Abstract
The present research is focused on the development of a cryogenically modulated (CM) comprehensive two-dimensional gas chromatography-high resolution time-of-flight mass spectrometry (GC × GC-HR ToFMS) method for the in-depth profiling of the unsaponifiable fraction of vegetable oils. Specifically, twelve vegetable oils (9 extra-virgin olive, soybean, peanut, and hazelnut oils) were subjected to analysis, with particular attention devoted to the higher molecular weight constituents (sterols, vitamin E, triterpenic alcohols). Peak assignment was carried out by using commercial unit-mass MS databases, accurate mass data, literature and on-line freely-available mass spectral information, as well as an in-lab-constructed HR ToFMS lipid database. The main objective of the work was to develop a method to define a well-characterized extra-virgin olive oil fingerprint, to be potentially exploited for vegetable oil differentiation. An additional objective of the investigation was to evaluate the performance of the HR ToFMS system (operated at a mass resolution of 25,000 FWHM) under the challenging analytical conditions of a CM GC × GC-based experiment. The HR ToFMS accurate-mass lipid database was (previously) constructed by acquiring, for the main part, the mass spectra of vegetable oil constituents, well-separated from both the matrix and column bleed background.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comprehensive two-dimensional gas chromatography (GC × GC) was first reported in 1991, by Liu and Phillips (Liu and Phillips 1991), whereas the first paper reporting the use of GC × GC in combination with mass spectrometry (MS) appeared in 1999 (Frysinger and Gaines 1999). In historical terms, the most popular MS choice, in the GC × GC field, has been low-resolution time-of-flight (ToF) MS, followed rather distantly by single quadrupole mass spectrometry (QMS). With regard to other forms of MS instrumentation, there have been a much lower number of descriptions involving triple-quadrupole and high-resolution (HR) ToFMS (Tranchida et al. 2018). For more general details on GC × GC technology, the reader is directed to the literature (Marriott et al. 2012).
In terms of mass spectrometry, the present contribution is focused on HR ToFMS; this powerful form of MS can be exploited in both untargeted and targeted GC-based experiments (Hernández et al. 2011). Qualitative information is attained through MS database matching and the study of accurate-mass ions (the molecular ion if present, along with the fragments). Pre-targeted analysis is performed by using highly selective accurate-mass extracted-ion chromatograms; the spectrum data can also be investigated at a later stage to pinpoint previously unsearched analytes (post-targeted analysis).
Recently, GC × GC experiments focused on the use of an HR ToFMS instrument, with a 200-Hz spectral generation capability at a mass resolution of 25,000 (FWHM), have been reported (Tranchida et al. 2015; Byer et al. 2016), with both directed to the analysis of S-containing compounds in petrochemical samples. The same type of HR ToFMS instrument has been used for the qualitative analysis of the unsaponifiable fraction of milk lipids (Tranchida et al. 2013a); specifically, GC-HR ToFMS was used to support the identification of several lipids, previously subjected to analysis by using GC × GC-QMS. Such a process was performed by a cross-comparison of the one- and two-dimensional chromatograms (the stationary phase used in the first GC × GC dimension was the same as that used in the GC experiment).
Instead of deriving detailed lipid information from the mutual use of GC × GC-QMS and GC-HR ToFMS, in the present study, it is attained directly from the GC × GC-HR ToFMS system. It is noteworthy that GC × GC with dual detection [flame ionization detection (FID) and QMS] has been used to study the entire unsaponifiable fraction of extra-virgin olive (8 samples), sunflower, and peanut oils (Tranchida et al. 2013b). Pre-fractionation of the unsaponifiable fraction was not performed due to the enhanced GC × GC separation space.
A main objective of the present investigation was to develop a method enabling a detailed characterization of the unsaponifiable fraction of vegetable oils [more in particular of extra-virgin olive oils, within the context of a national research project (see Acknowledgments)]. In such a respect, the samples subjected to study were nine extra-virgin olive oils, as well as a soybean, peanut, and hazelnut oil. With respect to the previous GC × GC-FID/QMS study, the analysis time was reduced by 43%, with more focus on the higher boiling point compounds; moreover, approx. 20–25% amounts of the reagents/solvents were used to isolate the unsaponifiable fraction as previously described (Tranchida et al. 2014). Finally, the performance of the HR ToFMS system (operated at a mass resolution of 25,000 FWHM) was evaluated in different aspects under the challenging analytical conditions of a cryogenic-modulation (CM) GC × GC-based experiment.
Material and Methods
Samples, Standard Compounds and Reagents
Nine genuine extra-virgin olive oils (EVOO1–9) were provided by participants of a national project on olive oil. The remaining three oils (soybean, peanut, hazelnut) were part of an in-lab collection of vegetable oils.
The BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide] + 1% TMCS (trimethylchlorosilane) kit was supplied by Merck Life Science (Merck KGaA, Darmstadt, Germany). Powdered anhydrous sodium sulfate, pyridine (anhydrous 99.8%), potassium hydroxide (KOH), diethyl ether (99.0%) and ethanol (LC grade) were supplied by Merck Life Science. Water was obtained from a Milli-Q SP Reagent Water System (Millipore, Bedford, MA, USA).
Sample Preparation
Approximately 1 g of exactly weighed vegetable oil was added to 10 mL of a 2 N KOH/EtOH solution, heated at 80 °C, under reflux, and magnetic stirring (for about 20 min after solution clarification).
After, extraction was performed three times: the first with 20 mL of diethyl ether, and the other two with 15 mL of the same solvent. The extracts were combined and washed with 10 mL of distilled water (washing was performed about 15 times per sample). The washed solution was dried with anhydrous sodium sulfate, and the solvent was evaporated under low-pressure conditions at 37 °C.
The unsaponifiable fraction isolated was dissolved in 500 μL of chloroform, and then treated with the derivatization mixture [200 μL of BSTFA (1% TMCS) and 200 μL of pyridine], and then heated at 70 °C for 20 min. The derivatized sample was then ready for GC injection.
Instrumental Conditions
All CM GC × GC-HR ToFMS applications were carried out on a Pegasus GC-HRT 4D system (LECO, Mönchengladbach, Germany). The first column was of low polarity, specifically an SLB-5ms [equivalent in polarity to poly(5%diphenyl/95%dimethyl siloxane)], with dimensions 30 m × 0.25 mm ID × 0.25 μm df; the second column was of intermediate polarity, namely, an SLB-35ms, with dimensions 1.9 m × 0.10 mm ID × 0.10 μm df [equivalent in polarity to poly(35%diphenyl/65%dimethyl siloxane)]. Both analytical columns were from Merck Life Science. The column connection was made by using a SilTite mini union (Trajan, Ringwood, Victoria, Australia).
In order to focus attention on the higher boiling point compounds, the following method was developed: temperature program, 90–280 °C at 15 °C min−1 and 280–360 °C at 2.5 °C min−1; modulation period was 4 s, with the hot jet held for 1.4 s and the cold jet for 0.6 s; injector temperature, 360 °C; injection mode and volume, split (10:1), and 3 μL; He flow (constant), 2.4 mL min−1; transfer line temperature, 360 °C; modulation heating and secondary oven temperatures, + 15 °C offset with respect to the temperature of the main GC oven; modulation cooling temperature, − 60 °C.
The HR ToFMS analyses were performed through electron ionization (70 eV), at a source temperature of 250 °C. Masses were analyzed over a range of m/z 45–600, at a spectral production frequency of 120 Hz, using the high resolution mode (25,000 FWHM). The mass spectral databases used were NIST 11, Lipids (Wiley), and an in-lab-constructed high resolution MS lipid database.
Results and Discussion
The combination of CM GC × GC and HR ToFMS generates a very powerful analytical platform, benefiting from the high sensitivity, selectivity, and resolving power, of both the GC and MS sides. Hence, GC × GC-HR ToFMS has the capability to perform in-depth investigations, and generate detailed fingerprints, of complex mixtures of volatile compounds.
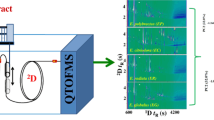
A GC × GC-HR ToFMS method was developed, it being characterized by a 44.7-min duration and a variable temperature gradient, namely, rapid in the first part (up until 280 °C) and slower after (to 360 °C at 2.5 °C min−1). The method provided a detailed view of the heavier molecular weight (MW) compounds, such as the sterols. Modulation period was 4 s, with cold and hot jet durations of 0.6 and 1.4 s, respectively. A total ion current (TIC) two-dimensional (2D) chromatogram of the EVOO1 fingerprint is illustrated in Fig. 1, highlighting its compositional complexity (acquisition started at 12 min). A spectral production frequency of 120 Hz was applied, it being sufficient both for the purposes of effective deconvolution and reliable quantification.
TIC GC × GC-HR ToFMS chromatogram of sample EVOO1. For peak identification refer to Table 1
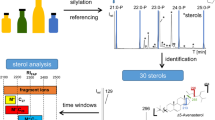
In general, peak identification was achieved by exploiting commercial unit-mass MS databases, accurate mass data, literature and on-line freely-available mass spectral information. Moreover, an in-lab HR MS lipid database (containing mainly derivatized sterol spectra) was exploited; such a database was in-the-past constructed, for the main part, through the analysis of a series of vegetable oils through CM GC × GC-HR ToFMS.
An MS database search was launched, with only the matches characterized by a minimum spectral similarity of 800 considered: 8 linear saturated hydrocarbons and 2 linear alcohols, practically all eluted along a diagonal (Fig. 1), and were tentatively identified with good spectral similarities (Table 1). Compound numbering is related to elution order, and to the overall number of peaks assigned in all the vegetable oils. Considering the moderate degree of polarity of the second dimension (2D), the elution pattern can be related to the 2D separation temperatures and to the fact that the alcohols are analyzed as trimethylsilyl ethers (polarity is greatly reduced): for example, heptacosane (peak 6—C27H56) is subjected to lower 2D temperatures compared with tetracosan-1-ol (peak 7—C27H58SiO), leading to very similar retention times in the second column. The accurate mass data was not investigated for the linear hydrocarbons and alcohols due to the satisfactory spectral similarities and organized elution pattern; instead, the accurate mass data was helpful to support the identification of higher MW compounds, in particular sterols.
As was expected, the largest peak of the 2D fingerprint was that correspondent to squalene (peak 9; similarity = 928). Additionally, its mass spectrum contained a molecular ion with a mass error of 1.7 ppm. With regard to the most information-rich part of the chromatogram (here defined as the “sterol zone”), enclosed in a rectangle in Fig. 1, thirteen acceptable MS database matches (HR ToFMS database) were attained. Valuable MW information was also attained in nine cases, and among these (peaks 28, 31, 32, 33, 35, 40), further identitied information was attained from the literature. In three cases (peaks 36, 38, 39) in which only an acceptable database match was attained, the literature was also consulted for MS information. For the remaining compound, cholesterol (peak 20), peak assignment of this low-concentration constituent was performed only by using the HR ToFMS database.
Among the thirteen compounds which refer to the sterol zone, there are six desmethylsterols, one methylsterol, three dimethylsterols, two triterpenic alcohols, and one triterpenic dialcohol. In general, mass accuracy for all 10 compounds (squalene is also considered) was satisfactory: an average value of 3.0 ppm (absolute value) was calculated. With regard to the HR ToFMS database similarity values, these were acceptable with an average calculated value of 887. The high-resolution mass spectrum of β-sitosterol (peak 29), the most abundant sterol in EVOO, is illustrated in Fig. 2a. The HR ToFMS database match for this compound (920) was satisfactory and an accurate-mass molecular ion (− 0.8 ppm) is evident. A high-resolution mass spectrum of parkeol (peak 32), a dimethylsterol, is shown in Fig. 2b. In this instance, a spectral similarity of 847 and a mass accuracy of 1.2 ppm were obtained.
Considering the sterol zone in the other 8 EVOO samples, an additional two compounds were detected: campestanol (a desmethylsterol) and uvaol (a triterpenic dialcohol). All fifteen compounds have been previously related to olive oil (Boskou et al. 2006; Tranchida et al. 2013b). Mass spectral information for the other 8 EVOO samples can be found in Supplementary Material (Tables S1–8); for samples EVOO2 (Table S1) and EVOO3 (Table S2), the information is related to the entire 2D fingerprint, while for the other EVOO samples, the information relates to the sterol zone. Average mass accuracies for the 8 samples were in the range 2.1–4.9 ppm (absolute values), while the average spectral similarities varied in the range 868–903.
A chromatogram expansion showing the sterol zone of hazelnut oil (HO) is illustrated in Fig. 3a, compared with the EVOO samples the sterol zone fingerprint was entirely different. In such a respect, the sterol zone of sample EVOO9 is illustrated in Fig. 3b, aligned below that of HO. In the latter, a dehydration product of β-sitosterol, namely, stigmasta-3,5-diene (peak 19), was identified exploiting MW accurate-mass information and the HR ToFMS database. Stigmasta-3,5-diene is a stigmastadiene, a class of compounds formed through refining processes (e.g., deodorizing) (Amelio et al. 1998). The circled zone in Fig. 3a (containing peak 19), in itself a smaller fingerprint inside a larger one, is totally absent in Fig. 3b. Furthermore, sitostanol (a desmethylsterol—peak 30) was found in the HO but not in the EVOO samples. It is noteworthy that the presence of sitostanol in olive oil has been reported (Boskou et al. 2006). Again, and for HO, mass accuracy and spectral similarity values were satisfactory; average values of 3.0 ppm (absolute value) and 882, respectively, were calculated (Table 2).
Compared to EVOO1, only cholesterol was not found in EVOO9. It is noteworthy that cholesterol must not be present in percentages exceeding 0.5% (with respect to the other sterols). Adulterations with palm oil or with a lipid of animal origin would lead to higher percentage values (Tranchida et al. 2013b). Finally, stigmasterol (peak 26), which was one of the sterols with the lowest abundance in Fig. 3b, was reconstructed with 34 data points meaning that it possessed a peak width at the base of circa 280 ms. Such a number of data points is more than sufficient for the purposes of effective deconvolution and reliable quantification (if performed). It is worthy of note that stigmasterol must be present in lower concentrations compared with campesterol, with this being in percentages not exceeding 4%. If, on the other hand, the % of stigmasterol is higher than that of campesterol, then there would be the possible suspected presence of soybean oil (Tranchida et al. 2013b).
With regard to the peanut oil (PO) and soybean oil (SO), information related to the entire 2D fingerprint is reported in Tables S9 and S10, respectively. Apart from stigmasta-3,5-diene, found in both oils, additional peaks assigned in the sterol zone, and not found in samples EVOO1–9, were as follows: Δ7-stigmastenol (a desmethylsterol) in both PO and SO, and brassicasterol (a desmethylsterol), 24-methylenecholesterol (a desmethylsterol), Δ7-campesterol (a desmethylsterol), and Δ7-avenasterol (a desmethylsterol) only in the SO. Even though not detected in samples EVOO1–9, the presence of Δ7-stigmastenol and brassicasterol is regulated in extra-virgin olive oils, with maximum % values of 0.5% and 0.1%, respectively. Both were found in the previous GC × GC-FID/QMS study on EVOO samples (Tranchida et al. 2013b). In such a respect, the total number of assigned peaks in the sterol zone in the previous and present study reached 24 and 22, respectively.
Conclusions
A CM GC × GC-HR ToFMS approach for the detailed qualitative profiling of vegetable oils has been developed. The method, a relatively-rapid one, will be exploited for the analysis of 100 s of samples of EVOO for the creation of a sample-specific fingerprint, within the context of an EVOO research project.
Apart from method development and application, a further objective of the present study consisted in the evaluation of the performance of the HR ToFMS system. Such instrumentation was found to be certainly suitable for the challenging circumstances of CM GC × GC analyses. In general, mass accuracy was acceptable, with poorer values observed for the less-abundant compounds.
References
Amelio M, Rizzo R, Varazini F (1998) Separation of stigmasta-3,5-diene, squalene isomers, and wax esters from olive oils by single high-performance liquid chromatography run. J Am Oil Chem Soc 75:527–530. https://doi.org/10.1007/s11746-998-0259-5
Boskou D, Blekas G, Tsimidou M (2006) Olive oil composition. In: Boskou D (ed) Olive oil, chemistry and technology, 2nd edn. AOCS Press, Champaign, Illinois, pp 41–72
Byer JD, Siek K, Jobst K (2016) Distinguishing the C3 vs CH4 mass split by comprehensive two-dimensional gas chromatography-high resolution time-of-flight mass spectrometry. Anal Chem 88:6101–6104. https://doi.org/10.1021/acs.analchem.6b01137
Frysinger GS, Gaines RB (1999) Comprehensive two-dimensional gas chromatography with mass spectrometric detection (GC×GC/MS) applied to the analysis of petroleum. J High Resolut Chromatogr 22:251–255. https://doi.org/10.1002/(SICI)1521-4168(19990501)22:5<251::AID-JHRC251>3.0.CO;2-V
Hernández F, Portolés T, Pitarch E, López FJ (2011) Gas chromatography coupled to high-resolution time-of-flight mass spectrometry to analyze trace-level organic compounds in the environment, food safety and toxicology. TrAC Trend Anal Chem 30:388–400. https://doi.org/10.1016/j.trac.2010.11.007
Liu Z, Phillips JB (1991) Comprehensive two-dimensional gas chromatography using an on-column thermal modulator interface. J Chromatogr Sci 29:227–231. https://doi.org/10.1093/chromsci/29.6.227
Marriott PJ, Chin ST, Maikhunthod B, Schmarr HG, Bieri S (2012) Multidimensional gas chromatography. TrAC Trend Anal Chem 34:1–21. https://doi.org/10.1016/j.trac.2011.10.013
Tranchida PQ, Salivo S, Bonaccorsi I, Rotondo A, Dugo P, Mondello L (2013a) Analysis of the unsaponifiable fraction of lipids belonging to various milk-types by using comprehensive two-dimensional gas chromatography with dual mass spectrometry/flame ionization detection and with the support of high resolution time-of-flight mass spectrometry for structural elucidation. J Chromatogr A 1313:194–201. https://doi.org/10.1016/j.chroma.2013.07.089
Tranchida PQ, Salivo S, Franchina FA, Bonaccorsi I, Dugo P, Mondello L (2013b) Qualitative and quantitative analysis of the unsaponifiable fraction of vegetable oils by using comprehensive 2D GC with dual MS/FID detection. Anal Bioanal Chem 405:4655–4663. https://doi.org/10.1007/s00216-013-6704-9
Tranchida PQ, Franchina FA, Salivo S, Russo M, Dugo P, Mondello L (2014) Flow-modulated comprehensive 2D gas chromatography-triple quadrupole MS elucidation of the fatty acids and unsaponifiable constituents of oil derived from lemon seeds, a food-industry waste product. LCGC N Am 32:24–29
Tranchida PQ, Salivo S, Franchina FA, Mondello L (2015) Flow-modulated comprehensive two-dimensional gas chromatography combined with a high-resolution time-of-flight mass spectrometer: a proof-of-principle study. Anal Chem 87:2925–2930. https://doi.org/10.1021/ac5044175
Tranchida PQ, Aloisi I, Giocastro B, Mondello L (2018) Current state of comprehensive two-dimensional gas chromatography-mass spectrometry with focus on processes of ionization. TrAC Trend Anal Chem 105:360–366. https://doi.org/10.1016/j.trac.2018.05.016
Acknowledgments
The research was performed within the context of the project AGER2-Rif 2016-0169, “Valorizzazione dei prodotti italiani derivanti dall’oliva attraverso tecniche analitiche innovative”—“Violin”. The authors thank LECO Corporation and Merck Life Science for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent is not applicable for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 59.3 kb)
Rights and permissions
About this article
Cite this article
Aloisi, I., Zoccali, M., Dugo, P. et al. Fingerprinting of the Unsaponifiable Fraction of Vegetable Oils by Using Cryogenically-Modulated Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry. Food Anal. Methods 13, 1523–1529 (2020). https://doi.org/10.1007/s12161-020-01773-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01773-9