MK843]; and Nanjing Bureau of Quality and Technical Supervision Fund [Grant number Kj2020005].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Conflict of Interest
Yao Yang declares that she has no conflict of interest. Yanrong Liu declares that she has no conflict of interest. Yonghong Shu declares that she has no conflict of interest. Wenxu Xia declares that he has no conflict of interest. Ranyang Xu declares that she has no conflict of interest. Ying Chen declares that she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Liu, Y., Shu, Y. et al. Modified PMA-qPCR Method for Rapid Quantification of Viable Lactobacillus spp. in Fermented Dairy Products. Food Anal. Methods 14, 1908–1918 (2021). https://doi.org/10.1007/s12161-021-02022-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02022-3