Abstract
Research in cactus (Opuntia ficus-indica (L.) Mill.) cladodes as a functional food increases because of their (poly)phenolic compounds and health-promoting ability. Extraction conditions are the basis to obtain the highest amount of bioactive compounds with antioxidant properties. Therefore, several individual and combination of extraction solvents and conditions were carried out. Even a great amount of (poly)phenols was extracted using 80% ethanol; the combination of solvents with different polarities (methanol, acetone and water) favored the extraction of flavonoids and antioxidant capacity (DPPH and ABTS radicals). The extract with the highest flavonoid content was that obtained by successive extractions. Additionally, in order to quantify (poly)phenols by HPLC-DAD, aglycones were released from glycosides by acid hydrolysis (0.6, 1.2, 1.5, and 1.7 M HCl) through reflux at 90 °C at different times (2 h and 3 h). Flavonoid aglycones (isorhamnetin, quercetin, and kaempferol) and phenolic acids (ferulic and 4-hydroxybenzoic acids) were identified and quantified. Acid hydrolysis with 1.5 M HCl during 2 h was the best for the release of flavonoid aglycones, without phenolic acid degradation. Likewise, less acidity (0.6 M HCl) does not completely release the aglycones whereas a higher acidity (1.7 M) and a longer time of reflux (3 h) resulted in the degradation of flavonoid aglycones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cactus Opuntia ficus-indica (L.) Mill. is an American endemic plant which can grow in Egypt, Turkey, Central and South Africa, and in the Middle East (Israel, Jordan) and India (Stintzing and Carle 2005; Aragona et al. 2018). In these countries, especially in Mexico, the cactus has been used fresh or processed for human consumption, as well as a functional constituent for food and pharmaceutical products due to the important content of bioactive compounds like (poly)phenols (El-Mostafa et al. 2014; Ventura-Aguilar et al. 2017; De Santiago et al. 2018a; Rocchetti et al. 2018). Cactus stems, known as cladodes, have been characterized by the presence of phenolic acids and flavonoids such as isorhamnetin, kaempferol, quercetin, and isoquercitrin, where most of them are usually in their glucoside, rutinoside, and xyloside forms (Ginestra et al. 2009; Astello-García et al. 2015; De Santiago et al. 2018b; Mena et al. 2018).
The (poly)phenolic compound extraction is the most important step before analysis and, consequently, characterization of bioactive compounds of cactus cladodes. A common issue regarding the extraction of (poly)phenols in cactus cladodes is that the dietary fiber is an essential part of the composition of this plant including pectin, lignin, mucilage, cellulose, and hemicellulose, where the bioactive compounds are linked and can be strongly retained (Hernández-Urbiola et al. 2011). Several studies with O. ficus-indica have used different solvents for their extraction like methanol (Sánchez et al. 2014; Mena et al. 2018), ethanol (Guevara-Figueroa et al. 2010), or a combination of several solvents such as acetone, methanol, and water (Ramírez-Moreno et al. 2013; Avila-Nava et al. 2014). It is also important to control factors like temperature, time, and solvent proportion used in the extraction methods to avoid the degradation of the bioactive compounds or undesirable reactions like oxidation (González-Montelongo et al. 2010; Bindes et al. 2019).
In addition, after the extraction, a continuous monitoring of the phenolic composition is necessary to guarantee the quality of the plant extracts. In laboratories where HPLC-DAD is the only one economically available, the (poly)phenolic glycosides of cactus cladodes are in most cases hydrolyzed to their respective aglycones prior to analysis. The most common method for breakdown the flavonoid glycosides is the acid hydrolysis with hydrochloric acid (HCl) under continuous heating (Moussa-Ayoub et al. 2011; Khoddami et al. 2013). Although there are different extraction conditions reported, there is not a consensus on the optimal methodology to apply for the extraction and hydrolysis for cactus cladodes.
Therefore, the main aim of this work was first to select the best extraction conditions of (poly)phenolic compounds from cactus cladodes, and then, the optimal hydrolysis terms for the control analysis of their composition by HPLC-DAD.
Material and methods
Chemical and reagents
Ethanol, methanol, acetone, and hydrochloric acid (HCl) were of analytical grade from Panreac (Barcelona, Spain). The acetonitrile and formic acid (HPLC grade) were also purchased from Panreac (Barcelona, Spain). Folin–Ciocalteu reagent, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2′-azinobis (3-ethylbenzothiazonile-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and rutin, as well as the standards used for identification and quantification of phenolic compounds (isorhamnetin, kaempferol, quercetin, ferulic acid, and hydroxybenzoic acid), were purchased from Sigma-Aldrich (Steinheim, Germany).
Sample preparation
Fresh cactus (O. ficus-indica (L.) Mill.) cladodes from the same harvest and equal stage of ripening were obtained from BioArchen Company located in Murcia, Spain. Cladodes were washed, and the thorns were removed manually. Then, they were cut into equal small pieces, mixed well, and lyophilized in a freeze dryer Cryodos-80 (Telstar, Terrasa, Spain), and stored at − 18 °C until analysis. Figure 1 shows a general diagram with the experimental design of the study.
Extraction methods
Four different extracts were prepared as follows:
-
1.
Water: The extract was prepared according to Siddiq et al. (2013)’s method with some modifications. Briefly, 30 mL of distilled water was added to 2 g of lyophilized cactus cladodes. The content was mixed on a mechanical shaker for 1 h at room temperature and then centrifuged at 4000 rpm for 10 min. Supernatant was collected, and residues were re-extracted twice using 10 mL of distilled water by vortexing (1 min) and centrifuged at 4000 rpm for 5 min. All three supernatants were combined and frozen at − 18 °C before analysis.
-
2.
Eighty percent ethanol (80% EtOH): A second extract was performed as previously described, but instead of adding distilled water, 30 mL of ethanol/water (80/20) was used. Likewise, after mixing the three supernatants, the extract was frozen at − 18 °C.
-
3.
Ethanol (EtOH): Lee et al. (2002)’s method was used for this extraction. Five grams of lyophilized cladodes was mixed with 50 mL of EtOH and shaken at room temperature for 72 h. Then, it was filtered and centrifuged at 4000 rpm 10 min. Supernatant was stored at − 18 °C before analysis.
-
4.
Successive extractions: The last extract was prepared based on the method of Avila-Nava et al. (2014) with modifications. A mix of 50 mL of methanol/water (50/50) solution was added to 4 g of lyophilized cladodes. This mixture was stirred for 2 h and then vacuum filtered. The resulting filtrate was saved and stored. The residue was subjected to a second extraction with 50 mL of acetone/water (70/30) solution and agitation for 2 h. The mixture was vacuum filtered and stored. A third extraction was performed stirring the residue in 50 mL of distilled water for 30 min and also vacuum filtered. The resulting filtrates were mixed together and stored at − 18 °C before analysis.
TPC
Total phenolic compounds (TPC) were measured using the Folin–Ciocalteu reagent according to Singleton and Rossi (1965)’s method. Each extract was properly diluted in demineralized water, and 100 μL was mixture with 500 μL of Folin–Ciocalteu reagent and 7.9 mL of demineralized water. After 2 min, 1.5 mL of a 7.5% sodium carbonate solution was added. Then, the samples were incubated in darkness for 90 min at room temperature. The absorbance of the sample was measured at 765 nm in a spectrophotometer Lambda 25 UV/VIS (Perkin Elmer Instruments, Madrid, Spain). Analysis was made in triplicate. Gallic acid (GA) was used as reference, and the results were expressed as milligrams of GA equivalent per gram of dry matter (mg GAE/g dm).
TFC
The aluminum chloride method was used (Lamaison and Carnet 1990). An aliquot of 100 μL of each extract properly diluted in distilled water was added to 1 mL of a 2% AlCl3·6H2O methanol solution. The mixture was shaken, and after 10 min of incubation at room temperature, the absorbance was read at 430 nm in a spectrophotometer Lambda 25 UV/VIS (Perkin Elmer). Analysis was made in triplicate. Rutin was used for calibration curve, and the results were expressed as milligram of rutin equivalent per gram of dry matter (mg rutin/g dm).
Antioxidant capacity by ABTS assay
The ABTS antioxidant capacity was performed according to the method of Re et al. (1999). The radicals ABTS•+ were generated by the addition of 0.36 mM potassium persulfate to a 0.9 mM ABTS solution prepared in phosphate-buffered saline (PBS) (pH 7.4), and the ABTS•+ solution was stored in darkness for 12 h. The ABTS•+ solution was adjusted with PBS to an absorbance of 0.700 (± 0.020) at 734 nm in a 3-mL capacity cuvette (1 cm length) at 25 °C (Lambda 25 UV–Vis spectrophotometer, Perkin-Elmer Instruments, Madrid, Spain). An aliquot of 100 μL of each extract sample properly diluted in demineralized water was added to 2 mL of ABTS•+ solution. After 18 min, the absorbance was measured at 734 nm. Analysis was made in triplicate. Calibration curve was performed with Trolox solution (a water-soluble vitamin E analog), and the results were expressed as micromoles of Trolox equivalent per gram of dry matter (μmol Trolox/g dm).
Antioxidant capacity by DPPH assay
The antioxidant capacity was also measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH•) decolorization assay with some modifications (Brand-Williams et al. 1995). A 6.1 × 10−5 M DPPH• methanolic solution was prepared immediately before use. In a 3-mL-capacity cuvette (1 cm length), the DPPH• solution was adjusted with methanol to an absorbance of 0.700 (± 0.020) at 515 nm (Lambda 25 UV–Vis spectrophotometer, Perkin-Elmer Instruments, Madrid, Spain). All the extracts were properly diluted in demineralized water, and 50 μL of samples was added to 1.95 mL of the DPPH• solution. The absorbance was measured at 515 nm after 18 min. Analysis was made in triplicate. Calibration was performed with Trolox solution and expressed as micromoles of Trolox equivalent per gram of dm (μmol Trolox/g dm).
Acid hydrolysis
Once the best extraction method was selected, acid hydrolysis was carried out through a reflux at 90 °C with different concentrations of HCl and times: 0.6 M for 3 h, 1.2 M for 3 h, 1.5 M for 2 and 3 h, and 1.7 M for 2 and 3 h. After hydrolysis, each extract was filtered and frozen at − 18 °C for chromatographic analysis.
(Poly)phenolic compounds by HPLC-DAD
(Poly)phenolic compounds were analyzed by HPLC following the method described by Juániz et al. (2016a) with some modifications. HPLC analysis was performed with an analytical HPLC unit model 1200 (Agilent Technologies, Palo Alto, CA, USA) equipped with a binary pump and an auto-sampler injector. The column used was a C18 5U Kinetex 100A (250 × 4.60 mm), and the volume of each sample injection was 20 μL. Chromatographic separation was carried out using a gradient of acid water with formic acid 1% (solvent A) and acetonitrile (solvent B) at a constant flow of 1 mL/min starting with 95% solvent A. Then, solvent A was decreased to 60% within 55 min, maintained until 60 min, and decreased to 10%. Then, it remained until 67 min and returned to the initial conditions (solvent A 95%, solvent B 5%). Detection was accomplished with a diode array detector (DAD) at 360 nm for flavonoids, at 325 nm for ferulic acid, and at 256 nm for hydroxybenzoic acid. Chromatograms were analyzed using the Agilent ChemStation software, and the identification of phenolic compounds was performed by comparison of the peak retention times and UV spectra with those of the reference standards. Analysis was made in triplicate. A calibration curve with reference standards of the identified compounds was carried out for quantification. Results were expressed as milligrams of each compound per gram of dry matter (mg/g dm).
Statistical analysis
Each parameter was analyzed in triplicate. Results are shown as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was applied for each parameter. A Tukey test was applied as a posteriori test with a level of significance of 95%. All statistical analyses were performed using the STATA v.12.0 software package.
Results and discussion
Cactus (O. ficus-indica) cladodes are rich in macromolecules like pectins, mucilages, and cellulose with high water capacity retention. Most (poly)phenols are usually ionically or covalently linked to these polymeric compounds. Therefore, the extraction of these bioactive compounds can be difficult. Several individual solvents such as ethanol and water, as well as successive extractions with methanol (50%), acetone (70%), and water, were used for the (poly)phenolic compound extraction of cactus cladodes. The main problem during extraction was the filtration time, taking few hours, especially when water and ethanol were used. The filtration time was significantly reduced when 80% ethanol was used. However, using the successive extraction with methanol/water (50/50), and subsequently acetone/water (70/30) and water, the filtration time was much shorter.
TPC and TFC
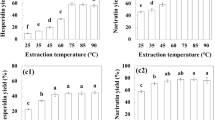
Figure 2 shows the total phenolic content (TPC) and total flavonoid content (TFC) of the different extracts of cactus (O. ficus-indica) cladodes. The highest TPC was obtained for the 80% EtOH extract with 39.26 mg GAE/g dm (250.86 mg GAE/100 g), followed by the successive extractions with 36.62 mg GAE/g dm (234.01 mg GAE/100 g). The extract with the least amount of phenolic compounds was the water extract with 14.18 mg GAE/g dm (90.62 mg GAE/100 g).
There is a great variation in the results reported by other authors due in part to the different methodology applied, but also to the different expression of results (fresh weight, dry matter, milliliter of extract). Nevertheless, the TPC found in 80% EtOH and successive extractions extracts were higher than those previously reported in the literature. In cactus cladodes, Sánchez et al. (2014) and Andreu et al. (2018) reported a total phenolic content of 4.27 mg GAE/g dm and 18.9 g GAE/Kg dm, using 70% and 80% methanol, respectively, while Guevara-Figueroa et al. (2010) reported 19.9 mg GAE/g when ethanol was used for extraction. Similar processes with successive extractions were carried out by Dib et al. (2013) and Ramírez-Moreno et al. (2013) for cactus cladodes. However, they reported less (poly)phenolic content than in the present study (26.7 and 4.58 mg GAE/g dm, respectively), which may be due to the methodology applied (less shaking time) but also to sample differences in the cladode maturity, cultivation region, and climate. Likewise, cactus cladodes presented a greater amount of TPC than other vegetables such as pepper, broccoli, spinach (Turkmen et al. 2005), cauliflower (Sikora et al. 2008), kale, and red cabbage (Murador et al. 2015).
On the other hand, the extract with the highest TFC was that obtained by successive extractions with 4.83 mg rutin/g dm (30.89 mg rutin/100 g). In this case, the 80% EtOH extract showed the lowest amount of TFC with 2.85 mg rutin/g dm (18.22 mg rutin/100 g). These flavonoids results are also difficult to compare with those reported by other authors (Guevara-Figueroa et al. 2010; Medina-Torres et al. 2011) because of the different standard compounds (rutin, quercetin and catechin) used for reporting results.
Methanol and ethanol are the most used solvents for the extraction of (poly)phenolic compounds in plants. However, the combination of different solvents, such as in the successive extraction, is more successful as a result of the increase of polarity which influence in the plant cell permeability (Oreopoulou et al. 2019). The efficiency in the extraction of (poly)phenols is affected by the polarity of the solvent used because hydroxylated forms of phenolic compounds are more soluble in alcohols such as methanol or ethanol mixing with water, while less polar solvents such as ethyl acetate, acetone, and chloroform are used for the less polar and methoxylated compounds (Robards 2003; Dorta et al. 2012). Consequently, the combination of solvents with different polarity may facilitate the extraction of different cactus (poly)phenols (flavonoids, phenolic acids, etc.), glycosylated or not, free and bound to polymeric compounds (fiber, etc.).
Antioxidant capacity
Antioxidant capacity measured by ABTS and DPPH radicals quenching activity of cactus cladodes extracts is shown in Fig. 3. For both radicals, the extract obtained by successive extractions had the highest antioxidant capacity with 778.03 and 72.17 μmol trolox/g dm (4791.63 and 461.14 μmol trolox/100g) respectively. This antioxidant capacity is much higher than that reported by other authors: 13.97 μmol trolox/g dm for ABTS radical using a similar successive extraction (Ramírez-Moreno et al. 2013) and 90 μmol trolox/100g for DPPH using non-polar solvents (hexane/dichloromethane) for extraction (Corral-Aguayo et al. 2008). In contrast, the extract with the least antioxidant capacity was that obtained with water, which accounted for 1.13 μmol trolox/g dm (7.25 μmol trolox/100g) for ABTS and 1.44 μmol trolox/g dm (9.21 μmol trolox/100g) for DPPH radical. In comparison with other vegetables, cactus cladodes showed a higher antioxidant capacity than kale, red cabbage (Murador et al. 2015), carrots (Guillén et al. 2017), onion, pepper, and cardoon (Juániz et al. 2016b).
The antioxidant activity of phenolic compounds is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. Cactus cladodes contain polysaccharides such as pectins and cellulose, which form a complex with (poly)phenols, making the extraction more difficult (Bordenave et al. 2014). Therefore, results suggest that the antioxidant activity is also dependent on the solvent used due to the different antioxidant potentials of compounds with different polarity. In addition, a mixture of acetone and water might also improve the extraction of bioactive compounds like (poly)phenols from the cell matrix more than just the use of ethanol. Hence, the successive extraction method was selected as the best to further identify and quantify (poly)phenolic compounds by HPLC-DAD.
(Poly)phenolic compounds by HPLC-DAD
In plants, (poly)phenols can be found free or bound to the polymeric compounds like fiber, carbohydrates, or proteins, being most of them in their glycoside forms. The stability of the glycosides depends on the kind and position of the sugar, as well as the compound union. Likewise, the number of aromatic moieties and hydroxyl groups present in phenolic compounds are important factors, because more aromatic rings tended to bind more strongly to macromolecules like cellulose than those compounds with a single aromatic ring (Bordenave et al. 2014).
The analysis of (poly)phenolic compounds by HPLC-MS is more and more extended, but its availability in many food quality control laboratories is limited due to its high price while a HPLC-DAD equipment is available (Vogeser and Seger 2008). The analysis of cactus cladode extract by HPLC-DAD showed the presence of several unidentified peaks (Fig. 1Sa), but the absence of flavonoid aglycones and free phenolic acids, because in vegetables, most of the phenolic compounds are present in the form of glycosides (Acosta-Estrada et al. 2014). In most cases, to release the aglycone from the glycoside, it is necessary to apply a hydrolysis, controlling the parameters (time, temperature, concentration, etc.) to avoid further breaking the aglycones or phenolic acids structures.
Therefore, acid hydrolysis was applied to hydrolyze the (poly)phenolic compounds in their corresponding aglycones and free forms, and thus be able to quantify them, based on previous studies (Santos-Zea et al. 2011; Avila-Nava et al. 2014; Juániz et al. 2016a, 2017) for the release of compounds from cell walls and polymeric structures. Six acid hydrolyses methods were carried out through a reflux at 90 °C with different HCl concentrations and times: 0.6 M for 3 h, 1.2 M for 3 h, 1.5 M for 2 and 3 h, and 1.7 M for 2 and 3 h.
Chromatograms obtained by HPLC-DAD analysis before and after hydrolysis show the release of the (poly)phenolic aglycones (Fig. 1S). After acid hydrolysis, HPLC-DAD showed the presence of quercetin, kaempferol, and isorhamnetin aglycones, indicating that (poly)phenolic compounds were released from the glycosides, but also from the food matrix bound to or associated with polysaccharides. In cactus cladodes, 3 flavonoids aglycones (quercetin, kaempferol and isorhamnetin) and 2 phenolic acids (ferulic and hydroxybenzoic acids) were identified and quantified (Table 1).
Flavonoids were the main (poly)phenolic compounds in cactus cladodes accounting for around 85% of total (poly)phenols. Isorhamnetin was the most abundant flavonol, followed by a smaller amount of quercetin and kaempferol, which is in agreement with literature (Ginestra et al. 2009; Jun et al. 2013; Avila-Nava et al. 2014). In contrast, Santos-Zea et al. (2011) did not find quercetin. Previous studies without hydrolysis have identified and quantified isorhamnetin glycosides as the main flavonoid glycosides in cactus cladodes by HPLC-MS (Ginestra et al. 2009; Moussa-Ayoub et al. 2014; De Santiago et al. 2018b), with kaempferol and quercetin glycosides also present (Guevara-Figueroa et al. 2010; De Santiago et al. 2018b).
Otherwise, ferulic acid was the predominant phenolic acid, followed by 4-hydroxybenzoic acid. Protocatechuic, chlorogenic, salicylic, gallic, and caffeic acids have been previously reported in Opuntia cactus cladodes (Guevara-Figueroa et al. 2010; Jun et al. 2013), but they were not detected in the present study.
Among different hydrolysis conditions, the highest amount of total (poly)phenolic compounds in cactus cladodes was that obtained after acid hydrolysis for 2 h of reflux at 90 °C with 1.5 M HCl (3.97 mg/g dm). Likewise, the highest amount of aglycones due to the deglycosylation of flavonoids was also observed at the same conditions. Results show that less acidity (0.6 M HCl) does not completely release the aglycones. Otherwise, a higher acidity (1.7 M) and a longer time of reflux (3 h) resulted in the degradation of flavonoid aglycones. A previous study in cactus cladodes which performed an acid hydrolysis with higher concentration of HCl (3.2M) for 20 min showed less amount of (poly)phenolic compounds (Santos-Zea et al. 2011) than in this study, demonstrating the degradation of the compounds. Likewise, enzymatic hydrolysis has been shown as less effective than the acid one, because isorhamnetin was the only aglycone identified in much smaller amount (0.003 mg/g dm) in cactus cladodes (Moussa-Ayoub et al. 2014).
When attention is focused on total phenolic acid content, the highest amount is shown after the 0.6M HCl hydrolysis, followed by the 1.2 M HCl concentration. When the acid concentration and the reflux time increased, ferulic acid was degraded. However, the highest content of 4-hydroxybenzoic acid was measured after 2 h of 1.5 M HCl hydrolysis and starting to decrease with longer time and higher acid concentration applied. In any case, the amount of the two phenolic acids found in the present study was higher than that found by Guevara-Figueroa et al. (Guevara-Figueroa et al. 2010) without hydrolysis. A similar work carried out in onion and spinach showed that the 2-h reflux at 80 °C with 1.2 M HCl gave the best results for quercetin and kaempferol aglycones, whereas phenolic acids were degraded under the same conditions (Nuutila et al. 2002). Therefore, because phenolic acids are more susceptible than flavonoids to be degraded, it is necessary to choose the acid hydrolysis conditions that favored the release of aglycones without puting at risk the identification and quantification of phenolic acids. For that reason, acid hydrolysis with 1.5 M HCl for 2 h of reflux (90 °C) has been shown as the most effective for further identification and quantification of (poly)phenolic compounds in cactus cladodes by using HPLC-DAD equipment. After extraction, acid hydrolysis provides a helpful method for the complex (poly)phenolic profile of cactus cladodes for the identification and quantification by HPLC-DAD as a result of the glycosylated and esterified phenolics transformation into their aglycones produced from deglycosylation, demethylation, and demethoxylation.
Conclusions
The present study evidences the need to use the combination of solvents with different polarities to extract bioactive compounds from cactus cladodes, as well as the most adequate acid hydrolysis conditions in order to further identify and quantify their (poly)phenolic compounds by HPLC-DAD. This technique is quite common in food control laboratories where HPLC-MS is not available. This study also showed that long acid hydrolysis leads to the degradation of (poly)phenolic compounds, specifically phenolic acids. Therefore, the choice of the best method of extraction is very important for obtaining the highest amount of bioactive compounds, especially antioxidants, from the plant material, as well as to further control its (poly)phenolic composition, in order to use cactus cladode extracts for the development of functional food ingredients.
References
Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO (2014) Bound phenolics in foods, a review. Food Chem 152:46–55. https://doi.org/10.1016/j.foodchem.2013.11.093
Andreu L, Nuncio-Jáuregui N, Carbonell-Barrachina ÁA, Legua P, Hernández F (2018) Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J Sci Food Agric 98:1566–1573. https://doi.org/10.1002/jsfa.8628
Aragona M, Lauriano ER, Pergolizzi S, Faggio C (2018) Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat Prod Res 32:2037–2049. https://doi.org/10.1080/14786419.2017.1365073
Astello-García MG, Cervantes I, Nair V, Santos-Díaz MS, Reyes-Agüero A, Guéraud F, Negre-Salvayre A, Rossignol M, Cisneros-Zevallos L, Barba de la Rosa AP (2015) Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J Food Compos Anal 43:119–130. https://doi.org/10.1016/j.jfca.2015.04.016
Avila-Nava A, Calderón-Oliver M, Medina-Campos ON, Zou T, Gu L, Torres N, Tovar AR, Pedraza-Chaverri J (2014) Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. J Funct Foods 10:13–24
Bindes MMM, Cardoso VL, Reis MHM, Boffito DC (2019) Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J Food Eng 241:97–104. https://doi.org/10.1016/j.jfoodeng.2018.08.006
Bordenave N, Hamaker BR, Ferruzzi MG (2014) Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct 5:18–34. https://doi.org/10.1039/C3FO60263J
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Leb und-Technologie 28:25–30
Corral-Aguayo RD, Yahia EM, Carrillo-Lopez A, González-Aguilar G (2008) Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J Agric Food Chem 56:10498–10504
De Santiago E, Domínguez-Fernández M, Cid C, De Peña MP (2018a) Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem 240:1055–1062. https://doi.org/10.1016/j.foodchem.2017.08.039
De Santiago E, Pereira-Caro G, Moreno-Rojas JM et al (2018b) Digestibility of (poly)phenols and antioxidant activity in raw and cooked cactus cladodes (Opuntia ficus-indica). J Agric Food Chem 66:5832–5844. https://doi.org/10.1021/acs.jafc.8b01167
Dib H, Beghdad MC, Belarbi M et al (2013) Antioxidant activity of phenolic compounds of the cladodes of Opuntia ficus-indica Mill. from Northwest Algeria. Int J Med Pharm Sci 3:147–158
Dorta E, Lobo MG, Gonzalez M (2012) Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 77:80–88. https://doi.org/10.1111/j.1750-3841.2011.02477.x
El-Mostafa K, El Kharrassi Y, Badreddine A et al (2014) Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 19:14879–14901
Ginestra G, Parker ML, Bennett RN, Robertson J, Mandalari G, Narbad A, Lo Curto RB, Bisignano G, Faulds CB, Waldron KW (2009) Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J Agric Food Chem 57:10323–10330
González-Montelongo R, Gloria Lobo M, González M (2010) Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem 119:1030–1039. https://doi.org/10.1016/j.foodchem.2009.08.012
Guevara-Figueroa T, Jiménez-Islas H, Reyes-Escogido ML, Mortensen AG, Laursen BB, Lin LW, de León-Rodríguez A, Fomsgaard IS, Barba de la Rosa AP (2010) Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J Food Compos Anal 23:525–532
Guillén S, Mir-Bel J, Oria R, Salvador ML (2017) Influence of cooking conditions on organoleptic and health-related properties of artichokes, green beans, broccoli and carrots. Food Chem 217:209–216. https://doi.org/10.1016/j.foodchem.2016.08.067
Hernández-Urbiola MI, Pérez-Torrero E, Rodríguez-García ME (2011) Chemical analysis of nutritional content of prickly pads (Opuntia ficus indica) at varied ages in an organic harvest. Int J Environ Res Public Health 8:1287–1295
Juániz I, Ludwig IA, Bresciani L, Dall'Asta M, Mena P, del Rio D, Cid C, de Peña MP (2016a) Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J Funct Foods 27:201–213. https://doi.org/10.1016/j.jff.2016.09.006
Juániz I, Ludwig IA, Huarte E, Pereira-Caro G, Moreno-Rojas JM, Cid C, de Peña MP (2016b) Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem 197:466–473. https://doi.org/10.1016/j.foodchem.2015.10.139
Juániz I, Ludwig IA, Bresciani L, Dall'Asta M, Mena P, del Rio D, Cid C, de Peña MP (2017) Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J Funct Foods 32:195–207. https://doi.org/10.1016/j.jff.2017.02.033
Jun HI, Cha MN, Yang EI et al (2013) Physicochemical properties and antioxidant activity of Korean cactus (Opuntia humifusa) cladodes. Hortic Environ Biotechnol 54:288–295
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375
Lamaison J, Carnet A (1990) Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm Acta Helv 65:315–320
Lee JC, Kim HR, Kim J, Jang YS (2002) Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem 50:6490–6496
Medina-Torres L, Vernon-Carter EJ, Gallegos-Infante JA, Rocha-Guzman NE, Herrera-Valencia EE, Calderas F, Jiménez-Alvarado R (2011) Study of the antioxidant properties of extracts obtained from nopal cactus (Opuntia ficus-indica) cladodes after convective drying. J Sci Food Agric 91:1001–1005
Mena P, Tassotti M, Andreu L, Nuncio-Jáuregui N, Legua P, del Rio D, Hernández F (2018) Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res Int 108:301–308. https://doi.org/10.1016/j.foodres.2018.03.062
Moussa-Ayoub TE, El-Samahy SK, Kroh LW, Rohn S (2011) Identification and quantification of flavonol aglycons in cactus pear (Opuntia ficus indica) fruit using a commercial pectinase and cellulase preparation. Food Chem 124:1177–1184
Moussa-Ayoub TE, Abd El-Hady ESA, Omran HT et al (2014) Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res Int 64:864–872
Murador DC, Mercadante AZ, De Rosso VV (2015) Cooking techniques improve the levels of bioactive compounds and antioxidant activity in kale and red cabbage. Food Chem 196:1101–1107. https://doi.org/10.1016/j.foodchem.2015.10.037
Nuutila AM, Kammiovirta K, Oksman-Caldentey KM (2002) Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem 76:519–525. https://doi.org/10.1016/S0308-8146(01)00305-3
Oreopoulou A, Tsimogiannis D, Oreopoulou V (2019) Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. In: Watson R (ed) Polyphenols in plants, 2nd edn. Academic Press, London, pp 243–260
Ramírez-Moreno E, Córdoba-Díaz D, Sánchez-Mata M d C et al (2013) Effect of boiling on nutritional, antioxidant and physicochemical characteristics in cladodes (Opuntia ficus indica). LWT Food Sci Technol 51:296–302. https://doi.org/10.1016/j.lwt.2012.10.005
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Robards K (2003) Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J Chromatogr A 1000:657–691. https://doi.org/10.1016/S0021-9673(03)00058-X
Rocchetti G, Pellizzoni M, Montesano D, Lucini L (2018) Italian Opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 7:1–12. https://doi.org/10.3390/foods7020024
Sánchez E, Dávila-Aviña J, Castillo SL et al (2014) Antibacterial and antioxidant activities in extracts of fully grown cladodes of 8 cultivars of cactus pear. J Food Sci 79:659–664
Santos-Zea L, Gutiérrez-Uribe JA, Serna-Saldivar SO (2011) Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J Agric Food Chem 59:7054–7061
Siddiq M, Roidoung S, Sogi DS, Dolan KD (2013) Total phenolics, antioxidant properties and quality of fresh-cut onions (Allium cepa L.) treated with mild-heat. Food Chem 136:803–806. https://doi.org/10.1016/j.foodchem.2012.09.023
Sikora E, Cieslik E, Leszczynska T et al (2008) The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem 107:55–59. https://doi.org/10.1016/j.foodchem.2007.07.023
Singleton V, Rossi J (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Stintzing FC, Carle R (2005) Cactus stems (Opuntia spp.): a review on their chemistry, technology, and uses. Mol Nutr Food Res 49:175–194. https://doi.org/10.1002/mnfr.200400071
Turkmen N, Sari F, Velioglu YS (2005) The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem 93:713–718. https://doi.org/10.1016/j.foodchem.2004.12.038
Ventura-Aguilar RI, Bosquez-Molina E, Bautista-Baños S, Rivera-Cabrera F (2017) Cactus stem (Opuntia ficus-indica Mill): anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J Sci Food Agric 97:5065–5073. https://doi.org/10.1002/jsfa.8493
Vogeser M, Seger C (2008) A decade of HPLC-MS/MS in the routine clinical laboratory - goals for further developments. Clin Biochem 41:649–662. https://doi.org/10.1016/j.clinbiochem.2008.02.017
Funding
This study was funded by the Spanish Ministry of Science and Innovation (AGL2014-52636-P). E. De Santiago and I. Juániz had received research grants from the Association of Friends of the University of Navarra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elsy De Santiago declares that she has no conflict of interest. Isabel Juániz declares that she has no conflict of interest. Concepción Cid declares that she has no conflict of interest. María-Paz De Peña declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 131 kb)
Rights and permissions
About this article
Cite this article
De Santiago, E., Juániz, I., Cid, C. et al. Extraction of (Poly)phenolic Compounds of Cactus (Opuntia ficus-indica (L.) Mill.) Cladodes. Food Anal. Methods 14, 1167–1175 (2021). https://doi.org/10.1007/s12161-020-01946-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01946-6