Abstract
The potential of mid-infrared (MIR), near-infrared (NIR), and low-field nuclear magnetic resonance (LF-NMR) techniques combined with chemometrics for reliable and rapid determination of soluble solids content (SSC) and moisture in jams was investigated. Forty-four different jam samples with SSC ranging from 17.49 to 73.91 (°Brix) and moisture ranging from 20.44 to 81.03% were used in this study. Principal component analysis (PCA) showed that the three spectroscopic techniques were able to distinguish the jams based on the SSC and moisture content. Partial least squares (PLS) regression exhibited a good correlation between the reference values and the MIR, NIR, and LF-NMR predicted ones, with low errors of prediction and high coefficients of determination. An F test at 95% confidence level did not indicate significant differences between the accuracy of the PLS models obtained using MIR and NIR spectroscopic techniques. However, significant differences were observed comparing MIR with LF-NMR and NIR and LF-NMR. The residual prediction deviation (RPD) up to 2.5 indicated that all models are good.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Analytical instrumentation has provided simple, rapid, non-destructive, and, in some cases, automated methods for quality control (QC) and quality assurance (QA) of food products in all production stages in industry. Among these techniques, mid-infrared (MIR) and near-infrared (NIR) vibrational spectroscopies have received considerable attention due to the great potential for on-line/at-line food quality control (Porep et al. 2015). These techniques have been successfully applied to rapid assessment of the quality attributes and/or authenticity of meat (Prieto et al. 2009), fish (Cheng et al. 2013), dairy products (Karoui and Baerdemaeker 2007), beverages (Cozzolino et al. 2011), fruits and vegetables (Nicolai et al. 2007), and others (Karoui et al. 2010; Lohumi et al. 2015). All the results have demonstrated that MIR and NIR are powerful analytical techniques for the development of non-destructive, simultaneous, and environmentally friendly (require no sample preparation) methods.
Low-field nuclear magnetic resonance (NMR) spectrometers, based on benchtop instruments cryogen-free magnets, have also been used in QC/QA in food industry for more approximately five decades (Colnago et al. 2014). However, a sharp increase in applications has been seen in the last two decades with the uses of relaxametric and diffusometric methods (Guthausen 2016; Blumich 2016). These instruments are also known as time-domain NMR (TD-NMR) because the analyses are performed in time-domain signal, without Fourier transformation. Most of the analyses are performed with the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence that yield an exponential decay with a time constant T2 called transverse or spin-spin relaxation times. This sequence has been used to predict the quality of fresh fruits (Pereira et al. 2013; Møller et al. 2013), meat (Micklandery et al. 2002), vegetable oil (Zhu et al. 2017; Santos et al. 2017), and dairy products (Castell-Palou et al. 2013; Santos et al. 2016). Compared to the vibrational spectroscopy, the main advantage of LF-NMR is that the analysis is not restricted to the sample surface and can be performed through package (Santos et al. 2017; Pereira et al. 2015).
Although MIR, NIR, and LF-NMR techniques have been widely used in food analysis and some procedures have already accepted by the regulatory agencies, there are no reports about the use of these techniques to evaluate the quality of jam. Jam is one of the most popular shelf-stable products made from fruit. The jam’s production is one way to minimize fruit losses and increase its availability to consumers during the off-season. Traditionally, jams are prepared by cooking fruits with sugar, extracted acids, and pectin in adequate proportions until reaching the required final soluble solids content (SSC). SSC, a measurement based on grams of sucrose/100 g of mixture, is usually performed in refractometers and expressed in °Brix (AOAC 1990). Alternatively, SSC can be measured using MIR, NIR, and LF-NMR. Currently, these techniques have been successfully applied to determinate SSC (°Brix) in a wide variety of fruit and juices, such as apples (Giovanelli et al. 2014), strawberries (Sánchez et al. 2012), orange (Cayuela and Weiland 2010), tomatoes (Ayvaz et al. 2016), and plums (Pereira et al. 2013).
The objective of this study was to develop calibration models using MIR, NIR, and LF-NMR techniques to predict the amount of SSC (°Brix) and moisture in jams. The performances of the models were evaluated based on the root mean square error of calibration (RMSEC), cross-validation (RMSECV), and prediction (RMSEP), and residual prediction deviation (RPD).
Materials and Methods
Samples
A total of 44 commercial jam samples (26 traditional, 10 without sugar addition, 3 light, and 5 diet), from 21 commercial brands and 4 different fruits (strawberry, grape, guava, and raspberry), were used in this study. Among the 21 commercial brands analyzed, 67% were made in Brazil, 14% were made in Italy, 9% were made in France, and the less 10% were made in Poland and Turkey. The jams were obtained from a local market (São Carlos, SP, Brazil) and stored at 5 °C.
SSC (°Brix) and Moisture Determination
SSC (°Brix) and moisture were determined according to the analytical methods described by Association of Official Analytical Chemists (AOAC). SSC (°Brix) was performed using a refractometer (RX5000α, Atago, USA) at 25 °C (AOAC 1990). Moisture content (grams of water per gram of product) was determined by drying the sample to a constant weight in a vacuum oven (440-2D, Nova Ética, Brazil) at 60 °C (AOAC 1990). All the samples were analyzed in triplicate.
LF-NMR Measurements
LF-NMR experiments were evaluated in the SLK 100 TD-NMR benchtop spectrometer (Spinlock Magnetic Resonance Solution, Cordoba, Argentine) equipped with a 0.23-T permanent magnet (8.9 MHz for 1H). 1H transverse relaxation time, T2, of the samples was performed using CPMG pulse sequence with a π/2 pulse width of 6.0 μs, time between echoes of 100 μs, 3000 echoes, and a recycle delay of 3 s, in triplicate.
MIR Measurements
A Cary 630 FTIR spectrometer (Agilent Technologies Inc., USA) equipped with a single-bounce attenuated total reflectance (ATR) diamond crystal interface, ZnSe beamsplitter, and DTGS detector was used in the MIR measurements. MIR spectra of the samples were acquired in the region 4000–650 cm−1 with a resolution of 4 cm−1 and using 32 scans, in triplicate.
NIR Measurements
NIR spectra were collected in the 100N FT-NIR spectrometer (PerkinElmer, USA), using a NIR infrared reflectance accessory (NIRA) (PerkinElmer, USA). The sample spectra were collected in a frequency range of 10,000–4000 cm−1 using a spectral resolution of 4 cm−1 and 32 scans, in triplicate.
Multivariate Analysis
The MIR, NIR, and LF-NMR data were analyzed by principal component analysis (PCA) and partial least squares (PLS) regression using MatLab 7.2 (The MathWorks, Natick, MA, USA) installed with the PLS toolbox version 6.5 (Eigenvector Technologies, Manson, WA, USA). MIR spectra and LF-NMR data were previously mean centered. The baseline deviations on the NIR spectra were corrected by applying the first derivative with Savitzky-Golay smoothing (15 points in the filter and first-order polynomial fit) and then, the spectra were mean centered (Rinnan et al. 2009).
For the development of PLS models, the 132 (44 samples × 3 triplicate) MIR, NIR, and LF-NMR data were divided into 99 for the calibration set and 33 for the validation set by using the Kennard-Stone algorithm. A homemade routine was employed for the detection of outliers (Silva et al. 2012). The best number of latent variables (LV) was chosen by random subset (20 splits and 20 iterations) cross-validation, based on the value of RMSEC and RMSECV. The PLS model evaluations were based on RMSEC, RMSECV, RMSEP, and RPD. The RPD has been defined as the ratio of standard deviation (SD) of reference values of the calibration and validation sets by the RMSECV and RMSEP, respectively (Botelho et al. 2010). It is useful for evaluating the performance of multivariate calibration models in absolute terms. Models with RPD values higher than 2.4 are considered desirable for good calibration equations, whereas equations with RPD less than 1.5 are considered unusable (Botelho et al. 2010). The presence of trends in the prediction residuals was assessed by a nonparametric permutation test (Filgueiras et al. 2014).
In order to compare the performance of the PLS models obtained using the three spectroscopic techniques (MIR, NIR, and LF-NMR), an F test was evaluated with a confidence level of 95%. The F-values were obtained by the ratio of the variances (RMSEP squared), where RMSEP1 > RMSEP2. If Fcalculated is greater than the \( {F}_{\upnu 1,\upnu 2,\alpha}^{\mathrm{critical}} \), the two RMSEPs are significantly different.
Results and Discussion
SSC (°Brix) and Moisture Content
Table 1 shows the results of SSC (°Brix) and moisture content obtained for the 44 commercial jams. As can be observed, a significant difference for both properties was observed among the four types of jams: traditional, without sugar addition, diet, and light. The results showed that the differences among the different kinds of jams (traditional, without sugar addition, diet, and light) were more important than the observed within the group.
Traditional jams showed the highest SSC, ranging from 55.44 to 73.91 °Brix (mean = 63.97 and SD = 3.82) and the lowest values to moisture, ranging from 20.44 to 40.18% (mean = 30.84 and SD = 4.21). Conversely, diet samples showed the lowest SSC, ranging from 17.49 to 32.90 °Brix (mean = 27.00 and SD = 6.59) and highest moisture, ranging from 65.56 to 81.03% (mean = 71.21 and SD = 7.09).
Exploratory Analysis
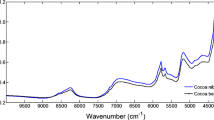
The MIR and NIR spectra and the CPMG relaxation curves obtained with the 44 commercial jams, gray-scaled colored according to the SSC (°Brix), are shown in Fig. 1a–c together with the corresponding PCA score plots (Fig. 1d–f).
Figure 1a shows that the main changes in the MIR spectra of traditional, light and diet jam samples are highlighted in the fingerprint region (1500–900 cm−1), where it is possible to observe an increase in absorption in the samples with high SSC (°Brix) (i.e., traditional jams). This region contains unique information about the carbohydrate absorption, including several bands that could be associated with C–C and C–O stretching modes (1180–900 cm−1) and C–O–H, C–O–C, and O–C–H bending vibrational modes (1500–1200 cm−1). The MIR spectra also showed two prominent absorption bands centered at 3274 and 1643 cm−1 associated with the strong O–H stretching vibrations (Ayvaz et al. 2016).
NIR reflectance spectra (Fig. 1b) show strong bands between 7200 and 6400 cm−1 and between 5400 and 4900 cm−1 which are associated with the first overtone of O–H stretching and O–H combination bands of water, respectively. Other small features in the spectral regions between 5800 and 5400 cm−1 and between 4900 and 4000 cm−1 are related to the first overtone of C–H stretching and C–H + C–H and C–H + C–C combination bands, respectively, both attributed to vibrations of the molecules of sugars (Giovanelli et al. 2014).
LF-NMR experiments showed that the samples with high sugar concentration (i.e., traditional jams) had significantly lower T2 values than the samples with low sugar concentration (i.e., diet jams) (Fig. 1c). This is in agreement with some previously published studies (Pereira et al. 2013), which showed that the increase of SSC (°Brix) restricted the motion of the water molecules, due to exchange with sugar OH groups, and resulted in a lower relaxation time. Thus, the short T2 of jams samples could be assigned to the water that is strongly associated with the carbohydrates, while the long T2, obtained for the diet jellies, could be attributed the most mobile water.
Figure 1d shows the score plot of the first (PC1) and the second (PC2) principal components obtained with MIR spectra. PC1 explains 93.17% of the total variance and clearly separates the traditional jam samples (positive side of PC1) from the jams without sugar addition, light, and diet (negative side of PC1). Inspection of the graph of loadings of the first PC (Fig. S1, supplementary material) revealed that the main peak responsible for the sample distribution along this PC is associated with the sugar signal (band around 1030 cm−1) in the fingerprint region.
The PCA score plot obtained with NIR spectra (Fig. 1e) and CPMG relaxation curves (Fig. 1f) showed similar performance than that obtained with MIR spectra (Fig. 1d), allowing the discrimination of the jams in two groups: (1) traditional and (2) jams without sugar addition, light, and diet. Traditional samples were grouped at one end of PC1, whereas jams without sugar addition, light, and diet were grouped at the other end. The NIR loadings (Fig. S2, supplementary material) showed that the regions effecting the sample distribution are related to sugar (4900–4000 cm−1) and water (5400–5200 cm−1) absorption bands. In the LF-NMR loading plot (Fig. S3, supplementary material), it is possible to observe that samples with T2 higher than 0.05 ms (jams without sugar addition, light, and diet) show positive scores for PC1. Conversely, samples with T2 lower than 0.05 ms (traditional jams) show negative scores for this PC.
Calibration Models
MIR, NIR, and LF-NMR techniques allowed the development of calibration models for quantification of SSC (°Brix) and moisture in jams. All PLS models for SSC (°Brix) determination were developed using two latent variables that describe 94.41, 94.68, and 99.26% of the data variance using MIR, NIR, and LF-NMR techniques, respectively. PLS models for moisture determination were also developed using two latent variables and describe 95.45, 94.55, and 99.22% of the data variance applying MIR, NIR, and LF-NMR techniques, respectively. The results obtained with the three spectroscopic techniques are summarized in Table 2. It is possible to observe that all models exhibited excellent results, with low prediction errors. The MIR, NIR, and LF-NMR calibration models for SSC (°Brix) determination gave values of RMSEP equal to 1.16, 1.50, and 2.65 °Brix, respectively. For moisture determination, RMSEP values range from 1.50 to 3.19%.
An F test at 95% of confidence level indicated that the differences in the RMSEP values of the PLS models obtained with MIR and NIR spectroscopic techniques were not significant differences either for SSC (°Brix) (\( {F}_{\upnu 1=23,\upnu 2=29,\alpha =0.05}^{\mathrm{critical}} \) = 1.91 > Fcalculated = 1.67) or moisture (%) (\( {F}_{\upnu 1=23,\kern0.5em \upnu 2=29,\alpha =0.05}^{\mathrm{critical}} \) = 1.91 > Fcalculated = 1.55) models. However, comparing the performance of the PLS models obtained using MIR and LF-NMR spectroscopic techniques revealed that the differences in the RMSEP values are significant (Fcalculated > \( {F}_{\upnu 1,\upnu 2,\alpha}^{\mathrm{critical}} \)). The F test evaluated with the RMSEP values obtained in the MIR and LF-NMR PLS models exhibited Fcalculated values of 5.22 and 4.44 for SSC (°Brix) and moisture (%), respectively, and \( {F}_{\upnu 1=26,\kern0.5em \upnu 2=29,\alpha =0.05}^{\mathrm{critical}} \) = 1.88. Similar results were obtained comparing the PLS models obtained using NIR and LF-NMR. An F test evaluated with the RMSEP values obtained in the NIR and LF-NMR PLS models exhibited Fcalculated values of 3.12 and 2.86 for SSC (°Brix) and moisture (%), respectively, and \( {F}_{\upnu 1=23,\upnu 2=29,\alpha =0.05}^{\mathrm{critical}} \) = 1.99. These results indicated that the MIR and NIR models present better accuracy than those obtained with LF-NMR. Although the models showed different accuracy, all the models showed RPD > 2.5; it is considered good for calibration equations. In particular, MIR and NIR techniques showed RPD up to 6.5, indicating that the models are adequate for quality control and process monitoring (Igne and Hurburgh Jr 2010).
The PLS regression curves for prediction of SSC (°Brix) and moisture content are shown in Figs. 2 and 3, respectively. Good agreement between the measured values and those predicted from models was observed, with coefficient of determination near 1 in all the cases. Nonparametric permutation test confirmed that there are no trends in residuals, with p values greater than 0.05 for all PLS models.
Conclusion
Fast, simple, and accurate methods for determination of SSC (°BRix) and moisture content in jams were demonstrated by using MIR, NIR, and LF-NMR techniques combined with multivariate analysis. Both infrared (MIR and NIR) techniques generated PLS models with superior performance than those obtained with LF-NMR, as shown in the results obtained from F test at 95% of confidence level. RPD higher than 2.4 indicated that all models are good. Comparing the three spectroscopic techniques, the main advantage of LF-NMR is the possibility of a direct measurement through glass or plastic vials, allowing the costumer to check the product quality before buying.
References
AOAC (1990) Official methods of analysis, 4th edn. Association of Official Analytical Chemists, Washington, DC
Ayvaz H, Sierra-Cadavid A, Aykas DP, Mulqueeney B, Sullivan S, Rodriguez-Saona LE (2016) Monitoring multicomponent quality traits in tomato juice using portable mid-infrared (MIR) spectroscopy and multivariate analysis. Food Control 66:79–86. https://doi.org/10.1016/j.foodcont.2016.01.031
Blumich B (2016) Introduction to compact NMR: a review of methods. Trend Anal Chem 83:2–11. https://doi.org/10.1016/j.trac.2015.12.012
Botelho BG, Mendes BAP, Sena MM (2010) Development and analytical validation of robust near-infrared multivariate calibration models for the quality inspection control of mozzarella cheese. Food Anal Methods 6:881–891. https://doi.org/10.1007/s12161-012-9498-z
Castell-Palou A, Rosselló C, Femenia A, Simal S (2013) Simultaneous quantification of fat and water content in cheese by TD-NMR. Food Bioprocess Technol 6:2685–2664. https://doi.org/10.1016/j.foodchem.2007.04.079
Cayuela JA, Weiland C (2010) Intact orange quality prediction with two portable NIR spectrometers. Postharvest Biol Technol 58:113–120. https://doi.org/10.1016/j.postharvbio.2010.06.001
Cheng JH, Dai Q, Sun DW, Zeng XA, Liu D, Pu HB (2013) Applications of non-destructive spectroscopic techniques for fish quality and safety evaluation and inspection. Trends Food Sci Technol 34:18–31. https://doi.org/10.1016/j.tifs.2013.08.005
Colnago LA, Andrade FD, Souza AA, Azeredo RB, Lima AA, Cerioni LM, Osán TM, Pusiol DJ (2014) Why is inline NMR rarely used as industrial sensor? Challenges and opportunities. Chem Eng Technol 37:191–203. https://doi.org/10.1002/ceat.201300380
Cozzolino D, Cynkar W, Shah N, Smith P (2011) Technical solutions for analysis of grape juice, must, and wine: the role of infrared spectroscopy and chemometrics. Anal Bioanal Chem 401:1475–1484. https://doi.org/10.1007/s00216-011-4946-y
Filgueiras PR, Alves JCL, Sad CMS, Castro EVR, Dias JCM, Poppi RJ (2014) Evaluation of trends in residuals of multivariate calibration models by permutation test. Chemometr Intell Lab 133:33–41. https://doi.org/10.1016/j.chemolab.2014.02.002
Giovanelli G, Sinelli N, Beghi R, Guidetti R, Casiraghi E (2014) NIR spectroscopy for the optimization of postharvest apple management. Postharvest Biol Technol 87:13–20. https://doi.org/10.1016/j.postharvbio.2013.07.041
Guthausen G (2016) Analysis of food and emulsions. Trend Anal Chem 83:103–106. https://doi.org/10.1016/j.trac.2016.02.011
Igne B, Hurburgh Jr CR (2010) Local chemometrics for samples and variables: optimizing calibration and standardization processes. J Chemom 24:75–86. https://doi.org/10.1002/cem.1274
Karoui R, Baerdemaeker JD (2007) A review of the analytical methods coupled with chemometric tools for the determination of the quality and identity of dairy products. Food Chem 102:621–640. https://doi.org/10.1016/j.foodchem.2006.05.042
Karoui R, Downey G, Blecker C (2010) Mid-infrared spectroscopy coupled with chemometrics: a tool for the analysis of intact food systems and the exploration of their molecular structure-quality relationships—a review. Chem Rev 110:6144–6168. https://doi.org/10.1021/cr100090k
Lohumi S, Lee S, Lee H, Cho BK (2015) A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci Technol 46:85–98. https://doi.org/10.1016/j.tifs.2015.08.003
Micklandery E, Peshlovy B, Purslow PP, Engelseny SB (2002) NMR-cooking: monitoring the changes in meat during cooking by low-field 1H-NMR. Trends Food Sci Technol 13:341–346. https://doi.org/10.1016/S0924-2244(02)00163-2
Møller SM, Travers S, Bertram HC, Bertelsen MG (2013) Prediction of postharvest dry matter, soluble solids content, firmness and acidity in apples (cv. Elshof) using NMR and NIR spectroscopy: a comparative study. Eur Food Res Technol 237:1021–1024. https://doi.org/10.1007/s00217-013-2087-6
Nicolai BM, Beullens K, Bobelyn E, Peirs A, Saeys W, Theron KI, Lammertyn J (2007) Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol Technol 46:99–118. https://doi.org/10.1016/j.postharvbio.2007.06.024
Pereira FMV, Carvalho AS, Cabeça LF, Colnago LA (2013) Classification of intact fresh plums according to sweetness using time-domain nuclear magnetic resonance and chemometrics. Microchem J 108:14–17. https://doi.org/10.1016/j.microc.2012.12.003
Pereira FMV, Rebellato AP, Pallone JAL, Colnago LA (2015) Through-package fat determination in commercial samples of mayonnaise and salad dressing using time-domain nuclear magnetic resonance spectroscopy and chemometrics. Food Control 48:62–66. https://doi.org/10.1016/j.foodcont.2014.02.028
Porep JU, Kammerer DR, Carle R (2015) On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci Technol 46:211–230. https://doi.org/10.1016/j.tifs.2015.10.002
Prieto N, Roehe R, Lavín P, Batten G, Andrés S (2009) Application of near infrared reflectance spectroscopy to predict meat and meat products quality: a review. Meat Sci 83:175–186. https://doi.org/10.1016/j.meatsci.2009.04.016
Rinnan A, van den Berg F, Engelsen SB (2009) Review of the most common pre-processing techniques for near-infrared spectra. Trend Anal Chem 28:1201–1222. https://doi.org/10.1016/j.trac.2009.07.007
Sánchez MT, Haba MJ, Benítez-López M, Fernández-Novales J, Garrido-Varo A, Pérez-Marín D (2012) Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J Food Eng 110:102–108. https://doi.org/10.1016/j.jfoodeng.2011.12.003
Santos PM, Pereira-Filho ER, Colnago LA (2016) Detection and quantification of milk adulteration using time domain nuclear magnetic resonance (TD-NMR). Microchem J 124:15–19. https://doi.org/10.1016/j.microc.2015.07.013
Santos PM, Kock FVC, Santos MS, Lobo CMS, Carvalho AS, Colnago LA (2017) Non-invasive detection of adulterated olive oil in full bottles using time-domain NMR relaxometry. J Braz Chem Soc 28:385–390. https://doi.org/10.5935/0103-5053.20160188
Silva MAM, Ferreira MH, Braga JWB, Sena MM (2012) Development and analytical validation of a multivariate calibration method for determination of amoxicillin in suspension formulations by near infrared spectroscopy. Talanta 89:342–351. https://doi.org/10.1016/j.talanta.2011.12.039
Zhu W, Wang X, Chen L (2017) Rapid detection of peanut oil adulteration using low-field nuclear magnetic resonance and chemometrics. Food Chem 216:268–274. https://doi.org/10.1016/j.foodchem.2016.08.051
Funding
This study was funded by Fapesp (grant number 2013/22970-1, 2014/22126-9, and 2017/12864-0) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Poliana Macedo dos Santos declares that she has no conflict of interest. Luiz Alberto Colnago declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic supplementary material
ESM 1
(DOCX 70 kb)
Rights and permissions
About this article
Cite this article
Santos, P.M., Colnago, L.A. Comparison Among MIR, NIR, and LF-NMR Techniques for Quality Control of Jam Using Chemometrics. Food Anal. Methods 11, 2029–2034 (2018). https://doi.org/10.1007/s12161-018-1195-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1195-0