Abstract
In this study, the molecularly imprinted nano-polymer of minocycline was polymerized on the surface of a metal organic framework material to synthesize a novel composite. This composite was used as absorbent to develop a dispersive solid phase microextraction method for extraction of 7 tetracyclines in chicken muscle followed by determination with ultra performance liquid chromatography. The composite achieved high absorption capacities (2200–3000 ng/mg) and high recoveries (> 92%) for the 7 tetracyclines, and could be reused for 8 times. Due to the high enrichment factors (18–37), the limits of detection for the 7 drugs were in the range of 0.2–0.6 ng/g, and the limits of quantification were in the range of 0.5–2.0 ng/g. The recoveries of the 7 drugs from standard-fortified blank chicken muscle sample were in the range of 69.6–94.7%. Therefore, this method could be used as a practical tool for multi-detection of the residues of tetracyclines in meat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a class of broad spectrum antibiotics, tetracycline drugs (TCs) are commonly used to treat various bacterial infections in human beings and animals. However, their residues in foods of animal origin may cause different risks to the consumers, e.g., allergic reaction, tetracycline pigmentation teeth, and gastrointestinal disturbance (Michalova et al. 2004; Roesch et al. 2006). For protection of consumer health, many countries including the People’s Republic of China and the European Union have set the maximum residue limit (MRL) of 100 ng/g for single or total TCs in different animal derived food samples (European Commission 1999; Ministry of Agriculture of China 2002).
Therefore, many analytical methods have been reported to determine TCs residues (Pérez-Rodríguez et al. 2018). Among the reported analytical methods, the key step is to extract and purify the samples in order to remove the sample impurities and increase the analyte concentrations. The usually used sample extraction and purification methods include solid phase extraction, matrix solid phase dispersion, stir bar sorptive extraction, solid phase microextraction, dispersive solid phase microextraction, single drop microextraction, and dispersive liquid-liquid microextraction.
Among these sample preparation methods, dispersive solid phase microextraction method (DSPME) is very attractive (Chisvert et al. 2019). For this method, the absorbent is put into the sample extract to be shaken for several minutes, and then the analytes absorbed on the absorbent surface are desorbed for analysis. Therefore, its operation is simple, and the extraction and purification processes can be accomplished in one step. Furthermore, this method can achieve different enrichment effects depending on the used absorbent. Therefore, the used absorbent plays the crucial role for a DSPME method because its absorption capacity, absorption specificity, and surface area directly influence the method performance. By now, some materials including polyaniline-SiO2 nanofiber (He et al. 2015), multi-walled carbon nanotubes (Du et al. 2012), graphene (He et al. 2017), and nanoparticles (Roman et al. 2011) have been used to develop DSPME methods for extraction of different analytes.
During the past few years, metal organic frameworks (MOF) have attracted the interests of many researchers because this kind of material is a class of novel synthetic materials that contain large surface area, porous surface, and high adsorption affinity (Hashemi et al. 2017). Therefore, many MOF materials have been used to develop DSPME methods for extraction of different analytes (Rocío-Bautista et al. 2017), such as organic pollutants (Rocío-Bautista et al. 2018), and herbicides (Li et al. 2015). For TCs, there has been only one paper reporting the preparation of a MOF-based magnetic stir cake to extract TCs from food samples (Du et al. 2019). However, all of the abovementioned sample preparation methods employ the non-specific absorbents, so the competitive absorption and interferential absorption may occur during the extraction and purification process when a real sample simultaneously contains different classes of compounds.
Molecularly imprinted polymer (MIP) is a type of synthetic material that has specific recognition ability, so this kind of material has been used to develop many extraction methods for the different analytes (Kubo and Otsuka 2016). By now, there have been some papers reporting the use of MIP as absorbent to develop the extraction methods for TCs (Feng et al. 2016; Jing et al. 2009, 2010; Lv et al. 2012; Lian et al. 2012; Sánchez-Polo et al. 2015), but there have been only two MIP-based dispersive solid phase extraction methods reported to extract TCs (Chen et al. 2009; Kong et al. 2012). As discussed above, a sample preparation method simultaneous containing the merits of DSPME, MOF, and MIP is desirable.
Until recently, there are several articles reporting the use of MIP-MOF composite to develop matrix solid phase dispersion methods for extraction of pyrethroids (Liang et al. 2019) and TCs (Wang et al. 2018). As far as we know, there has been no article reporting the use of MIP-MOF composite to develop the DSPME method for extraction and purification of veterinary drugs in poultry products so far. In the present study, a MOF material was synthesized, and a type of nano-MIP for TCs was polymerized on the MOF surface to produce a MIP-MOF composite. This composite was used as absorbent to develop a DSPME method for extraction of TCs in chicken muscle sample followed by determination with ultra performance liquid chromatography/photo-diode array detector (UPLC-PDA).
Materials and Methods
Chemicals and Reagents
Minocycline (MC), chlortetracycline (CTC), tetracycline (TC), oxytetracycline (OTC), demeclocycline (DMC), and doxycycline (DC) were obtained from Sigma-Aldrich (St. Louis, USA). Methacycline (MTC) was purchased from J&K Scientific Ltd. (Beijing, China). Methacrylic acid (MA) was obtained from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). N-t-Butylacrylamide (TBAm) was purchased from TCI (Tokyo Japan). Ammonium persulfate (APS) was purchased from Fuchen Chemical Company (Tianjin, China). Acrylamide (AA), N,N-methylenebisacrylamide (BIS), N,N,N,N-tetramethylethylenediamine (TEMED), and sodium dodecyl sulfate (SDS) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). ZrCl4 and 2-amino-1,4-benzene-dicarboxylic acid (H2N-H2bda) were obtained from TCI Chemicals (Tokyo Japan). Tetrabutyl titanate (TBT) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Liquid chromatographic grade acetonitrile was purchased from Dikma (Richmond Hill, USA). Other common chemical reagents of analytical grade were purchased from Beijing Chemical Company (Beijing, China). The stock solutions of the 7 TCs (100 μg/mL) and their working solutions with concentrations of 0.1–200 ng/mL were prepared with methanol, respectively.
Synthesis of MOF Material UiO-66
The MOF material UiO-66 was synthesized according to a previous literature (Katz et al. 2013). Briefly, 60 mL dry N,N-dimethylformamide (DMF) and 10 mL concentrated hydrochloric acid were added into a 100-mL Teflon-lined stainless steel autoclave, and then 1.25 g ZrCl4 and 0.1812 g H2N-H2bda were dissolved in the mixture. The autoclave was sealed and heated in an oven at 80 °C for 12 h under autogenous pressure. After cooling down to room temperature, the reaction system was washed with DMF, and the sample was purified with anhydrous methanol for several times to eliminate DMF. The yellow product was dried at 80 °C for 12 h to obtain UiO-66.
Synthesis of MIP-MOF Composite
The nano-MIP was synthesized according to a recent report (Cenci et al. 2015) that was directly polymerized on the surface of UiO-66 to produce the composite MIP-MOF. Briefly, 8 mg AA, 8 mg MA, 4 mg TBAm, 80 mg BIS, and 0.02 mg SDS were dissolved in 10 mL water, and the solution was filtered with a 0.2-μm filter. Then, 0.1 mmol template MC was added into the above solution, and the mixture was sonicated for 10 min and purged with nitrogen stream for 30 min. After that, 4 mg APS, 3.87 μL TEMED, and 80 mg UiO-66 were added, and the mixture was stirred at room temperature for 20 h. The suspension was centrifuged at 10,000 rpm for 15 min, and the obtained sediments were transferred into a Soxhlet apparatus to be extracted for 6 h with methanol/acetic acid (9:1, v/v). Finally, the suspension was filtered and the particles were washed with methanol and dried to obtain the MIP-MOF. At the same time, a type of controlled MIP without addition of UiO-66 and a type of controlled non-imprinted polymer composite without addition of the template MC (NIP-MOF) were also synthesized for comparison with the MIP-MOF. The MIP, MOF, and MIP-MOF were characterized with Fourier transform infrared (FT-IR) and transmission electron microscope (TEM) techniques, respectively.
Evaluation of MIP-MOF Absorption Ability
For comparing the absorption ability, MIP-MOF, NIP-MOF, MOF, and MIP (20 mg) were added into 5 mL of the standard solution of 5 test drugs respectively to be incubated for different times (MC, sulfadiazine, amoxicillin, chloramphenicol, and clenbuterol; 10 μg/mL, 5–240 min). After incubation and centrifugation, the analyte amounts of each drug absorbed by the four absorbents were estimated respectively by measuring the supernatants based on their calibration curves (developed on a UV spectrophotometer at their respective maximum absorption wavelengths).
Development of the DSPME Method
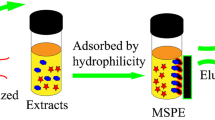
The schematic representation of the MIP-MOF-DSPME procedure is shown in Fig. 1. The isolation of TCs from the chicken muscle sample was according to a previous method (Nikolaidou et al. 2008). Briefly, the chicken muscle samples were homogenized before extraction, and 1 g sample and 10 mL 0.4 M oxalate buffer (pH 4.0) were added into a centrifuge tube. Then, the tube was vortexed for 5 min and centrifuged at 6000 rpm for 5 min. The supernatant was decanted into a clean tube, and 5 mg MIP-MOF was added. The mixture was shaken for 15 min and centrifuged at 10,000 rpm for 5 min. The supernatant was decanted, and 1 mL methanol was added to desorb the analyte from MIP-MOF by vortex for 5 min. After centrifugation at 10,000 rpm for 5 min, the eluate was transferred into a clean tube and evaporated to dryness in a 45 °C water bath under cold nitrogen gas. The dry residue was dissolved in 200 μL methanol for UPLC analysis. Finally, the MIP-MOF particles were washed with 5 mL methanol for the next use.
In this study, the 7 TCs were diluted with the extracts of blank chicken samples to prepare the matrix-matched solutions (100 ng/mL) for evaluation of the enrichment effect. The enrichment factor (EF) for each drug was calculated as C/C0, where C is the analyte concentration after DSPME and C0 is 100 ng/mL (He et al. 2017). During the experiments, the MIP-MOF amount, sample pH, absorption time, and elution solvent were optimized respectively with MC as the representative drug and with EF as the criterion. In addition, the recycle performance of the composite was tested.
Sample Determination
Some blank chicken muscle samples were obtained from a controlled slaughterhouse in Baoding, Hebei China. The 7 TCs were diluted with the blank extracts to evaluate the MIP-MOF-DSPME-UPLC method. The limit of detection (LOD) for each drug was calculated as the drug concentration corresponding to S/N = 3, and the limit of quantification (LOQ) was calculated as S/N = 10. Then, the 7 TCs were fortified into the blank chicken samples (1, 10, and 100 ng/g) to be analyzed respectively as described above. The intra-day recovery (six repetitions at each fortification level in a single day) and the inter-day recovery (duplicate injections at each fortification level on six successive days) for each drug were calculated respectively. Finally, 80 chicken muscle samples were collected from some supermarkets in China and analyzed by the developed method.
UPLC Conditions
The UPLC system consisted of ACQUITY H-CLASS liquid chromatography, PDA detector and HSS T3 column (2.1 × 100 mm, 1.8 μm) (Waters, USA). The mobile phase consisted of (A) acetonitrile/methanol (3:2, v/v) and (B) 0.01% oxalic acid with gradient elution. The gradient elution program was: started with 5% (A), linearly increased to 40% (A) in 5.0 min, further increased to 80% (A) in 5.0 min and maintained for 2 min, finally brought back to 5% (A). The flow rate was 0.3 mL/min, the injection volume was 10 μL, and the detection wavelength was 350 nm.
Results and Discussions
Optimization of UPLC Conditions
For simultaneous determination of the 7 TCs with UPLC, the separation conditions were optimized for shortening the analysis time and obtaining the optimal peak shape. During the experiments, acetonitrile and methanol incorporating different proportions of acid at different gradient programs were compared. Results showed that the separation conditions in “UPLC Conditions” achieved the best separation. The retention times of the 7 TCs are shown in Table 1, and the representative chromatograms are shown in Fig. 2.
Characterization of MOF and MIP-MOF
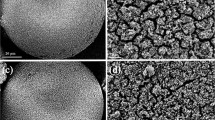
In the past few years, many MOF materials have been reported (Hashemi et al. 2017; Rocío-Bautista et al. 2017). In the present study, the MOF material UiO-66 was synthesized according to a previous method with Zr as metal ion and H2N-H2bda as organic ligand (Katz et al. 2013). As shown in Fig. 3a, the results from Fourier transform infrared analysis (FT-IR) showed that the MOF contained the characteristic peaks of NH2 and O-H bond (around 3449 cm−1), phenyl ring (1655–1264 cm−1), and Zr (770, 482 cm−1), indicating UiO-66 was obtained. The MIP-MOF composite contained the peaks from MOF (Zr, 770, 482 cm−1) and MIP (alkyl group, 2929, 1446, and 711 cm−1, C=C bond 903 cm−1), indicating a new composite was obtained. At the same time, the characteristic peaks of phenyl ring and Zr in MIP-MOF decreased due to the coated polymer shells (Fig. 3a). As shown in Fig. 3b, the results from transmission electron microscopy (TEM) showed that the MIP and the MOF were nano-sized particles, and the MIP-MOF was core-shell structured composite. These results revealed that the MIP-MOF was obtained, so it was used to develop the DSPME method.
Evaluation of MIP-MOF
For evaluation of absorption ability, MOF, MIP, MIP-MOF, and NIP-MOF were used to absorb 5 test drugs respectively (MC, sulfadiazine, amoxicillin, clenbuterol, and chloramphenicol). As shown in Fig. 4a, MOF showed comparably high absorption amounts for the 5 drugs, NIP-MOF showed comparably low absorption amounts for the 5 drugs, and MIP and MIP-MOF only showed high absorption amounts for MC. These results indicated that MIP and MIP-MOF showed specific absorption whereas MOF and NIP-MOF showed non-specific absorption. The absorption amount for MC from MIP-MOF was lower than that from MOF (Fig. 4a), which was because the entire MOF surface could absorb the analyte whereas only the MC-imprinted pores on MIP-MOF surface could absorb the analyte. In addition, the adsorption kinetic curves shown in Fig. 4b illustrated that MIP-MOF achieved much higher adsorption amount for MC than NIP-MOF at different absorption times, indicating the formed cavities were beneficial for capturing MC molecules. Therefore, the synthesized MIP-MOF could be used to develop the specific extraction method for TCs.
Optimization of MIP-MOF Amount
For a DSPME method, the first step is to select the optimum absorbent amount. In this study, different amounts of MIP-MOF (2–20 mg) were used to perform the DSPME procedure by using the matrix matched MC solution (10 mL, 100 ng/mL). As shown in Fig. 5a, the EFs of MC were generally comparable (26–29), indicating the MIP-MOF contained large absorption capacity and its amount showed negligible influence on analyte absorption at low analyte concentration. For convenient operation, 5 mg MIP-MOF was selected for the subsequent experiments.
Enrichment factors for MC when using a different amounts of MIP-MOF (absorption 30 min, pH 4.0, elution with methanol), b different sample pH (MIP-MOF 5 mg, absorption 30 min, elution with methanol), c different absorption times (MIP-MOF 5 mg, pH 4.0, elution with methanol), and d different elution solvents (MeOH = methanol, ACN = acetonitrile, X = 10% formic acid, Y = 10% acetic acid, MIP-MOF 5 mg, pH 4.0, absorption 15 min)
Optimization of Sample pH
For development of a DSPME method for TCs, the pH value is a critical factor because it influences the ionic form of TCs molecule and the absorbed analyte amount. TCs are the amphoteric compounds that have several dissociation constants: pKa 2.8–3.3, 7.3–8.3, 9.3–10.2 (Samanidou et al. 2007). In this study, the matrix-matched MC solution was adjusted to different pH values (2.0–11.0) for performing the DSPME procedure. As shown in Fig. 5b, the EFs of MC at pH 4.0, 8.0, and 9.0 (29, 27, and 26) were generally higher than those at other pH values (10–23). This was because the MC molecules at the three pH values were mainly as non-dissociation forms, thus obtaining a high absorption amount and high EFs. For convenience, the sample extracts (oxalate buffer, pH 4.0) were used for DSPME procedure directly.
Optimization of Absorption Time
For shortening the sample pretreatment time, the absorption time was optimized. During the experiments, 5 mg MIP-MOF was added into matrix-matched MC solution to be shaken for different times (1–30 min). As shown in Fig. 5c, the EF of MC reached a plateau after 15 min shaking (26–28), so 15 min was selected as the optimal absorption time.
Optimization of Elution Solvent
For a MIP-based extraction method, the critical factor is to find an optimal elution solvent to elute the absorbed analyte from the absorbent. In this study, several kinds of elution solvents including methanol, acetonitrile, and their mixtures with formic acid and acetic acid at different volume proportions were used to elute MC from MIP-MOF. As shown in Fig. 5d, the EFs of MC when adding acid were higher than those when using pure methanol and pure acetonitrile. However, the use of acid in elution solvent damaged the MOF structure, and there was a large impurity peak in UPLC chromatogram. General consideration, methanol was selected as the optimal elution solvent.
Performance of the MIP-MOF-DSPME-UPLC Method
Under the optimal conditions, the matrix-matched solutions of the 7 TCs (10 μg/mL, 10 mL) were used to estimate the MIP-MOF absorption capacity according to the procedures in “Evaluation of MIP-MOF” absorption ability. As shown in Table 1, MIP-MOF simultaneously recognized the 7 drugs, and the absorption capacities were in the range of 2200–3000 ng/mg. The previously reported MIP materials for TCs recognized at most 4 drugs (Feng et al. 2016; Jing et al. 2009, 2010; Lv et al. 2012; Lian et al. 2012; Sánchez-Polo et al. 2015; Chen et al. 2009; Kong et al. 2012; Wang et al. 2018), so the recognition spectrum of the present MIP material was broader than those MIPs.
Furthermore, the matrix-matched solutions of the 7 TCs (100 ng/mL) were used to evaluate the MIP-MOF-DSPME method. As shown in Table 1, the DSPME method showed high recoveries (92%– 97%) and high EFs (18–37) for the 7 drugs. As shown in Fig. 2, the chromatograms of TC standards before and after DSPME procedure illustrated the high enrichment effect. Due to the high enrichment effect, the LODs of the MIP-MOF-DSPME-UPLC method for the 7 drugs were in the range of 0.2–0.6 ng/mL and the LOQs were in the range of 0.5–2.0 ng/mL (Table 1).
During the experiments, the matrix-matched MC solution (100 ng/mL) was used to perform the DSPME procedure for successive 10 times. Results showed that the recoveries were generally stable when repeating the experiments for 8 times (RSD < 4.7%), and decreased about 26% when repeating for 9 times, indicating the MIP-MOF was a durable and recyclable absorbent. Then, the 7 TCs were fortified into the blank chicken muscle samples to evaluate the MIP-MOF-DSPME-UPLC method. As shown in Table 2, the inter-assay recoveries were in the range of 69.6–94.6% with the coefficients of variation of 5.2–11.6%, and the intra-assay recoveries were in the range of 72.8–94.7% with the coefficients of variation of 5.1–9.8%. As shown in Fig. 2, the UPLC chromatograms of the blank chicken and the TC-fortified blank chicken indicated the satisfactory purification effect.
Sample Analysis
The 80 real chicken muscle samples were analyzed with the present method. Results showed that one sample contained TC residue (21 ng/g) and another sample contained CTC residue (49 ng/g), but their residual levels were all lower than their maximum residue limits (100 ng/g). Other real samples were all determined as negative samples by the method. Therefore, this MIP-MOF-DSPME-UPLC method could be used as a useful tool for multi-determination of the 7 TCs in meat samples.
Comparison with Related Methods
The present study for the first time reported a MIP-MOF composite–based DSPME method for extraction of TCs in poultry products. For comparison with the related methods, the details of the previously reported MOF-based sorptive method (Du et al. 2019), MIP-DSPME methods (Chen et al. 2009; Kong et al. 2012), and MIP-MOF-based matrix solid phase dispersion method (Wang et al. 2018) for extraction of TCs are summarized in Table 3. General considerations on sample pretreatment time, multi-determination ability, and enrichment effect and sensitivity, the authors encourage the use of the MIP-MOF-DSPME-UPLC method for the determination of TCs in poultry products.
Conclusion
As the promising absorbents, MOF and MIP have been used to develop many sample preparation methods for extraction and purification of different analytes. In this study, a type of nano-MIP for TCs was directly polymerized on the surface of a MOF material to generate a novel composite. A simple dispersive solid phase microextraction method was developed for extraction of 7 TCs in the chicken muscle sample followed by UPLC determination. Results showed that this method was simple, rapid, sensitive, and accurate, so it could be used as a routine tool for multi-detection of the residual TCs in meat sample.
References
Cenci L, Andreetto E, Vestri A, Bovi M, Barozzi M, Iacob E et al (2015) Surface plasmon resonance based on molecularly imprinted nanoparticles for the picomolar detection of the iron regulating hormone Hepcidin-25. J Nanobiotechnol 13:51–65
Chen LG, Liu J, Zeng QL, Wang H, Yu AM, Zhang HQ, Ding L (2009) Preparation of magnetic molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and tissue samples. J Chromatogr A 1216:3710–3719
Chisvert A, Cárdenas S, Lucena R (2019) Dispersive micro-solid phase extraction. TrAC-Trend Anal Chem 112:226–233
Du XD, Wu YL, Yang HJ, Yang T (2012) Simultaneous determination of 10 β2-agonists in swine urine using liquid chromatography-tandem mass spectrometry and multi-walled carbon nanotubes as a reversed dispersive solid phase extraction sorbent. J Chromatogr A 1260:25–32
Du F, Sun L, Tan W, Wei Z, Nie H, Huang Z et al (2019) Magnetic stir cake sorptive extraction of trace tetracycline antibiotics in food samples: preparation of metal-organic framework-embedded polyHIPE monolithic composites, validation and application. Anal Bioanal Chem 411:2239–2248
European Commission (1999) Off J Eur Union 60:16–52
Feng MX, Wang GN, Yang K, Liu HZ, Wang JP (2016) Molecularly imprinted polymer-high performance liquid chromatography for the determination of tetracycline drugs in animal derived foods. Food Control 69:171–176
Hashemi B, Zohrabi P, Raza N, Kim KH (2017) Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. TrAC-Trend Anal Chem 97:65–82
He XM, Zhu GT, Zheng HB, Xu SN, Yuan BF, Feng YQ (2015) Facile synthesis of polyaniline-coated SiO2 nanofiber and its application in enrichment of fluoroquinolones from honey samples. Talanta 140:29–35
He X, Wang GN, Yang K, Liu HZ, Wu XJ, Wang JP (2017) Magnetic graphene dispersive solid phase extraction combining high performance liquid chromatography for determination of fluoroquinolones in foods. Food Chem 221:1226–1231
Jing T, Gao XD, Wang P, Wang Y, Lin YF, Hu XZ, Hao QL, Zhou YK, Mei SR (2009) Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography tandem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction. Anal Bioanal Chem 393:2009–2018
Jing T, Wang Y, Dai Q, Xia H, Niu J, Hao Q, Mei S, Zhou Y (2010) Preparation of mixed-templates molecularly imprinted polymers and investigation of the recognition ability for tetracycline antibiotics. Biosens Bioelectron 25:2218–2224
Katz MJ, Brown ZJ, Colon YJ, Siu PW, Scheidt KA, Snurr RQ et al (2013) A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem Commun 49:9449–9451
Kong J, Wang Y, Nie C, Ran D, Jia X (2012) Preparation of magnetic mixed-templates molecularly imprinted polymer for the separation of tetracycline antibiotics from egg and honey samples. Anal Methods 4:1005–1011
Kubo T, Otsuka K (2016) Recent progress in molecularly imprinted media by new preparation concepts and methodological approaches for selective separation of targeting compounds. TrAC-Trend Anal Chem 81:102–109
Li N, Zhang L, Nian L, Cao B, Wang Z, Lei L, Yang X, Sui J, Zhang H, Yu A (2015) Dispersive micro-solid-phase extraction of herbicides in vegetable oil with metal-organic framework MIL-101. J Agric Food Chem 63:2154–2161
Lian W, Huang J, Yu J, Zhang X, Lin Q, He X et al (2012) A molecularly imprinted sensor based on β-cyclodextrin incorporated multiwalled carbon nanotube and gold nanoparticles-polyamide amine dendrimer nanocomposites combining with water-soluble chitosan derivative for the detection of chlortetracycline. Food Control 26:620–627
Liang T, Wang S, Chen L, Niu N (2019) Metal organic framework molecularly imprinted polymer as adsorbent in matrix solid phase dispersion for pyrethroids residue extraction from wheat. Food Anal Method 12:217–228
Lv YK, Wang LM, Yang L, Zhao CX, Sun HW (2012) Synthesis and application of molecularly imprinted poly(methacrylic acid)-silica hybrid composite material for selective solid-phase extraction and high-performance liquid chromatography determination of oxytetracycline residues in milk. J Chromatogr A 1227:48–53
Michalova E, Novotna P, Schlegelova J (2004) Tetracyclines in veterinary medicine and bacterial resistance to them. Vet Med Czech 49:79–100
Ministry of Agriculture of China (2002) Regulation No. 235 of Ministry of Agriculture, Ministry of Agriculture of China, Peoples Republic of China
Nikolaidou KI, Samanidou VF, Papadoyannis IN (2008) Development and validation of an HPLC method for the determination of seven tetracycline antibiotics residues in chicken muscle and egg yolk according to 2002/657/EC. J Liq Chromatogr R T 31:2141–2158
Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR (2018) An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182:1–21
Rocío-Bautista P, Gonzalez-Hernandez P, Pino V, Pasan J, Afonso AM (2017) Metal-organic frameworks as novel sorbents in dispersive-based micro-extraction approaches. TrAC-Trend Anal Chem 90:114–134
Rocío-Bautista P, Pino V, Pasan J, Lopez-Hernandez I, Ayala JH, Ruiz-Perez C et al (2018) Insights in the analytical performance of neat metal organic frameworks in the determination of pollutants of different nature from waters using dispersive miniaturized solid-phase extraction and liquid chromatography. Talanta 179:775–783
Roesch M, Perreten V, Doherr MG, Schaeren W, Schallibaum M, Blum JW (2006) Comparison of antibiotic resistance of udder pathogens in dairy cows kept on organic and on conventional farms. J Dairy Sci 89:989–997
Roman IP, Alberto AC, Canals A (2011) Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J Chromatogr A 1218:2467–2475
Samanidou VF, Nikolaidou KI, Papadoyannis IN (2007) Advances in chromatographic analysis of tetracyclines in foodstuffs of animal origin-A review. Sep Purif Rev 36:1–69
Sánchez-Polo M, Velo-Gala I, López-Penlver JJ, Rivera-Utrilla J (2015) Molecular imprinted polymer to remove tetracycline from aqueous solutions. Micropor Mesopor Mat 203:32–40
Wang S, Zhang J, Li C, Chen L (2018) Analysis of tetracyclines from milk powder by molecularly imprinted solid-phase dispersion based on a metal-organic framework followed by UHPLC-MS/MS. J Sep Sci 41:2604–2612
Funding
The authors are financially supported by the National Natural Science Foundation of China (31801645, 21603054), Hebei Key Research and Development Project (18226628D), and Hebei Layer/Broiler Industry Technology System (HBCT2018150210).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ning Ma declares no conflict of interest. Cheng Feng declares no conflict of interest. Ping Qu declares no conflict of interest. Ge Wang declares no conflict of interest. Jing Liu declares no conflict of interest. Ju Xiang Liu declares no conflict of interest. Jian Ping Wang declares no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with humans.
Informed Consent
All the authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, N., Feng, C., Qu, P. et al. Determination of Tetracyclines in Chicken by Dispersive Solid Phase Microextraction Based on Metal-Organic Frameworks/Molecularly Imprinted Nano-polymer and Ultra Performance Liquid Chromatography. Food Anal. Methods 13, 1211–1219 (2020). https://doi.org/10.1007/s12161-020-01744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01744-0