Abstract
A rapid and sensitive method was established to simultaneously determine multiple pesticide residues in celery through gas chromatography-mass spectrometry (GC-MS). Samples were extracted through a modified quick, easy, cheap, effective, robust, and safe method (modified QuEChERS) and then refined and preconcentrated through dispersive liquid-liquid microextraction (DLLME) by using CHCl3 as extractive solvent and acetonitrile (ACN) as dispersive solvent. The main factors, including type of extraction solvent, volume of extraction solvent, volume of dispersive solvent, extraction time, salt concentration, vortex velocity, and pH of aqueous solution, influencing DLLME were initially evaluated by performing single-factor variable experiments; three significant factors, particularly volume of extraction solvent, volume of dispersive solvent, and extraction time, were thoroughly analyzed through response surface methodology. The following optimized extraction conditions were obtained: 100 μL of CHCl3, 900 μL of ACN, and 1.62-min extraction time. The optimized method was validated with average recoveries ranging from 70.8 to 93.2 % (with relative standard deviations of <15 %) at three spiked levels for all of the pesticides. Good linearity with determination coefficients of >0.9974 was obtained on the basis of the matrix-matched calibration curve of each pesticide; limits of detection ranging from 2.4 to 14.2 μg/kg indicated high sensitivity. Malathion with concentrations varying from 0.009 to 0.012 mg/kg was detected in all of the samples; other pesticides were not detected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Celery is an essential part of daily human diet; this vegetable can supply trace Fe and vitamins crucial for human health, and the Fe content of celery is almost 20 times higher than that of tomato. Demands for vegetables of individuals worldwide are gradually increasing; to ensure high vegetable production, farmers and producers apply chemical control as one of the most effective measures. However, chemicals not only promote high yields but also contaminate the environment and potentially threaten human health. With current improvements in human lifestyle, the required quality of life has been extensively investigated. Methylparathion, ethion, diazinon, and malathion residues have been found in tomato samples (Bidari et al. 2011). Thiacloprid (5.9 ng g−1) and acetamiprid (4.1 ng g−1) residues have also been detected in cucumber, although these concentrations remain lower than maximum residue levels (MRLs) set by the EU (Zhang et al. 2012). By contrast, carbamate pesticide residues in tomatoes and grapes have been detected in amounts almost similar to EU and Codex Alimentarius MRLs (Fernández et al. 2000). As such, stricter MRLs of agrochemicals have been defined by all countries of the world. Accurate and sensitive methods should also be established to determine trace pesticides in fruits and vegetables. To the best of our knowledge, few studies have investigated pesticide residues in celery; thus, celery was chosen as the experimental subject in this work.
Sample preparation is necessary to detect pesticides in fruits and vegetables. Some of these preparation methods include solid-phase extraction (SPE), matrix solid-phase dispersion (MSPD), and dispersive solid-phase extraction (dSPE). Compared with conventional liquid-liquid extraction, SPE and MSPD require less solvent consumption and manual labor, but still demand time-consuming manual steps (Cunha and Fernandes 2011). In contrast to those techniques, dSPE is characterized by simple performance and time-saving manual steps. Despite all this, the absorbent used in dSPE removes impurities from a specific matrix but may simultaneously absorb some target analytes when multi-residue samples are analyzed. Hence, accurate and simple sample preparation methods should be developed to simultaneously determine different classes of agrochemicals in a complex matrix.
In recent years, dispersive liquid-liquid microextraction (DLLME) has emerged as an advanced technique that permits efficient extraction using a tertiary component solvent system on a microliter scale, i.e., an aqueous solution, a water-immiscible solvent (extraction solvent), and a water-miscible solvent (dispersive solvent) (Rezaee et al. 2006). Compared to dSPE, DLLME could yield larger enrichment factor using less extraction solvent and be capable of handling a large number of samples in a short time. Therefore, DLLME has been applied to treat samples of biological, pharmacological, and environmental analytes, such as curcuminoid compounds (Caballero-Casero et al. 2014), Sudan dyes (Yan et al. 2011), antibiotics (Gao et al. 2012), and heavy metals (Berton et al. 2010; Farajzadeh et al. 2008), because of high sample throughput and rapid and steady sample enrichment. Likewise, DLLME has also been employed to analyze pesticides in liquid and solid samples. Moreover, DLLME has been mainly utilized to analyze multiple pesticides in liquid samples, such as environmental water, bottled water, drinking water, wastewater, and rainwater (Zhang and Lee 2013; Wang et al. 2011; Wu et al. 2010; Pena et al. 2009; Ma et al. 2014; Matsadiq et al. 2011), but rarely employed to evaluate juice (Zhang et al. 2013), red wine (You et al. 2013), and honey (Pirard et al. 2007) samples. Furthermore, this technique has been used to investigate a limited range of solid samples, such as apples (Zhou et al. 2011), peaches (Matsadiq et al. 2011), as well as soil samples (Asensio-Ramos et al. 2011). Besides, DLLME has been combined with QuEChERS to analyze pesticides in few solid samples, such as oranges (Andraščíková et al. 2013) and tomatoes (Melo et al. 2013). Similarly, DLLME has been rarely applied to screen vegetables (Bidari et al. 2011; Qiao et al. 2010). Therefore, it’s of great significance to extensively carry out the study of determining pesticide residues in solid samples using DLLME.
This work aimed to develop a reliable sample preparation method with a combination of a modified quick, easy, cheap, effective, robust, and safe (modified QuEChERS) method and DLLME to determine nine pesticides in celery samples through gas chromatography-mass spectrometry (GC-MS). These different chemical classes of pesticides, such as metalaxyl, atrazine, pyrimethanil, propiconazole, hexaconazole, phorate, chlorpyrifos-methyl, malathion, and parathion, were chosen because these chemicals are extensively used to treat celery diseases and pests. In particular, response surface methodology was applied to optimize DLLME procedure and obtain optimum extraction conditions for these nine pesticides in celery by considering the integrated effect of amounts of extractive and dispersive solvents and extraction time. The optimized method was carefully validated and applied to evaluate the presence of the investigated pesticides in celery samples obtained from different supermarkets.
Materials and Methods
Chemicals and Reagents
Pesticide standards of metalaxyl, atrazine, pyrimethanil, propiconazole, and hexaconazole, with ≥96.5 % purity, were purchased from Beijing Dike Ma Technology Co., Ltd. (Beijing, China). Phorate, chlorpyrifos-methyl, malathion, and parathion (1000 mg/L of each substance in acetonitrile (ACN)) were obtained from Guangdong Province Pesticide Verification Place (Guangzhou, China). Individual standard stock solutions of each pesticide (metalaxyl, atrazine, pyrimethanil, propiconazole, and hexaconazole) were prepared using ACN to dissolve each compound to a final concentration of 1000 mg/L and stored at 4 °C in the dark. A 100-mg/L mixture of the nine pesticides was also prepared in ACN and stored at 4 °C in the dark. Standard working solutions were prepared daily by using ACN to dilute the mixture.
Dichloromethane (CH2Cl2), chloroform (CHCl3), carbon tetrachloride (CCl4), and chlorobenzene (C6H5Cl) were HPLC-grade solvents purchased from Beijing Dike Ma Technology Co., Ltd. ACN was an AR-grade solvent from Tianjin Wealthy Fine Chemical Co. (Tianjin, China). Sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium chloride (NaCl), and anhydrous magnesium sulfate (anhydrous MgSO4) were from the Tianjin Fu Chen Chemical Reagent Factory (Tianjin, China).
Apparatus and GC-MS Conditions
A 6890-N gas chromatograph (Agilent, Little Falls, DE, USA) equipped with an electronically controlled split/splitless injection port and a 7683B series injector/autosampler was linked to a single-quadrupole mass selective detector (5975B, Agilent) with an electron impact ionization chamber.
GC separation was conducted using a DB-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness, J&W Scientific, Folsom, CA, USA). Helium (purity, 99.999 %) was used as a carrier gas at a constant pressure of 10.52 psi. Injection was set in a spitless mode of 0.5 min and an injector temperature of 260 °C. The oven temperature program was set as follows: 100 °C as initial temperature, ramped to 170 °C at 20 °C/min, ramped to 250 °C at 5 °C/min, ramped to 270 °C at 15 °C/min, and maintained for 5.0 min. Total run time was 25.83 min. MS transfer line temperature was maintained at 280 °C.
MS parameters were set as follows: electron impact ionization, 70 eV energy; ion source temperature, 230 °C; and quadrupole temperature, 150 °C. The MS system was routinely set in a selective ion monitoring (SIM) mode, and each compound was quantified on the basis of the peak area by using one target and one or two qualifier ions. Table 1 summarizes the target pesticides with respective retention time (t R), ions selected in the SIM mode, and individual molecular weight (MW).
Sample Preparation
Celery samples were randomly purchased from the local markets of Guangzhou. Samples were reduced, chopped using a DS-1 organization masher (Shanghai, China), and stored in sample bags at less than −20 °C. Pesticides were extracted from celery through a modified QuEChERS method coupled with DLLME.
The extraction procedure included the following steps: (i) 10 ± 0.1 g of the sample was accurately weighed in a 50-mL polypropylene centrifuge tube, (ii) 10 mL of ACN was added and the tube was vortex-mixed for 1 min to adequately mix ACN with the sample, (iii) extraction was conducted in SK 2200 HP ultrasonic bath (Shanghai, China) for 15 min, (iv) 4 g of anhydrous MgSO4 and 1 g of NaCl were added to each tube (the former can remove water from the organic phase and the latter can induce the separated layer between organic and inorganic phases), and the mixture was immediately vortex-mixed for 0.5 min, (v) the tubes were centrifuged at 4000 rpm for 10 min (aqueous phase and impurities from celery samples co-extracted with analytes formed a sediment at the bottom of the tubes; the organic phase with analytes was found in the supertratum), and the upper layer was extracted and subjected to DLLME.
DLLME was conducted as follows: (i) 4 mL of ultrapure water was added to a 5-mL screw cap glass tube with a conical bottom, (ii) 900 μL of the extract solution (described in the extraction procedure) and 100 μL of CHCl3 were rapidly added to the tube (the extract solution simultaneously served as sample and dispersive solvent; CHCl3 was used as extraction solvent), (iii) cloudy solution from the dispersion of fine droplets of CHCl3 in the aqueous solution was produced in the glass tube by vortexing at 2500 rpm for 1.62 min; thus, analytes were extracted in CHCl3 droplets, (iv) the glass tube was then centrifuged at 3000 rpm for 10 min (the extraction solvent formed sediments at the bottom of the glass tube), and (v) the sediment was transferred to a vial by using a 100-μL microsyringe, and 1-μL was injected into a GC-MS system for analysis.
Results and Discussion
Selection of Experimental Parameters
In the modified QuEChERS stage, ultrasonic-assisted extraction was applied to enhance the extraction efficiency of targeted pesticides from the celery matrix. Ultrasound was conducted for 5 and 30 min. The results revealed that all of the analytes exhibited high recovery at 15 min, but no significant increase was observed after 15 min. ACN was selected as a dispersive solvent based on a previous study (Rezaee et al. 2006); this solvent could also provide clear phase distinction. Although surface foaming was observed in celery, this side effect can be overlooked and did not cause any difference.
The preparation method mainly involved DLLME. In preliminary experiments, the influence of several factors, such as type of extraction solvent, volume of extraction solvent, volume of dispersive solvent, extraction time, salt concentration, vortex velocity, and pH of extraction solution, on the extraction recovery of DLLME were carefully analyzed.
Selection of Extraction Solvent
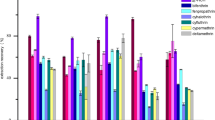
The selection of extraction solvent is the most crucial step in DLLME because the physical properties of a specific solvent determine the ability to extract targeted analytes. Selected organic solvents should satisfy the following requirements: (a) immiscible with water and of higher density than water, (b) good analyte extraction ability, and (c) good chromatographic behavior. Thus, four chlorinated organic solvents, namely, CH2Cl2, CHCl3, CCl4, and C6H5Cl, were selected in this work. The experiments using targeted analyte-free spiked celery were performed in triplicate. Spiked samples were initially subjected to QuEChERS, and 1 mL of extraction solution was added to a 5-mL conic glass test tube filled with 4 mL of ultrapure water. Afterward, 100 μL of the extractant was rapidly added to the tube and vortex-mixed for 1 min at 2200 rpm. After centrifugation was performed at 3000 rpm for 10 min, 1 μL of the sediment phase was analyzed through GC-MS. Figure 1 shows that CHCl3 was the most effective extractant with recoveries ranging from 60 to 88 %, followed by C6H5Cl (46–73 %), CH2Cl2 (31–65 %), and CCl4 (20–58 %). Therefore, the subsequent experiments were performed using CHCl3 as the extraction solvent.
Selection of Extraction Solvent Volume
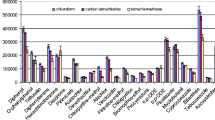
Extraction solvent volume is one of the most important factors affecting the extraction recovery of DLLME. Excess extractant exhibits higher recovery but at a lower enrichment factor because of dilution; by contrast, insufficient extractant prevents thorough recovery and leads to inconvenient autoinjection because of low extraction solution volume. Therefore, different volumes of CHCl3 were investigated to choose the optimum volume of extractant. Experiments were conducted as described in “Selection of Extraction Solvent” section, and the results are illustrated in Fig. 2. Recovery increased as the amount of CHCl3 increased. The optimum recovery of the analytes at 100 μL ranged from 62 to 85 %. By contrast, the optimum recovery of the analytes at 120 μL ranged from 65 to 83 %; however, no significant difference was observed between these two recovery values. Furthermore, 100 μL of CHCl3 was used in the subsequent experiments by considering enrichment factor.
Selection of Dispersive Solvent Volume
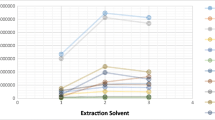
The volume of ACN solution (as dispersive solvent) used in DLLME influences analyte solubility and tertiary mixture emulsification because dispersers exhibit an organic property. As the volume of dispersive solvent increased, a greater portion of the analytes was attracted to the aqueous solution, whereas less amount of the disperser cannot induce valid emulsification. Different volumes of ACN solution (500, 800, 1000, 1200, and 1500 μL) were selected to determine the optimized level, to achieve effective emulsification, and to minimize disperser volume. As disperser volume increased, extraction recovery initially increased and then decreased because analytes exhibited high affinity to the aqueous phase (Fig. 3). Furthermore, 1500 μL of the ACN solution provided a remarkable decline in the extraction yield. In this work, 800 μL was chosen as the optimized disperser volume for the succeeding experiments.
Effect of Extraction Time
The extraction time of DLLME is commonly defined as the interval between the time at which dispersive solvent and extraction solvent are added to the ultrapure water and the time at which centrifugation is initiated. In this work, vortex auxiliary extraction was performed at extraction time of 0.5, 1.0, 1.5, 3.0, and 5.0 min to promote the rapid formation of the emulsification system, including tertiary compounds (extraction solvent, dispersive solvent, and water). Extraction recovery increased as extraction time was extended, and recovery of most of the analytes was enhanced at 1.5 min (Fig. 4); no variation was observed when extraction time was further extended. Hence, 1.5 min was chosen as the optimum extraction time for subsequent experiments.
Effect of Other Parameters
In this work, other parameters, such as salt concentration, vortex velocity, and pH of aqueous solution, were investigated to select the optimum levels of these factors for response surface methodology. In terms of salt concentration, 0, 1, 3, 5, and 8 % NaCl (w/v) were selected to estimate the effect of salt on extraction recovery. And the results revealed that recovery slightly decreased as NaCl concentration increased, and the organic phase floated on 8 % NaCl aqueous solution. Thus, no salt was added in this work. Concerning vortex velocity, a series of values, such as 1000, 1400, 1800, 2200, and 2500 rpm, was evaluated, and the results showed that all of the analytes reached the maximum recovery at 2500 rpm. Therefore, 2500 rpm was set as optimum vortex velocity and used in DLLME. The pH of aqueous solution ranging from 4 to 9 was evaluated. The results showed that aqueous solutions with neutral pH provided the maximum recovery of the studied analytes. Thus, pH of the aqueous solution was not adjusted in this work.
Experimental Design
Based on the preliminary trials in “Selection of Experimental Parameters” section, a Box-Behnken design with three factors and three levels was further investigated to determine the optimized DLLME procedure with comprehensive factors and levels. Table 2 shows the levels of the factors in the Box-Behnken design. The 17 resulting experiments were randomly performed using CHCl3 as extraction solvent and ACN as dispersive solvent and then vortex-mixed at a rate of 2500 rpm.
The individual peak area of each pesticide and the sum of the peak areas of all of the pesticides were introduced separately as the response in the statistical program. The results of the experimental design were firstly analyzed in terms of individual pesticide. These results are consistent with those of the preliminary experiments. As disperser volume increased, recovery initially increased and then decreased. Recovery also increased as extraction solvent volume was increased and extraction time was extended; after peak volume and time were obtained, recovery slightly changed and remained almost constant. In this work, the mean recovery percentage was chosen as a compromise response. Figure 5 shows the response surfaces of the extraction of the studied pesticides. Overall, high extraction solvent volume, long extraction time, and moderate disperser volume provided high recovery yield. The following theoretical optimized extraction conditions were obtained: 101.78 μL of CHCl3, 899.50 μL of ACN, and 1.62 min of extraction time. In consideration of unmeasured volume of CHCl3 and ACN solution, 100 μL of CHCl3 and 900 μL of ACN were modified, and it was observed that recoveries almost remained unchanged. Therefore, 100 μL of CHCl3, 900 μL of ACN, and 1.62 min of extraction time were determined as the optimized extraction conditions in this work.
Matrix Effect
Compared with the response in a matrix-free solution, the response of the analytes in a complicated sample matrix is usually enhanced through GC or GC-MS. This enhancement may result from the injection liner or column active sites blocked by matrix compounds which reduce analyte adsorption by active sites and protect analytes from thermal degradation. This work adopted the ratio of the slopes in matrix-matched standard solutions and in solvent standard solutions to evaluate matrix effects. Matrix effects were classified into three types according to a decrease or an increase in the percentage of the slope: mild signal suppression or enhancement effects (−20 to 0 % or 0 to 20 %), moderate effects (−50 to −20 % or 20 to 50 %), and strong matrix effects (less than −50 % or greater than 50 %). Among the nine studied pesticides, two of the analytes (atrazine and pyrimethanil) presented moderate signal suppression effect, two of the analytes (chlorpyrifos-methyl and malathion) showed moderate enhancement effects, and the remaining analytes elicited mild effects (Fig. 6). Matrix-matched standard solutions were used in this work to compensate for the matrix effect induced by the sample matrix and to accurately control the quantity of analytes.
Method Validation
Linearity and Limits of Detection (LODs)
Matrix-matched calibration curves were obtained using five matrix calibration solutions (standards added to the prepared blank sample extract, as described in “Sample Preparation” section). The linearity of the proposed method was evaluated by calculating the determination coefficient (R 2) of the linear regression equations. Each pesticide exhibited good linearity in the studied concentration range (Table 3) with good R 2 varying from 0.9974 (malathion) to 0.9996 (propiconazole).
LODs of this method were obtained by successively analyzing the decreasing amounts of standards of spiked blank sample extract until three times the signal-to-noise ratio was obtained, while limits of quantification (LOQs) were calculated based on the response of signal with 10 times that of noise. Chlorpyrifos-methyl yielded the lowest LOD and LOQ, 2.4 × 10−3 and 7.9 × 10−3 mg/kg, respectively, and propiconazole exhibited the highest LOD and LOQ, 1.4 × 10−2 and 4.7 × 10−2 mg/kg, respectively, (Table 3).
Recovery and Repeatability
The recovery and repeatability of the method were evaluated by performing recovery experiments (n = 5) at three spiked concentration levels (0.01, 0.05, and 0.1 mg/kg). Table 3 shows the average recovery and repeatability (expressed as relative standard deviation, RSD). Average recoveries ranged from 74.0 (hexaconazole) to 93.2 % (phorate) with RSDs between 4.1 (propiconazole) and 10.6 % (atrazine). The recovery rates of all of the analytes ranged from 70 to 120 % with RSDs of <20 %. Hence, the preparation method exhibited high accuracy and good repeatability. The chromatograms of blank and spiked blank celery samples (0.1 mg/kg) showed that the compounds of the celery matrix did not display evident interference on the targeted pesticides (Figs. 7a, b).
GC-MS chromatograms of a non-spiked and b spiked celery sample at 0.1 mg/kg for each pesticide in SIM mode using the optimized DLLME procedure. Peak identification: (1) phorate, (2) atrazine, (3) pyrimethanil, (4) chlorpyrifos-methyl, (5) metalaxyl, (6) malathion, (7) parathion, (8) hexaconazole, and (9) propiconazole
Analysis of Celery Samples
Ten celery samples were randomly purchased from local markets to verify the potential application of the proposed method. The samples were subjected to the optimized treatment procedure and then analyzed through GC-MS in a SIM mode. Malathion was detected in all of the samples, but other pesticides were not detected. The amount of malathion residue in the samples ranged between 0.009 and 0.012 mg/kg.
Comparison of DLLME with Other Reported Methods
The efficiency of the developed DLLME is comparable with or even better than other reported analytical methods using SPE, dSPE, and SPME applied to fruits and vegetables (Table 4). Compared with SPE technique, much smaller sample and solvent volume are required in DLLME, as well as easier equipment and handling. Compared to SPME, less extraction time is needed in this method because of a long analyte equilibration time needed in SPME. DLLME has a comparable performance with dSPE. Compared with the relatively expensive adsorbents needed in dSPE, only accessible solvent is required in DLLME. In general, DLLME is a rapid, easy, labor-saving, and alternative pretreatment method for the determination of pesticide residues in fruits and vegetables.
Conclusions
A simple, quick, stable, and sensitive method was established to simultaneously determine nine pesticides in celery samples by QuEChERS-DLLME-GC-MS. The proposed method is characterized by the following advantages: small amount of extractive solvent, short extraction time, no cleanup procedure, and good repeatability. Through method validation, it was proved that this method was of high accuracy and good repeatability. Moreover, the developed method was successfully applied to analyze real samples. Therefore, this method can be a feasible alternative method applied to the routine analysis of pesticides studied in celery samples. Furthermore, the method has the potential to analyze different pesticides in various vegetables and fruits.
References
Andraščíková M, Hrouzková S, Cunha SC (2013) Combination of QuEChERS and DLLME for GC-MS determination of pesticide residues in orange samples. Food Addit Contam A 30:286–297
Asensio-Ramos M, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MÁ (2011) Ionic liquid-dispersive liquid–liquid microextraction for the simultaneous determination of pesticides and metabolites in soils using high-performance liquid chromatography and fluorescence detection. J Chromatogr A 1218:4808–4816
Berton P, Martinis EM, Wuilloud RG (2010) Development of an on-line temperature-assisted ionic liquid dispersive microextraction system for sensitive determination of vanadium in environmental and biological samples. J Hazard Mater 176:721–728
Bidari A, Ganjali MR, Norouzi P, Hosseini MRM, Assadi Y (2011) Sample preparation method for the analysis of some organophosphorus pesticides residues in tomato by ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chem 126:1840–1844
Caballero-Casero N, Ocak M, Ocak Ü, Rubio S (2014) Quick supramolecular solvent-based microextraction for quantification of low curcuminoid content in food. Anal Bioanal Chem 406:2179–2187
Cunha SC, Fernandes JO (2011) Multipesticide residue analysis in maize combining acetonitrile-based extraction with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. J Chromatogr A 1218:7748–7757
Di MA, Fidente P, Barbini DA, Dommarco R, Seccia S, Morrica P (2006) Application of solid-phase extraction and liquid chromatography-mass spectrometry to the determination of neonicotinoid pesticide residues in fruit and vegetables. J Chromatogr A 1108:1–6
Farajzadeh MA, Bahram M, Mehr BG, Jönsson JA (2008) Optimization of dispersive liquid–liquid microextraction of copper (II) by atomic absorption spectrometry as its oxinate chelate: application to determination of copper in different water samples. Talanta 75:832–840
Fernández M, Picó Y, Manes J (2000) Determination of carbamate residues in fruits and vegetables by matrix solid-phase dispersion and liquid chromatography–mass spectrometry. J Chromatogr A 871:43–56
Gao S, Yang X, Yu W, Liu Z, Zhang H (2012) Ultrasound-assisted ionic liquid/ionic liquid-dispersive liquid–liquid microextraction for the determination of sulfonamides in infant formula milk powder using high-performance liquid chromatography. Talanta 99:875–882
Lambropoulou DA, Albanis TA (2003) Headspace solid-phase microextraction in combination with gas chromatography-mass spectrometry for the rapid screening of organophosphorus insecticide residues in strawberries and cherries. J Chromatogr A 993:197–203
Ma H, Li Y, Zhang H, Shah SM, Chen J (2014) Salt-assisted dispersive liquid–liquid microextraction coupled with programmed temperature vaporization gas chromatography–mass spectrometry for the determination of haloacetonitriles in drinking water. J Chromatogr A 1358:14–19
Matsadiq G, Hu HL, Ren HB, Zhou YW, Liu L, Cheng J (2011) Quantification of multi-residue levels in peach juices, pulps and peels using dispersive liquid–liquid microextraction based on floating organic droplet coupled with gas chromatography-electron capture detection. J Chromatogr B 879:2113–2118
Melo A, Mansilha C, Pinho O, Ferreira IMPLVO (2013) Analysis of pesticides in tomato combining QuEChERS and dispersive liquid-liquid microextraction followed by high-performance liquid chromatography. Food Anal Method 6:559–568
Pena MT, Casais MC, Mejuto MC, Cela R (2009) Development of an ionic liquid based dispersive liquid–liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6356–6364
Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt L, Haubruge E, De Pauw E, Focant JF (2007) Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid–liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1152:116–123
Qiao F, Zhang X, Wang M, Kang Y (2010) Rapid extraction of imidacloprid in tomatoes by ultrasonic dispersion liquid–liquid microextraction coupled with LC determination. Chromatographia 72:331–335
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Wang C, Wu Q, Wu C, Wang Z (2011) Application of dispersion–solidification liquid–liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J Hazard Mater 185:71–76
Wu C, Liu N, Wu Q, Wang C, Wang Z (2010) Application of ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of some organophosphorus pesticides in water samples. Anal Chim Acta 679:56–62
Yan H, Wang H, Qiao J, Yang G (2011) Molecularly imprinted matrix solid-phase dispersion combined with dispersive liquid–liquid microextraction for the determination of four Sudan dyes in egg yolk. J Chromatogr A 1218:2182–2188
You X, Wang S, Liu F, Shi K (2013) Ultrasound-assisted surfactant-enhanced emulsification microextraction based on the solidification of a floating organic droplet used for the simultaneous determination of six fungicide residues in juices and red wine. J Chromatogr A 1300:64–69
Zawiyah S, Che Man YB, Nazimah SAH, Chin CK, Tsukamoto I, Hamanyza AH, Norhaizan I (2007) Determination of organochlorine and pyrethroid pesticides in fruit and vegetables using sax/psa clean-up column. Food Chem 102:98–103
Zhang Y, Lee HK (2013) Low-density solvent-based vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction combined with gas chromatography–mass spectrometry for the fast determination of phthalate esters in bottled water. J Chromatogr A 1274:28–35
Zhang S, Yang X, Yin X, Wang C, Wang Z (2012) Dispersive liquid–liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem 133:544–550
Zhang J, Liang Z, Guo H, Gao P, Lu R, Zhou W, Zhang S, Gao H (2013) Ionic liquid-based totally organic solvent-free emulsification microextraction coupled with high performance liquid chromatography for the determination of three acaricides in fruit juice. Talanta 115:556–562
Zhou YW, Han LT, Cheng J, Guo F, Zhi XR, Hu HL, Chen G (2011) Dispersive liquid–liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Anal Bioanal Chem 399:1901–1906
Zhou X, Cao S, Li X, Tang B, Ding X, Xi C, Hu J, Chen Z (2015) Simultaneous determination of 18 preservative residues in vegetables by ultra high performance liquid chromatography coupled with triplequadrupole/linear ion trap mass spectrometry using a dispersive-SPE procedure. J Chromatogr B 989:21–26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by The National Natural Science Foundation of China (31071702).
Conflict of Interest
Yaru Wang declares that she has no conflict of interest. Xuexue Miao declares that she has no conflict of interest. Haifeng Wei declares that he has no conflict of interest. Deyun Liu declares that he has no conflict of interest. Gaofeng Xia declares that he has no conflict of interest. Xiaoyun Yang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent is not applicable in this study.
Rights and permissions
About this article
Cite this article
Wang, Y., Miao, X., Wei, H. et al. Dispersive Liquid-Liquid Microextraction Combined with Gas Chromatography-Mass Spectrometry for the Determination of Multiple Pesticides in Celery. Food Anal. Methods 9, 2133–2141 (2016). https://doi.org/10.1007/s12161-015-0390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0390-5