Abstract
In this study, a simple and rapid dispersive liquid-liquid microextraction method was established, and the residuals of four isomers of hexachlorocyclohexane and six kinds of pyrethroid pesticides in milk were simultaneously determined by gas chromatography electron capture detector (GC-ECD). The milk sample was first extracted with acetonitrile and cleaned with primary secondary amine (PSA). Then 0.5 mL of acetonitrile was mixed with 140 μL of cyclohexane and rapidly injected into 3 mL of pure water. After vortexing and centrifugation, the floating phase was removed with a 0.1-mL pipette into the GC-ECD. The type and volume of extraction solvent, volume of disperser solvent, volume of water, vortex time, and amount of salt were optimized. Under optimal extraction conditions, the ten pesticides showed a good linear relationship in a certain concentration range in milk matrix, and the correlation coefficients were greater than 0.99. The limits of detection ranged from 0.07 to 2 μg/kg, and the limits of quantitation ranged from 0.2 to 5 μg/kg. The average recovery rates were between 70.1% and 106.3%, and the relative standard deviations were less than 15.2%. This method can be used for the determination of hexachlorocyclohexane and pyrethroid pesticides in milk and for subsequent research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk is very important in the human diet and contains nutrients, such as proteins, that are necessary for human health. Because some pesticides are distributed in crops and even contaminate soil and water, these compounds are enriched in the food chain and are therefore present in milk (Dagnac et al. 2009).
Hexachlorocyclohexane was widely applied in the world in the 1960s and 1970s. The use of this pesticide stopped in the 1980s due to its high toxicity, long persistence, difficult decomposition, wide distribution, heavy damage, and difficulty of remediation in the environment (Singh and Nelapati 2017). Lindane is still used in a few areas (Berger et al., 2016). After cows ingest feed, forage, water, and soil contaminated with pesticides, these pesticides are converted and accumulate in the cows, so some harmful pesticide residues are detected in the milk (Salas et al. 2003). The maximum residue limit for lindane in milk, as prescribed by the Codex Alimentarius Commission (CAC), is 0.001 mg/kg (CAC 2018).
Pyrethroid pesticides are a kind of insecticide with excellent bioactivity and good environmental compatibility compared with those of organochlorine, organophosphorus, and carbamate pesticides. Pyrethroids have high efficiency, a broad spectrum, low toxicity, and low residual energy and have become one of the main pillars of agricultural and hygienic insecticides due to their biodegradation (Yu et al. 2016). Pyrethroid pesticides can be covalently bonded to some proteins in milk and therefore remain in the milk (Gao et al. 2010). The CAC has stipulated the following maximum residue limits for the studied pyrethroid pesticides: 0.2 mg/kg for bifenthrin, 0.2 mg/kg for cyhalothrin, 0.01 mg/kg for cyfluthrin, 0.2 mg/kg for cypermethrin, 0.01 mg/kg for fenpropathrin, and 0.05 mg/kg for deltamethrin (CAC 2018).
Sample pretreatment is a prerequisite for actual sample analysis. The analytes in the sample are extracted, purified, and concentrated for detection (Liu 2014). Common methods for processing milk samples are liquid-liquid extraction (LLE) (Jank et al. 2012; Knobel et al. 2013) and solid-phase extraction (SPE) (Chung et al. 2010; Maragou et al. 2006), but these methods have some drawbacks, such as the need for a large amount of organic solvent and a long time for analysis. At present, research on sample pretreatment methods is developing in the direction of simplicity, time savings, miniaturization, and the reduction of organic solvents (Wang et al. 2012). Therefore, rapid and environmentally friendly methods such as the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method (Cao et al. 2013; Rúbies et al. 2016), solid-phase microextraction (SPME) (Aguinaga et al. 2007; Campillo et al. 2010), and suspended droplet microextraction (SDME) (Liu et al. 2016) have been developed. In recent years, dispersive liquid-liquid microextraction (DLLME) has also been used as a milk pretreatment method (Asensio-Ramos et al. 2011).
Dispersive liquid-liquid microextraction is a liquid-phase microextraction technique that was proposed by Berijani et al. (2006). DLLME is based on a ternary solvent system such as that used in homogeneous liquid-liquid extraction (Cao et al. 2016). In this method, a suitable extraction solvent and a dispersion solvent mixture are rapidly injected into water by a syringe to form a cloudy solution. The analyte in the sample is extracted into the fine droplets of the extraction solvent. After extraction, the precipitated phase or the enriched phase collected after separation is subjected to chromatography or spectral detection by centrifugation (Rezaee et al. 2010). This method increases the dispersion of the organic extraction solvent in the aqueous phase due to the addition of the dispersant, increases the contact surface between the aqueous phase and the extraction solvent, and enables the target compound to rapidly transfer between the sample solution and the extraction solvent. The method integrates extraction and enrichment, uses less organic solvent, and has a short extraction time and high extraction efficiency (Zgoła-Grześkowiak and Grześkowiak 2011).
The use of trace extraction solvents is a highlight of this technology, which is simple to operate and low in cost. However, commonly applied extraction solvents such as carbon tetrachloride (Liang et al. 2008) and tetrachloroethane (Li et al. 2017) are highly toxic and volatile, easily cause serious harm to operators and the environment, and are mainly employed for the separation and analysis of analytes in water. For food, biological samples, drugs, and other samples with complex matrices, the extraction efficiency is low and the selectivity is poor. Therefore, exploring low-toxicity extractants, studying the combination of this technology with other sample pretreatment techniques, reducing the amount of organic solvent, and improving sample purification and enrichment efficiency have become the main research direction of this technology in recent years (Kokosa 2013; Cao et al. 2015).
The application of dispersive liquid-liquid microextraction has now been extended to the analysis of targets in milk samples. Farajzadeh et al. (2012) and Tuncel & Şenlik. (2016) studied the method of DLLME for the determination of phthalate in milk. A method for the extraction and quantification of four polycyclic aromatic hydrocarbons in milk sample has been developed by Mahmoudpour et al. (2016) using dispersive liquid-liquid microextraction followed by HPLC. Liu et al. (2011) developed a method of dispersive liquid-liquid microextraction to determine polychlorinated biphenyls and polybrominated diphenyl ethers in milk. Campillo et al.(2013) established a dispersive liquid-liquid microextraction method for detecting macrocyclic lactones in milk. Farajzadeh et al. (2011) studied the application of DLLME for the detection of triazole pesticides in milk. And with the development of DLLME, some methods have been improved which were combined with other sample pretreatment methods for milk residual research. Shamsipur et al. (2016) have combined SPE with the DLLME method for the extraction and determination of 19 pesticides in water, milk, honey, and fruit juice. Karaseva et al. (2014) and Miao et al. (2015) have used QuEChERS in combination with DLLME, the two methods were proposed for determining aflatoxins B1 and M1 and organophosphorus pesticides in milk, respectively. Considering environmentally friendly techniques, Farajzadeh and Mogaddam (2016), Miao et al. (2015), and Gao et al. (2018) have developed the DLLME methods for the determination of synthetic phenolic antioxidants, organophosphorus pesticides, and pyrethroid pesticides in milk by floating organic solvent as extract solvent, respectively. Dispersive liquid-liquid microextraction is suitable for the analysis of trace organics and is a promising environmentally friendly separation and enrichment technology. It has the advantages of easy operation, a short analysis time, a low limit of quantitation, and a low consumption of organic solvents.
By optimizing the DLLME method, this paper utilized an organic solvent with a lower density than water as an extraction solvent which was collected by a pipette. Moreover, this study provides an environmentally friendly method of dispersive liquid-liquid microextraction followed by gas chromatography electron capture detector (GC-ECD) for the simultaneous determination of four isomers of hexachlorocyclohexane and six pyrethroid pesticides in milk.
Materials and Methods
Reagents and Materials
Four isomers of hexachlorocyclohexane (α-HCH, β-HCH, γ-HCH, and δ-HCH) of analytical standard grade with purities of > 99% and six kinds of pyrethroid (bifenthrin, fenpropathrin, cyhalothrin, cyfluthrin, cypermethrin, and deltamethrin) with purities of > 92% were provided by the Department of Applied Chemistry, China Agricultural University. Stock standard solutions (1000 mg/L) for each compound were prepared in acetonitrile stored at − 20°C in the dark. The solvents were all HPLC grade and were obtained from various sources as follows: n-hexane was obtained from Fisher Scientific (New Jersey, USA) and cyclohexane, isooctane, 1-octanol, toluene, and acetonitrile were obtained from Mreda Technology Inc. (USA). Primary secondary amine was supplied by Agela Technologies (Tianjin, China). Milk samples were purchased from local supermarkets and stored under the recommended storage conditions.
Instrumentation
Chromatographic analyses were performed on a Shimadzu GC-2010 (Kyoto, Japan) GC system, equipped with an electron capture detector and an AOC-20i auto-injector in splitless mode (sampling time, 1 min). The separation was carried out on a ZB-5 fused silica capillary column (30 m × 0.25 mm I.D., film thickness 0.25 μm), and nitrogen was used as the carrier gas at a flow rate of 0.72 mL/min. The injector was held at 260°C, and the detector was maintained at 280°C. The GC oven temperature was initially held at 100°C for 1 min, increased to 280°C at a rate of 20°C/min and held for 15 min.
Preparation of Standard Solution and Sample
A standard solution containing 1000 mg/L for each compound was prepared by dissolving them in acetonitrile and was stored in the dark at − 20°C until use. Mixed working solutions were prepared by dilution of standard stock solution with acetonitrile. All solutions were stored in the dark at − 20°C.
Experimental samples should be universal and typical, so pesticide-free pure whole milk was selected as the experimental material. The commercial milk samples were acquired in their original packaging and stored under the recommended storage conditions.
DLLME Procedure
The samples were prepared by weighing 5 g of milk into a 50-mL centrifuge tube. Next, 5 mL of acetonitrile was added, 3 g NaCl was placed in the tube, and the mixture was vortexed vigorously for 3 min. Then, the sample was centrifuged at 3800 rpm for 5 min. Protein, fat, and other substances were settled at the bottom of the tube, and the analytes were collected in the supernatant phase. Then, 3 mL of the upper acetonitrile layer was transferred to another centrifuge tube; the residual carbohydrates, fatty acids, and other impurities were removed by mixing with 200 mg PSA and vortexing for 1 min immediately. The tube was centrifuged at 10,000 rpm for 1 min, and the supernatant was filtered through a 0.22-μm filter.
Then, 0.5 mL of the supernatant was mixed with 140 μL of cyclohexane and quickly injected into a 10-mL centrifuge tube containing 3 mL of pure water. After vortexing for 3 min, the tube was centrifuged at 3800 rpm for 10 min, and the extraction solvent floating in the aqueous phase was aspirated with a 0.1-mL pipette and transferred to a 250-μL conical vial.
Calculation of Extraction Recovery (ER)
ER is defined as the percentage of total analyte extracted (n0) to the total amount of analyte in the extraction solvent (nflo).
where cflo and c0 are the concentration of analyte in the floating phase and the initial acetonitrile phase, respectively, and vflo and v0 are the volumes of the extraction solvent and the acetonitrile solution in DLLME, respectively. In this paper, ER was operated to evaluate the extraction efficiency of each parameter.
Results and Discussion
Optimization of the DLLME Procedure
In this study, DLLME was utilized to determine four hexachlorocyclohexane and six pyrethroid pesticides in milk. To obtain the highest extraction efficiency, some important parameters affecting the DLLME procedure were optimized. All experiments used initial spiked blanks (α-HCH, γ-HCH, and δ-HCH were spiked at 0.004 mg/L; β-HCH was spiked at 0.008 mg/L; cyhalothrin was spiked at 0.02 mg/L; bifenthrin and fenpropathrin were spiked at 0.04 mg/L; cyfluthrin and cypermethrin were spiked at 0.08 mg/L; and deltamethrin was spiked at 0.1 mg/L). All samples were run in triplicate for each experiment.
Selection of Extraction Solvent
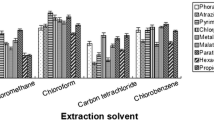
The selection of extraction solvent is particularly important in DLLME. The extraction solvent should meet the following requirements: low solubility in water, high solubility of target compounds, density higher or lower than that of water, and good compatibility with instrumental analysis (Farajzadeh et al. 2014). In previous studies, halogenated hydrocarbons have often been used as extraction solvents, but because of the requirements of green chemistry and the limitations of ECD, in this study, the extraction efficiencies of five extraction solvents (n-hexane, isooctane, cyclohexane, 1-octanol, and toluene) were explored. The dispersity of the five diverse solvents in the liquid-liquid dispersion system is different, and the solubility of the target analytes is also distinct in different extract solvents, so the optimal extraction solvent should be screened. A total of 100 μL of different extraction solvents was mixed with 2 mL of acetonitrile, rapidly injected into 5 mL of pure water, and vortexed for 5 min to compare the extraction efficiency for each spiked sample. Figure 1 shows that the extraction efficiency of 1-octanol and toluene for pyrethroid pesticides was poor, while in n-hexane, isooctane, and cyclohexane, the ERs of n-hexane and cyclohexane to hexachlorocyclohexane was significantly higher. Additionally, cyclohexane extracted more efficiently on pyrethroid; most of the analytes reached the best ERs when cyclohexane was used as the extraction solvent. Therefore, cyclohexane was chosen as the best extraction solvent in this DLLME procedure.
Effect of Extraction Solvent Volume
The extraction solvent volume is another important factor affecting the extraction efficiency. Under the same experimental conditions, the effects of different volumes (100, 110, 120, 130, 140, 150, 160, 170, and 180 μL) of cyclohexane on extraction efficiency were compared. As the volume of the extraction solvent increases, more organic phase is present in the upper layer after centrifugation and the easier it is to remove the solvent. In general, the extraction efficiency increases with the volume of the extract solvent, but when it exceeds a certain volume, the extraction efficiency will decrease (Caldas et al. 2010). If the volume of the extraction solvent is too small, the analytes may not be sufficiently extracted, while if the volume of the extraction solvent is too large, the enrichment effect on the analytes will be poor and does not meet the requirements of green chemistry. The results in Fig. 2 show that when 140 μL of cyclohexane was used as the extraction solvent, the ERs of β-HCH, cypermethrin, cyhalothrin, and deltamethrin reached maximum values; when the volume of the extraction solvent was 100 μL, the ERs of γ-HCH and δ-HCH reached maximum values; when the extraction solvent was 110 μL, only the ER of α-HCH reached a maximum value; when the extraction solvent was 130 μL, the ERs of cyfluthrin and cypermethrin reached maximum values; and the ER of bifenthrin reached a maximum at an extraction solvent volume of 150 μL. In summary, when the extraction solvent volume was 140 μL, the extraction efficiency of each target compound was high, so 140 μL was selected as the best volume of extraction solvent in DLLME.
Effect of Disperser Solvent Volume
Since the extraction solvent employed to analytes in milk was used as a disperser solvent in this DLLME process, the effect of the volume of the disperser solvent (acetonitrile phase) on the extraction efficiency can be directly studied. Disperser solvent volumes of 0.5, 1, 1.5, 2, 2.5, and 3 mL were mixed with 140 μL of cyclohexane, quickly injected into 5 mL of pure water, vortexed for 5 min, extracted, and determined. The effects of different volumes of disperser solvent on the extraction efficiency of DLLME were compared; when the disperser solvent volume was small, the micro droplets formed by the organic solvent in the aqueous phase were more conspicuous. As the volume of the disperser solvent increased, the dispersion formed by the aqueous phase and the organic phase became more turbid. As shown in Fig. 3, the ERs of most analytes decreased with the increase in disperser solvent volume, possibly because the disperser solvent increased and the solubility of a portion of the analyte in the aqueous phase increased accordingly. It can also be seen from Fig. 3 that only the ERs of bifenthrin, cyhalothrin, and cyfluthrin did not reach the maximum value when the volume of the disperser solvent was 0.5 mL, and the other seven analytes reached maximum extraction efficiencies under this condition; hence, the disperser solvent used in this experiment was 0.5 mL acetonitrile.
Effect of Water Volume
The volume of water also influences the dispersion state of the dispersive liquid-liquid microextraction procedure, while the volume of water will alter the partition coefficient of the analytes between the extraction solvent and the aqueous sample solution, which relates the extraction efficiency. To study the effect of water volume, the ERs of the ten analytes were compared for different volumes (3, 4, 5, 6, 7, and 8 mL) of pure water under the same conditions. The results showed that with an increase in the pure water volume in DLLME, the ERs of the four isomers of hexachlorocyclohexane increased until reaching a water volume of 3 mL and then remained constant, while those of the six pyrethroid pesticides increased until reaching a water volume of 3 mL and then slowly decreased. In the liquid-liquid microextraction process, the volume of water indirectly affected the contact area between the extraction solvent and the aqueous phase, thereby affecting the distribution of the analyte between the extraction solvent and the aqueous phase. As shown in Fig. 4 except for α-HCH and γ-HCH, the other eight analytes reached maximum ERs in a water volume of 3 mL; in consequence, 3 mL was the optimal water volume in the DLLME procedure.
Optimization of Vortex Time
The vortex time plays an important role in the DLLME process and has a certain influence on the dispersion state of the extraction solvent. The effect of vortex time (1, 2, 3, 4, and 5 min) on extraction efficiency was compared while maintaining the other experimental conditions constant. Due to the difference in physicochemical properties between hexachlorocyclohexane and pyrethroids, the ER trends of the four isomers of hexachlorocyclohexane and the six pyrethroid pesticides varied with increasing vortex time. The ERs of hexachlorocyclohexane increased slowly or stabilized with increasing vortex time, while the ERs of the six pyrethroid pesticides increased rapidly with increasing vortex time and gradually became stable after reaching a maximum at 3 min (Fig. 5). Consequently, the vortex time was selected to be 3 min.
Effect of Salt Content
In general, the addition of salt to the solution facilitates the transfer of the analytes to the organic phase; salt concentration is relevant to the condition of the dispersion and the solubility of the target analytes in organic solvents, so the effect of adding different amounts (0, 0.1, 0.2, 0.3, 0.4, and 0.5 g) of sodium chloride on the extraction efficiency during the DLLME procedure was investigated, and the results indicated that the salt content has no significant impact on extraction efficiency.
Method Validation
The milk sample contained none of the target pesticides which were used for validation of the DLLME method. Linearity, accuracy, and precision were verified under optimized experimental conditions. A total of 140 μL of cyclohexane was mixed as an extraction solvent with 0.5 mL of a disperser solvent, rapidly injected into 3 mL of pure water, without salt, vortexed for 3 min, and centrifuged; the upper organic phase was collected with a pipette and then injected into the separation system.
Linearity, Limits of Detection, and Limits of Quantitation
In this experiment, the stock standard solutions of the ten target pesticides were mixed, diluted with acetonitrile to a certain concentration of the standard working solution. To eliminate the influence of the matrix effect, the linearities of the target compounds were obtained using milk matrix standard solutions. A standard curve was drawn with the injection concentration as the abscissa and the peak area as the ordinate. Linear equations and correlation coefficients were derived. The linear ranges, linear equation correlation coefficients, limits of detection, and limits of quantitation of the ten target compounds in milk are shown in Table 1.
The results showed that the correlation coefficients of the four isomers of hexachlorocyclohexane and six pyrethroid pesticides in milk were between 0.9951 and 0.9981; the limits of detection (LODs), which were calculated at a signal-to-noise ratio of three, ranged from 0.07 to 2 μg/kg, and the limits of quantitaion (LOQs), which were calculated at a signal-to-noise ratio of ten, varied from 0.2 to 5 μg/kg. Within a certain concentration range, the mass concentrations of all analytes had a good linear relationship with the corresponding peak areas.
Recovery Studies
According to the sensitivity of DLLME, all the analytes were spiked at three different levels for the blank milk sample, with five replicates. The above analysis method was used to obtain the recovery rates and relative standard deviations of the four isomers of hexachlorocyclohexane and six pyrethroid pesticides in milk; the results are shown in Table 2 and Fig. 6. The average recovery rates are between 70.1% and 106.3 %, and the relative standard deviations (RSDs) are less than 15.2%.
Typical GC-ECD chromatograms of a blank milk sample and b milk sample spiked with the analytes (medium spiked level); the provided method was performed and 1 μL of the ultimate floating phase was injected into the GC system. Peak identification: 1, α-HCH; 2, β-HCH; 3, γ-HCH; 4, δ-HCH; 5, bifenthrin; 6, cyhalothrin; 7, cyfluthrin; 8, cypermethrin; 9, fenpropathrin; 10, deltamethrin
These results indicated that the proposed method has very high sensitivity and accuracy which can be used for the determination of very low levels of target pesticides in the milk sample.
Application to Real Samples
The developed DLLME method was applied to analyze four isomers of hexachlorocyclohexane and six pyrethroid pesticides of six milk samples purchased from the China Agricultural University supermarkets. Trace amount of the target pesticides were below the LOQ values. The residual analysis in real samples were confirmed by the traditional methods of the Chinese national standard GB/T 23210-2008 (Determination of 511 pesticides and related chemicals residues in milk and milk powder—GC-MS method). The target pesticides in the milk samples were also lower than the LOQs.
Comparison of the Proposed Method with Other Analytical Methods
Previously, there have been reports on the residual analysis of hexachlorocyclohexane and pyrethroids pesticides in milk and other samples, such as high-performance size exclusion chromatography (HPSEC) (Di Muccio et al. 1997), SPE (Khay et al. 2009; Bordet et al. 2002; Morelli-Cardoso et al. 1999), matrix solid-phase dispersion (MSPD) (Abhilash et al. 2009), QuEChERS (Anand et al. 2018; Gao et al. 2010), and so on. The proposed DLLME method in this work has been compared with some other methods of hexachlorocyclohexane and pyrethroid pesticide residue analysis reported in literature. The provided procedure shows obvious advantages in terms of operational complexity, organic solvent usage and LODs. In Table 3, the volume of organic solvent in this study was 5.14 mL, which was less than the volume, 11 mL, used in other literatures. Therefore, the developed DLLME presented an environmentally friendly characteristic. Also, the LODs of the proposed method ranged from 0.07 to 2 μg/kg, and the LODs of the reported methods were between 1 μg/kg and 8100 μg/kg, so the DLLME was a high-sensitivity method. The HPSEC and SPE methods showed the disadvantages of long time and complicated operation. Consequently, the proposed DLLME method was rapid, sensitive, and meets the requirements of green chemistry.
Conclusion
This paper developed a method based on dispersive liquid-liquid microextraction to extract four isomers of hexachlorocyclohexane and six kinds of pyrethroid from milk with acetonitrile. After being dispersed in water, the analytes were finally enriched in several microliters of cyclohexane and detected by GC-ECD. Compared with the Chinese national standard GB/T 23210-2008, the developed method uses a small amount of the environmentally friendly solvent as the extractant, meeting the requirements of green chemistry. The present method is simple, fast, and has low LODs, good reproducibility for the simultaneous determination of hexachlorocyclohexane and pyrethroid pesticide residues in milk.
References
Abhilash PC, Singh V, Singh N (2009) Simplified determination of combined residues of lindane and other HCH isomers in vegetables, fruits, wheat, pulses and medicinal plants by matrix solid-phase dispersion (MSPD) followed by GC–ECD. Food Chem 113:267–271. https://doi.org/10.1016/j.foodchem.2008.07.004
Aguinaga N, Campillo N, Viñas P, Hernández-Córdoba M (2007) Determination of 16 polycyclic aromatic hydrocarbons in milk and related products using solid-phase microextraction coupled to gas chromatography-mass spectrometry. Anal Chim Acta 596:285–290. https://doi.org/10.1016/j.aca.2007.06.005
Anand N, Kundu A, Ray S (2018) A validated method for the determination of neonicotinoid, pyrethroid and organochlorine residues in human milk. Chromatographia 81:315–325. https://doi.org/10.1007/s10337-017-3436-6
Asensio-Ramos M, Ravelo-Pérez LM, González-Curbelo MÁ, Hernández-Borges J (2011) Liquid phase microextraction applications in food analysis. J Chromatogr A 1218:7415–7437. https://doi.org/10.1016/j.chroma.2011.05.096
Berger M, Löffler D, Ternes T, Heininger P, Ricking M, Schwarzbauer J (2016) The effect of distribution processes on the isomeric composition of hexachlorocyclohexane in a contaminated riverine system. Int J Environ Sci Technol 13:995–1008. https://doi.org/10.1007/s13762-016-0940-4
Berijani S, Assadi Y, Anbia M, Milani Hosseini MR, Aghaee E (2006) Dispersive liquid-liquid microextraction combined with gas chromatography-flame photometric detection. Very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J Chromatogr A 1123:1–9. https://doi.org/10.1016/j.chroma.2006.05.010
Bordet F, Inthavong D, Fremy JM (2002) Interlaboratory study of a multiresidue gas chromatographic method for determination of organochlorine and pyrethroid pesticides and polychlorobiphenyls in milk, fish, eggs, and beef fat. J AOAC Int 85:1398–1409
CAC (Codex Alimentarius Commission) (2018) Codex Online Database Pesticides Residues in Food. Link: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/commodities-detail/en/?lang=en&c_id=187.
Caldas SS, Costa FP, Primel EG (2010) Validation of method for determination of different classes of pesticides in aqueous sample by dispersive liquid-liquid microextraction with liquid chromatography-tandem mass spectrometric detection. Anal Chim Acta 665:55–62. https://doi.org/10.1016/j.aca.2010.03.004
Campillo N, Peñalver R, Hernández-Córdoba M (2010) Determination of dimethylselenide and dimethyldiselenide in milk and milk by-products by solid-phase microextraction and gas chromatography with atomic emission detection. Talanta 80:1856–1861. https://doi.org/10.1016/j.talanta.2009.10.034
Campillo N, Viñas P, Férezmelgarejo G, Hernández-Córdoba M (2013) Dispersive liquid-liquid microextraction for the determination of macrocyclic lactones in milk by liquid chromatography with diode array detection and atmospheric pressure chemical ionization ion-trap tandem mass spectrometry. J Chromatogr A 1282:20–26. https://doi.org/10.1016/j.chroma.2013.01.086
Cao H, Chen XZ, Zhu Y, Li ZG, Wu XG, Zhu Y (2013) Determination of sulfonamides and quinolones in milk by QuEChERS and ultra performance liquid chromatography-tandem mass spectrometry. Food Sci Technol 38:323–329. https://doi.org/10.13684/j.cnki.spkj.2013.06.025
Cao JP, Xie QL, Zhou JM, Yi ZH (2015) Application progress of dispersive liquid-liquid microextraction in food analysis. J Instrum Anal 34:616–624. https://doi.org/10.3969/j.issn.1004-4957.2015.05.020
Cao JP, Di HW, Zhou JM, Liang YF, Xie QL (2016) A review on dispersive liquid-liquid microextraction method. J Instrum Anal 35:913–921. https://doi.org/10.3969/j.issn.1004-4957.2016.07.025
Chung TL, Liao CJ, Chen MF (2010) Comparison of liquid–liquid extraction and solid-phase extraction for the determination of polycyclic aromatic hydrocarbons in the milk of Taiwan. J Taiwan Inst Chem E 41:178–183. https://doi.org/10.1016/j.jtice.2009.07.003
Dagnac T, Garcia-Chao M, Pulleiro P, Garcia-Jares C, Llompart M (2009) Dispersive solid-phase extraction followed by liquid chromatography-tandem mass spectrometry for the multi-residue analysis of pesticides in raw bovine milk. J Chromatogr A 1216:3702–3709. https://doi.org/10.1016/j.chroma.2009.02.048
Di Muccio A, Pelosi P, Barbini DA, Generali T, Ausili A, Vergori F (1997) Selective extraction of pyrethroid pesticide residues from milk by solid-matrix dispersion. J Chromatogr A 765:51–60. https://doi.org/10.1016/S0021-9673(96)01005-9
Farajzadeh MA, Djozan D, Mogaddam MRA, Bamorowat M (2011) Extraction and preconcentration technique for triazole pesticides from cow milk using dispersive liquid-liquid microextraction followed by GC-FID and GC-MS determinations. J Sep Sci 34:1309–1316. https://doi.org/10.1002/jssc.201000928
Farajzadeh MA, Djozan D, Mogaddam MRA, Norouzi J (2012) Determination of phthalate esters in cow milk samples using dispersive liquid-liquid microextraction coupled with gas chromatography followed by flame ionization and mass spectrometric detection. J Sep Sci 35:742–749. https://doi.org/10.1002/jssc.201100853
Farajzadeh MA, Mogaddam MRA, Ghorbanpour H (2014) Development of a new microextraction method based on elevated temperature dispersive liquid–liquid microextraction for determination of triazole pesticides residues in honey by gas chromatography-nitrogen phosphorus detection. J Chromatogr A 1347:8–16. https://doi.org/10.1016/j.chroma.2014.04.067
Farajzadeh MA, Mogaddam MRA (2016) Low-density-solvent-based air-assisted liquid-liquid microextraction followed by gas chromatography with flame ionization detection for the determination of synthetic phenolic antioxidants in milk samples. J Sep Sci 39:1160–1167. https://doi.org/10.1002/jssc.201501210
Gao XS, Zhang Y, Wang SX, Hua R, Zhang XM (2010) Determination of pyrethroid pesticide residues in milk by QuEChERS-gas chromatography. China Dairy Cattle 8:56–60. https://doi.org/10.3969/j.issn.1004-4264.2010.08.020
Gao YL, Xu C, Liu SB, Sun P (2018) Determination of seven pyrethroid pesticides in liquid milk by dispersive liquid–liquid microextraction based on the solidification of a floating organic droplet followed by GC. Chromatographia 81:539–544. https://doi.org/10.1007/s10337-017-3457-1
Jank L, Hoff RB, Tarouco PC, Barreto F, Pizzolato TM (2012) β-Lactam antibiotics residues analysis in bovine milk by LC-ESI-MS/MS: a simple and fast liquid-liquid extraction method. Food Addit Contam Part A 29:497–507. https://doi.org/10.1080/19440049.2011.604044
Karaseva NM, Amelin VG, Tret’yakov AV (2014) QuEChERS coupled to dispersive liquid-liquid microextraction for the determination of aflatoxins B1 and M1 in dairy foods by HPLC. J Anal Chem 69:461–466. https://doi.org/10.1134/S1061934814030071
Khay S, El-Aty AMA, Choi JH, Shin EH, Shin HC, Kim JS, Chang BJ, Lee CH, Shin SC, Jeong JY, Shim JH (2009) Simultaneous determination of pyrethroids from pesticide residues in porcine muscle and pasteurized milk using GC. J Sep Sci 32:244–251. https://doi.org/10.1002/jssc.200800481
Knobel G, Calimag-Williams K, Campiglia AD (2013) Analysis of polycyclic aromatic hydrocarbon metabolites in cow's milk by liquid–liquid extraction and synchronous room-temperature fluorescence spectroscopy. Anal Methods 5:1577–1582. https://doi.org/10.1039/c3ay26114j
Kokosa JM (2013) Advances in solvent-microextraction techniques. Trends Anal Chem 43:2–13. https://doi.org/10.1016/j.trac.2012.09.020
Liang P, Xu J, Li Q (2008) Application of dispersive liquid-liquid microextraction and high-performance liquid chromatography for the determination of three phthalate esters in water samples. Anal Chim Acta 609:53–58. https://doi.org/10.1016/j.aca.2007.12.025
Li XJ, Yu H, Peng RF, Gan PS (2017) Determination of 19 sulfonamides residues in pork samples by combining QuEChERS with dispersive liquid–liquid microextraction followed by UHPLC–MS/MS. J Sep Sci 40:1377–1384. https://doi.org/10.1002/jssc.201601034
Liu D, Min SG, Ping H, Song XZ (2016) The application of directly suspended droplet microextraction for the evaluation of phthalic acid esters in cow's milk by gas chromatography mass spectrometry. J Chromatogr A 1443:66–74. https://doi.org/10.1016/j.chroma.2016.03.062
Liu HL (2014) Research progress of overview on sample pretreatment of pesticides residue in milk. J Food Saf Qual 5:1419–1426 http://www.chinafoodj.com/ch/reader/create_pdf.aspx?file_no=20140217003&flag=1&journal_id=spzljcxb
Liu XJ, Zhao AJ, Zhang AN, Liu HQ, Xiao WJ, Wang CJ, Wang XD (2011) Dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry determination of polychlorinated biphenyls and polybrominated diphenyl ethers in milk. J Sep Sci 34:1084–1090. https://doi.org/10.1002/jssc.201000767
Mahmoudpour M, Mohtadinia J, Ansarin M, Nemati M (2016) Dispersive liquid–liquid microextraction for HPLC-UV determination of PAHs in milk. J AOAC Int 99:527–533. https://doi.org/10.5740/jaoacint.15-0169
Maragou NC, Lampi EN, Thomaidis NS, Koupparis MA (2006) Determination of bisphenol A in milk by solid phase extraction and liquid chromatography-mass spectrometry. J Chromatogr A 1129:165–173. https://doi.org/10.1016/j.chroma.2006.06.103
Miao XX, Liu DB, Wang YR, Yang YY, Yang XY, Gong HR (2015) Modified QuEChERS in combination with dispersive liquid–liquid microextraction based on solidification of the floating organic droplet method for the determination of organophosphorus pesticides in milk samples. J Chromatogr Sci 53:1813–1820. https://doi.org/10.1093/chromsci/bmv089
Morelli-Cardoso MHW, Cardozo RTM, Mello JL, Abrantes S, Menezes KMP (1999) Extraction and clean-up method for the determination of twenty organochlorine pesticide residues in tomatoes by GLC-ECD. J Sep Sci 22:619–622. https://doi.org/10.1002/(SICI)1521-4168(19991101)22:11<619::AID-JHRC619>3.0.CO;2-0
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A 1217:2342–2357. https://doi.org/10.1016/j.chroma.2009.11.088
Rúbies A, Guo LL, Centrich F, Granados M (2016) Analysis of non-steroidal anti-inflammatory drugs in milk using QuEChERS and liquid chromatography coupled to mass spectrometry: triple quadrupole versus Q-Orbitrap mass analyzers. Anal Bioanal Chem 408:5769–5778. https://doi.org/10.1007/s00216-016-9679-5
Salas JH, González MM, Noa M, Pérez NA, Díaz G, Gutiérrez R, Zazueta H, Osuna I (2003) Organophosphorus pesticide residues in Mexican commercial pasteurized milk. J Agric Food Chem 51:4468–4471. https://doi.org/10.1021/jf020942i
Singh S, Nelapati K (2017) Effect of food processing on degradation of hexachlorocyclohexane and its isomers in milk. Vet World 10:270–275. https://doi.org/10.14202/vetworld.2017.270-275
Shamsipur M, Najmeh Y, Ghambarian M (2016) Combination of solid-phase extraction with dispersive liquid–liquid microextraction followed by GC–MS for determination of pesticide residues from water, milk, honey and fruit juice. Food Chem 204:289–297. https://doi.org/10.1016/j.foodchem.2016.02.090
Tuncel SG, Şenlik D (2016) Determination of phthalates in milk by ultrasound-assisted dispersive liquid–liquid microextraction and gas chromatography–mass spectrometry. Anal Lett 49:1334–1343. https://doi.org/10.1080/00032719.2015.1098654
Wang S, Yang S, Liu F, Xue J, You X (2012) Review on the application of liquid phase microextraction in pesticide residue analysis. Chinese J Pestic Sci 14:461–474. https://doi.org/10.3969/j.issn.1008-7303.2012.05.01
Yu X, Ang HC, Yang HS, Zheng C, Zhang YQ (2016) Low temperature cleanup combined with magnetic nanoparticle extraction to determine pyrethroids residue in vegetables oils. Food Control 74:112–120. https://doi.org/10.1016/j.foodcont.2016.11.036
Zgoła-Grześkowiak A, Grześkowiak T (2011) Dispersive liquid-liquid microextraction. Trends Anal Chem 30:1382–1399. https://doi.org/10.1016/j.trac.2011.04.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Yuanling Zhao declares no conflict of interest. Xi’ai Hou declares no conflict of interest. Dongmei Qin declares no conflict of interest. Dan Liu declares no conflict of interest.
Ethical Approval
This article does not contain any studies involving animals and human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Hou, X., Qin, D. et al. Dispersive Liquid-Liquid Microextraction Method for the Simultaneous Determination of Four Isomers of Hexachlorocyclohexane and Six Pyrethroid Pesticides in Milk by Gas Chromatography Electron Capture Detector. Food Anal. Methods 13, 370–381 (2020). https://doi.org/10.1007/s12161-019-01662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01662-w