Abstract
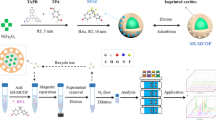
In this study, a core–shell magnetic dummy molecularly imprinted polymer (MDMIP) was prepared with a novel and simple method. The imprinting layer was synthesized directly on the surface of Fe3O4 by only one step, which avoided further modification on the exterior of magnetic core. 4,4′-Dihydroxybiphenyl (DDBP) was used as dummy template instead of bisphenol A (BPA) to eliminate the effect of template leakage on quantitative analysis. When used as solid-phase extraction sorbent, a rapid, sensitive, and accurate method for the simultaneous extraction, concentration, and determination of trace BPA in plastic bottled beverage samples by MDMIP-SPE coupled with high-performance liquid chromatography (HPLC) was developed. Advantages of such method may be counted as the mild working temperature during the synthesis, simplicity of extraction procedure, avoidance of leakage of template, time-saving, and high binding capacity and affinity. Several parameters affecting the extraction efficiency of the analytes including the sorbent mass, the pH of sample solution, the HAc percentage in the elute, and the desorption time were investigated. Under optimized conditions, the calibration graph was linear over the range of 11.4–4560 ng mL−1 with the limit of detection of 0.083 ng L−1. In addition, the higher enrichment factors (400-fold) and good recoveries (88.6–99.5%) with relative standard deviation (RSD) values less than 9.5% were achieved. Moreover, the developed extraction protocol simplified the process of traditional solid-phase extraction (SPE) provided the possibility for separation and enrichment of BPA from complicated food matrices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is an important industrial material used in the manufacture of many products such as microwave lunch boxes, baby bottles, water dispensers, and food packing boxes (Vandenberg et al. 2007). Because of easy migration from these products, BPA was usually found in a variety of foods and drinks. Exposure to BPA for a long period can potentially interfere with endocrine function and adversely influence the reproductive systems of wildlife and humans (Hiroi et al. 1999). Because of weak biological degradation of BPA and its resistance to chemical oxidation, conventional treatment process to remove it from drinking and food samples is difficult. Therefore, there has been increasing international concern over the monitoring of trace amounts of BPA released into the environment, especially in drinking and food samples.
Due to the low level of BPA in the food, it is usually necessary to concentrate the sample. To date, the common methods used for the determination of trace compound in different matrices include pipette tip extraction (PTE) (Arabi et al. 2016a), solid-phase microextraction (SPME) (Tan et al. 2009; Arabi et al. 2017a), stir bar sorptive extraction (SBSE) (Hu et al. 2010; Coensel et al. 2009; Cacho et al. 2012), solid-phase extraction (SPE) (Arabi et al. 2016b; Xu et al. 2014; Arabi et al. 2016c; Arabi et al. 2017b), matrix solid-phase dispersion (MSPD) (Arabi et al. 2016d), and microwave-assisted extraction (MSE) (Zhang et al. 2010) followed by HPLC or gas chromatography (GC). Among them, SPE is currently the leading technique for the extraction of pollutants from liquid samples (Cai et al. 2003; Rodriguez-Mozaz et al. 2004). However, the classical SPE sorbent does not have the ability to adsorb the target with high specificity and selectivity. As a kind of promising material with mechanical and chemical stability, desirable selectivity, large adsorption capacities, and low cost, molecularly imprinted polymers (MIPs) have attracted the attention of research community and were widely used to enrich and detect the trace amount of BPA in food samples, such as milk (Ji et al. 2009), fish (Wei et al. 2013), beverages, (Yang et al. 2014), and packed food (Xu et al. 2011). Unfortunately, these efforts may have the problems including the leakage of template, cumbersome process of packing the SPE column, and time-consuming process of loading a large-volume sample. To solve these problems, magnetic Fe3O4 was used as core to synthesize MIPs, which makes it easy for the sorbent to achieve rapid separation from complex matrix by applying an external magnet.
In addition, leakage of the residual template molecules is one of the biggest challenges for the application of magnetic molecularly imprinted polymers (MMIPs) in SPE. When MMIPs are used for sample separation and enrichment, even minute quantities of leakage will have a significant influence on the experimental result, especially in the analysis of trace samples. An effective solution to this problem is the utilization of dummy template molecules, whose chemical structure is similar to the target substance. By this way, any leakage of the virtual template will not interfere with the analysis results, as long as two analog molecules can be separated during the procedure. To the best of our knowledge, several dummy template molecules, such as 2,2-bis(4-hydroxyphenyl)hexafluoropropane (Yu et al. 2015; Hu et al. 2016), tetrabromobisphenol A (Li et al. 2016), and bisphenol F (Lin et al. 2012), have been used to prepare BPA-DMIPs. Considering the selectivity, adsorption capacity, toxicity, and cost of the dummy template in methanol–water system, the selection of dummy template was a very crucial step which can affect the final result and outcome of MISPE procedure.
Thus, in order to simplify the solid-phase extraction process and avoid interference from leached template molecules in the process of determination of trace BPA, DDBP was used as dummy template to synthesize magnetic dummy molecularly imprinted polymers (MDMIPs) on the surface of Fe3O4-COOH nanospheres through a one-step sol–gel method. The adsorption performance of MDMIPs was evaluated, and a method for MDMIP-SPE coupled with HPLC was developed and applied to the selective and sensitive determination of BPA from plastic bottled drinking products. The developed MDMIP-SPE-HPLC protocol significantly improved the sensitivity by loading a large volume of sample and eliminating the effect of template leakage on quantitative analysis.
Experimental
Chemicals and Reagents
3,3′,5,5′-Tetrabromobisphenol A (TBBPA, purity ≥98%), 4,4′-dihydroxybiphenyl (DDBP, purity >97%), bisphenol F (BPF, purity ≥98%), bisphenol A (BPA, purity ≥98%), and p-tert-butylphenol (PTBP, purity ≥96%) were purchased from Aladdin Reagent Co. (China). 3-Aminopropyltriethoxylsilane (APTES, purity ≥98%) was purchased from Nanjing Union Silicon Chemical Co. (China). Ferric chloride crystals (FeCl3·6H2O), tetraethoxysilane (TEOS), anhydrous sodium acetate (NaAc), ethylene glycol (EG), methanol, ethanol, trisodium citrate dihydrate (Na3Cit·2H2O), and ammonium hydroxide (28%) were purchased from Shanghai Chemical Reagent Co. (China). Doubly distilled water was obtained from a laboratory purification system. All of the chemicals were of at least analytical grade and without further purification.
Preparation of MDMIPs
Synthesis of Uniform Fe3O4 Nanospheres
The carboxyl-functionalized Fe3O4 nanospheres were prepared according to a previous method (Zhang et al. 2013) with some modifications. The resultant black product was washed several times with ethanol and doubly distilled water and dried at 60 °C for 8 h.
Synthesis of MDMIP and MNIP Beads
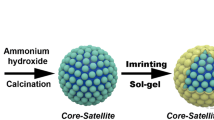
The imprinted layer was synthesized through sol–gel process on the surface of Fe3O4 by only one step, according to the method established by our groups previously (Chang et al. 2016). DDBP (0.5 mmol) and APTES (2 mL) were dissolved in methanol (10 mL) and kept stationary for 3 h to prepare the pre-assembly solution. Fe3O4 (200 mg) prepared previously was dispersed in methanol (30 mL) and doubly distilled water (5 mL) by ultrasonic vibration and mixed with NH3·H2O (1 mL, 28%) and TEOS (2 mL). The mixture was stirred for 5 min; then, the pre-assembly solution prepared was added, and copolymerization was carried out by stirring for 1 h at room temperature. The resultant product was separated by a magnet and dried at 60 °C for 12 h.
To remove DDBP, the polymers were washed several times with methanol/acetic acid solution (9:1, v/v) using ultrasonic vibration until no residual DDBP was detected. Finally, the resulted MDMIPs were washed with methanol to neutrality and then dried at 60 °C for 12 h. The magnetic non-imprinted polymers (denoted as MNIPs) were also prepared following the same procedure in the absence of DDBP.
Binding Experiments
To measure the binding kinetics of the materials, 10 mg of MDMIPs was dispersed in 10 mL of aqueous solution (containing 2% methanol, v/v) with 1 mmol L−1 BPA. The mixtures were mechanically shaken at room temperature, and HPLC-UV was used to monitor the temporal residual BPA concentration in solutions at certain time intervals ranging from 5 to 80 min. Isothermal adsorption experiments were carried out by changing the initial concentrations of BPA from 0.1 to 2.25 mmol L−1 while employing the same adsorption time of 30 min.
The sorption capacity, Q (μmol g−1), is calculated using the following formula:
where Co and Ce are the initial and final template concentrations (mmol L−1) of the solution, respectively, V (L) is the volume of the solution, and W (g) is the weight of the imprinted materials.
Evaluation of MDMIP Beads Used as SPE Sorbent
MDMIP-SPE Procedure
MDMIPs (50 mg) were dispersed into 100 mL of BPA aqueous solution. After being ultrasonicated for 15 min to ensure complete adsorption, the MDMIP beads were separated from the suspension with a magnet. Then, the BPA was eluted from the MDMIPs with 3 mL methanol (containing 10% acetic acid, v/v) under ultrasonication for 30 s. Finally, the eluate was evaporated at 323 K, and the residue was dissolved in 1 mL methanol.
Selective Extraction of BPA on MDMIP-SPE and DMNIP-SPE
TBBPA and PTBP were selected as BPA analogs. Their chemical structures are shown in Fig. 1. The sample solutions were a series of 100 mL of distilled water containing 10 nmol of BPA and different amounts of TBBPA and PTBP. The molar ratios of BPA to its analogs were 1:1:1, 1:2:2, 1:3:3, 2:1:1, and 3:1:1, respectively. The adsorption and elution procedures were similar to that described above.
Reusability and Enrichment Factor of MDMIP-SPE
Fifty-milligram MDMIPs were suspended in 100 mL of aqueous solution with 10 nmol BPA. A procedure similar to that in the “MDMIP-SPE Procedure” section was followed six times to observe the reusability of MDMIP-SPE.
To validate the new M-SPE method, the enrichment factor of MDMIPs was also investigated. Different sample volumes (100, 200, 300, and 400 mL) with 10 nmol of BPA were used. The same procedure mentioned in the “MDMIP-SPE Procedure” section was applied.
Method Validation and Application to Real Samples
MDMIP-SPE coupled to HPLC-UV was developed to monitor the trace BPA in real samples. The linearity of the analytical method was established by analyzing the elution of the BPA standard solutions after carrying out the MDMIP-SPE procedures using nine different concentrations (11.4, 22.8, 57, 114, 228, 570, 1140, 2280, and 4560 ng mL−1), respectively, as the process described in the “MDMIP-SPE Procedure” section. The mean peak areas from triplicate HPLC analyses of a range of solution concentrations were used to determine calibration curves for each standard. The plastic bottled beverages were spiked with four different amounts of BPA, resulting in final concentrations of 0.114, 0.57, 1.14, and 30 ng mL−1.
Samples of six plastic bottled drink products were collected in May 2017 in local stores in Hefei City: Fanta water, mineral water, green tea, pulsating drinks, Coca Cola, and orange juice; these samples covered a wide variety of products, such as diet, non-diet, and energy drinks. For the spiking experiments, analyte standard was added directly to the samples. All samples were sonicated for half an hour to remove carbon dioxide gases and then need to be further adjusted to about pH 6.0 by adding NaOH solution and filtered through 0.22-μm nylon membranes to remove suspended solids. Then, they were stored in a refrigerator at 4 °C before analysis to avoid any microbial degradation of the analytes.
Equipments and Conditions
The structures and morphologies of MDMIPs and MNIPs were observed using SIRION200 SEM (FEI, Holland) and a JEM-2011 TEM (JEOL, Japan) with a voltage of 5 and 200 kV, respectively. The infrared spectra were recorded on a Tensor-27 FT-IR instrument (Bruker, Germany) with a resolution of 2 cm−1 and a spectral range of 4000 to 400 cm−1. The magnetic properties were measured using a superconducting quantum interference device (SQUID) magnetometer at 300 K, and the magnetic field intensity was ±1 T.
The BPA amount was analyzed using a high-performance liquid chromatography (HPLC) system (Shimadzu, Japan), which contained a LC-15C pump, a SIL-10AF injector with a 50-μL loop, and a SPD-15C dual-wavelength absorbance detector. The mobile phase used for the HPLC experiments was a mixture of methanol and water (90:10, v/v). All separations were carried out on a GL Sciences C18 column (5 μm, 200 mm × 4.6 mm), and the sample injection volume was 10 μL. The detecting wavelength for the UV detector was 228 nm for BPA, with a flow rate of 1.0 mL min−1, and 276 nm for mixtures of BPA and analogs, with a flow rate of 0.8 mL min−1.
Results and Discussion
Preparation of MDMIPs
The synthesis of MDMIPs and the whole SPE process are illustrated in Fig. 2. First, the Fe3O4 whose surfaces were full of numerous carboxyl groups was successfully obtained via a modified one-step hydrothermal method. To eliminate template leakage and improve the accuracy of SPE process, the imprinting layer for specifically recognizing BPA was synthesized directly on the surface of Fe3O4 by siloxane copolymerization with DDBP as dummy template molecules, TEOS as cross-linkers, and APTES as functional monomer. The whole process was simple, convenient, and time-saving without further modification on the surface Fe3O4 supporters.
The key preparation procedure was the selection of dummy template molecule. To ensure the best imprinting effect, different MDMIPs were prepared using TBBPA, BPF, and DDBP as dummy templates. It should be noted that, as the most often used virtual template in the synthesis of BPA-MDMIP, TBBPA was not proper in this experiment, for a white precipitate was generated when the APTES was added to the solution containing TBBPA, which is not conductive to the pre-organization of APTES and TBBPA. In the meanwhile, when BPF was used as dummy template, the adsorption capacity was much lower and the cost of BPF was more expensive when compared with DDBP. In addition, the structural skeletons of DDBP were similar to that of BPA, while both of them contained two hydroxyl groups, which would form strong hydrogen bonds with the amino group of the functional monomer. Therefore, the DDBP was chosen as the final dummy template.
Compared with other reported surface-imprinted polymers for the capture of BPA, the procedure utilized in this work was more straightforward, cost-effective, and time-saving, which coated imprinting layer directly on the surface of Fe3O4. In addition, the developed extraction protocol based on the MDMIPs eliminated the effect of template leakage on quantitative analysis and simplified the process of traditional SPE by avoiding the need to pack the SPE column and the time-consuming process of loading a large-volume sample. It also makes it easy for the sorbent to achieve rapid separation from complex matrix by applying an external magnet. Such merits make MDMIPs one of the most promising candidates to the enrichment in the complex and large-volume drinking sample.
Characterization of the MDMIP Beads
Figure 3 shows the SEM and TEM images of the obtained Fe3O4 and MDMIP beads. As shown in Fig. 3a, c, Fe3O4 nanospheres synthesized by a solvothermal method were highly spherical and monodispersed, with an average diameter of 200 ± 25 nm. After the one-step sol–gel molecular imprinting process, the average diameter of the MDMIPs increased to approximately 280 ± 25 nm (Fig. 3d), corresponding to an imprinted layer thickness of 40 nm. The obvious core–shell structure shown in Fig. 3d implied that polymerization successfully occurred on the surface of Fe3O4.
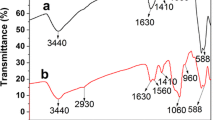
The FT-IR spectra of Fe3O4, DDBP, MNIPs, and MDMIPs are shown in Fig. 4a, providing evidences of the synthesis of Fe3O4 and magnetic dummy molecular imprinted polymer. The obvious absorption peak at 599 cm−1 belongs to the Fe–O stretching vibration. The bands at approximately 1398 and 912 cm−1 resulted from the –OH bending vibration of the carboxyl group, which demonstrated that there were numerous carboxyl groups on the surface of Fe3O4 synthesized by the solvothermal method. In spectra (b), the adsorptions observed around 1598 and 1505 cm−1 were the characteristic peaks of benzene ring and the sharp peak at 1229 cm−1 was attributed to the –OH connected to the benzene ring. In comparison with the spectrum of pure Fe3O4 and DDBP, the specific features of MDMIPs were the N–H bond at 1543 cm−1 and the Si–O–Si bond at 1065 cm−1 (Jiang et al. 2007), and characteristic peaks at 2930 cm−1 were attributed to C–H stretching. In addition, the obvious absorption peaks of benzene ring were not found in the MDMIP, and the main characteristic peaks of MDMIPs and MNIPs did not change significantly. These results implied that the imprinted layer was successfully bonded to the surface of Fe3O4 nanosphere by siloxane copolymerization of TEOS and APTES, and the DDBP was eluated completely from the polymer.
The magnetization curves of Fe3O4 and MDMIPs at room temperature are displayed in Fig. 4b. Superparamagnetism is an important property of MDMIPs, and the saturation magnetization is commonly used to measure the magnetic strength. As seen in Fig. 4b, the hysteresis loops showed negligible coercivity and remanence, which implied that both samples had superparamagnetic properties (Gao et al. 2014). The saturation magnetization values were approximately 59.04 and 47.60 emu g−1 for Fe3O4 and MDMIPs, respectively, which were much higher than that of the previous reported MMIP beads (Yuan et al. 2016; Guo et al. 2011; Li et al. 2011). The outstanding magnetic property makes it easy for MDMIPs to be separated from solution when used as dispersive adsorbents in SPE once the magnetic field was applied.
Evaluation of the Sorption Characteristics of MDMIPs
Kinetic Adsorption
The kinetic adsorption was characterized by monitoring the temporal concentration of BPA in the supernatant at different time intervals (Fig. 5a). It was shown that the adsorption capacities (Q) of BPA onto the MDMIPs increased rapidly within 10 min and then gradually increased. After 30 min, the adsorption had almost reached equilibrium. This result implied that the MDMIPs with specific recognition cavities on the surface had reduced mass transfer resistance, which led to easier diffusion for target molecule to access the recognition sites and make the MDMIPs very suitable as a SPE sorbent.
Adsorption Isotherms
The static adsorption capacities of the MDMIP and MNIP sorbents for BPA were determined using different concentrations that ranged from 0.1 to 2.25 mmol L−1. As seen in Fig. 5b, the adsorption capacities of MDMIPs increased quickly with increasing initial BPA concentration. The maximum BPA adsorption of MDMIPs was calculated to be 444.6 μmol g−1, which was 204.8 μmol g−1 higher than that of the corresponding MNIPs (239.8 μmol g−1). The results implied that the recognition sites on the outer surfaces of the MDMIPs had better chemical and steric matching with BPA. In contrast, the MNIPs had no imprinted sites, and the interaction with BPA was primarily through non-specific adsorption. In addition, it is worth noting that the adsorption capacities of the prepared MDMIPs were superior to values found in previous reports (Griffete et al. 2012; Zhu et al. 2010; Xu et al. 2012).
Optimization of the MDMIP-SPE Procedure
Amount of Sorbent
In the extraction process, MDMIP sorbent was dispersed in a water sample to rebind the target molecules. In order to get the efficient recovery under minimum amount of sorbent, different amounts of MDMIPs ranging from 20 to 100 mg were applied to extract BPA from 100 mL spiked water samples. The results showed that as the sorbent mass increased from 20 to 50 mg, the recovery of BPA continued to increase and 50 mg of sorbent enabled almost complete recovery of BPA (Fig. 6a). Further increasing the amount of MDMIPs gave no improvement on recovery of BPA. Therefore, the final amount of sorbent used in the SPE was fixed at 50 mg.
Effect of Sample pH
The effect of the sample pH was studied over the pH range from 4 to 9. The results showed that when the sample solution is close to neutrality (pH 6–7), the recovery reached the best value (Fig. 6b). This is probably because on one hand, the state of the BPA in the sample was affected by pH. Under mild conditions, most of the BPA existed in a molecular state, which is conducive to the adsorption by the MDMIP sorbent. On the other hand, hydrogen bonding which can promote the process of molecular recognition will be broken in strongly acidic or basic solutions due to the ionic state of BPA. Therefore, a sample pH of 6.0 was selected for subsequent experiments.
Elution Solvent
Because of its simple structure, high polarity, and easy volatilization, methanol was selected as the main elution agent and 3 mL of the methanol elution solvent containing acetic acid (0–10.0%, v/v) was evaluated with the assistance of sonication. It can be seen from Fig. 6c that the recovery constantly increased as acetic acid was added to the elution solvent from 0 to 10.0%, and it may be because the addition of acetic acid helped to disrupt the interactions between the polymers and template molecules. Therefore, 3 mL methanol containing 10% acetic acid was used as elution solvent in subsequent experiments.
Desorption Time
Magnetic solid-phase extraction mainly includes three processes: adsorption, separation, and elution. Total time is an important factor for measuring the efficiency of solid-phase extraction. For this purpose, the effect of different ultrasonic elution times on the recovery of BPA from 0.5 to 7 min was investigated. As can be seen from Fig.6d, the recovery with 30-s ultrasonic extraction time almost reached the maximum, and the recovery barely changed as the ultrasonication time further increased. Therefore, 30 s was chosen as the final ultrasonic elution time. Compared with the traditional solid-phase extraction, the elution time is significantly shorter.
Competitive Selectivity of MDMIP and MNIP Beads
To evaluate the competitive selectivity of MDMIP beads, aqueous solutions of BPA/TBBPA/PTBP at different molar ratios were prepared as extraction samples. As shown in Fig. 7a, the MDMIP beads only have selective retention for BPA, and even as the proportions of TBBPA and PTBA increased, the recoveries of PTBP and TBBPA were still lower than 58%, while the recoveries of BPA were 84.2–101.4%. Compared with Fig. 7b, BPA and its analogs were all poorly retained on the MNIP with low recovery from 18.5 to 55.4%. In addition, it is interesting to find that the non-specific bind of BPA is still the highest among three substances. The reason may be that there are two –OH groups in the BPA, while there is only one in the PTBP, which makes it easy for BPA to form hydrogen bonds with the amino group in the MNIP. Meanwhile, the structure of BPA is more simple without –Br groups when compared with TBBPA; this makes BPA to be easily adsorbed by MNIP without stereospecific blockade. Therefore, the experimental results showed that the prepared MDMIPs had good selectivity and an adequate ability of anti-interferences.
Enrichment Factor and Regeneration Studies of MDMIPs
Enrichment factor is an important factor in measuring the enrichment ability of MDMIP-SPE for the target analyte at very low concentration in large volumes. To determine the enrichment factor of MDMIPs, extraction samples with different volumes (100, 200, 300, and 400 mL) containing 10 nmol BPA were prepared. As shown in Fig. 8a, when the sample volume reached 400 mL and was redissolved in 1 mL methanol, the BPA recoveries still reached 89.3%, which indicated that the MDMIPs prepared in this experiment have potential to enrich and extract trace BPA from large volumes of water.
The reusability of MDMIP-SPE was often considered to have a great cost benefit for extending its practical applications. To this end, we evaluated the performance of the repeated use of MDMIP-SPE. As shown in Fig. 8b, the binding capacities of BPA retained a high recovery over 90% after 6 cycles. This demonstrated that the structure of materials has not been destroyed after several recycling. In this regard, MDMIPs have irreplaceable advantages when compared with the traditional disposable chromatography silica gel, which could save the costs for pre-treatment of samples.
Validation of Method and Application to BPA in Real Samples
Under optimized MDMIP-SPE conditions for BPA separation in water samples, the linearity of the total analytical method was estimated by analyzing BPA standard solution at various concentrations ranging from 11.40 to 4560 ng mL−1. The calibration graph was linear with a correlation coefficient >0.999. The regression equation was A = 20,601C + 58,764, where C is the concentration of BPA and A is the peak area. The LOD was 0.083 ng mL−1 (S/N = 3), and LOQ was 0.114 ng mL−1.
As a pre-condition, the dummy template of MDMIP should not interfere with the determination of targeted molecule. Our study verified that DDBP and BPA can be well separated by high-performance liquid chromatograph as displayed in Fig. 9a. In addition, to demonstrate the application of the method, real plastic bottled beverage samples, including Fanta water, pulsating drinks, green tea, mineral water, Coca Cola, and orange juice spiked with four different concentrations (0.114, 0.57, 1.14, and 30 ng mL−1) of BPA, were analyzed. As shown in Table 1, for all the six drinking samples, both in low concentration and high concentration, the obtained accuracy with recoveries of 88.6–99.5% and precision with RSD of 4.2–9.5% were satisfactory. Figure 9b, c shows the chromatograms of BPA in spiked plastic bottled beverage, which were obtained without SPE and with MDMIP-SPE, respectively. As can be seen, BPA in all the samples could not be detected by HPLC-UV without enrichment. After being enriched by MDMIP1-SPE 100 times, the concentration of BPA was high enough to be quantitatively analyzed. From Fig. 9a, b, we also found that there was no BPA detected in distilled water before and after SPE. So, distilled water did not affect the experimental results when it was used as solvent to prepare BPA standard solutions.
Table 2 compares the present method with other methods reported previously. It was clear that although the LOD in this work is higher than those of some methods (Muhammad et al. 2017; Lin et al. 2012; Yang et al. 2014), it is still lower than those of other approaches (Xu et al. 2014; Reyes-Gallardo et al. 2016; Li et al. 2016). Actually, the low LOD in Yang et al. (2014) is mainly attributed to the use of MS/MS method. In addition, when compared with Xu et al. (2014), Yang et al. (2014), Li et al. (2016), and Muhammad et al. (2017), the sorbent obtained in this work can be separated from solution with the help of external magnetic field and simplify the process of solid extraction in the meanwhile. Moreover, the BPA-imprinted sorbents used in Xu et al. (2014), Muhammad et al. (2017), Reyes-Gallardo et al. (2016), and Muhammad et al. (2017) all suffer from the leakage of residual BPA which is a crucial factor in the trace analysis. In addition, the procedure of obtained MDMIP was easy and time-saving, wrapping the imprinting layer directly on the magnetic core in the meanwhile. Therefore, this work provided a suitable approach to determine the trace BPA in the real samples.
Conclusion
In summary, we developed a simple and convenient method which used DDBP as dummy template to prepare MDMIPs. The materials obtained showed high superparamagnetism, large adsorption capacities, and fast binding kinetics for the recognition of BPA. When used as SPE sorbents, this reported MDMIP-SPE offers several advantages over conventional SPE: (i) simple and direct synthesis of the sorbent by one step on the exterior of Fe3O4 without further multi-step modification; (ii) avoidance of template molecule leakage during the extraction process, making the analysis results more accurate; (iii) avoidance of the need to pack the SPE column and the time-consuming process of loading large-volume samples which greatly simplifies the extraction process; (iv) it takes only 30 s to elute the template molecular in the solid-phase extraction process, which greatly increased the extraction efficiency; and (v) high selectivity, repeatability, and good recovery for the determination of BPA in drinking samples. However, the materials in this work only had selective absorption for BPA; there are still some shortcomings when compared with previous articles which can be used to adsorb a variety of phenolic compounds; at the same time, we will make some further improvement and optimization in the follow-up experiment. In summary, MDMIPs can be one of the most promising candidates for selective separation and fast enrichment of BPA from complicated plastic bottled beverage matrices.
References
Arabi M, Ghaedi M, Ostovan A, Tashkhourian J, Sadallahzadeh H (2016a) Synthesis and application of molecularly imprinted nanoparticles combined ultrasonic assisted for highly selective solid phase extraction trace amount of celecoxib from human plasma samples using design expert (DXB) software. Ultrason Sonochem 33:67–76
Arabi M, Ostovan A, Ghaedi M, Purkait M (2016b) Novel strategy for synthesis of magnetic dummy molecularly imprinted nanoparticles based on functionalized silica as an efficient sorbent for the determination of acrylamide in potato chips: optimization by experimental design methodology. Talanta 154:526–532
Arabi M, Ghaedi M, Ostovan A, Wang S (2016c) Synthesis of lab-in-a-pipette-tip extraction using hydrophilic nano-sized dummy molecularly imprinted polymer for purification and analysis of prednisolone. J Colloid Interface Sci 480:232–239
Arabi M, Ghaedi M, Ostovan A (2016d) Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem 210:78–84
Arabi M, Ghaedi M, Ostovan A (2017a) Water compatible molecularly imprinted nanoparticles as a restricted access material for extraction of hippuric acid, a biological indicator of toluene exposure, from human urine. Microchim Acta 184:879–887
Arabi M, Ghaedi M, Ostovan A (2017b) Synthesis and application of in-situ molecularly imprinted silica monolithic in pipette-tip solid-phase microextraction for the separation and determination of gallic acid in orange juice samples. J Chromatogr B 1048:102–110
Cacho IJ, Campillo N, Viñas P et al (2012) Stir bar sorptive extraction coupled to gas chromatography-mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables. J Chromatogr A 1247:146–153
Cai Y, Jiang G, Liu J, Zhou Q (2003) Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol A, 4n-nonylphenol, and 4tert-octylphenol. Anal Chem 75:2517–2521
Chang TT, Liu YX, Yan XY, Liu SM, Zheng HS (2016) One-pot synthesis of uniform and monodisperse superparamagnetic molecularly imprinted polymer nanospheres through a sol–gel process for selective recognition of bisphenol A in aqueous media. RSC Adv 6:66297–66306
Coensel ND, David F, Sandra P (2009) Study on the migration of bisphenol-A from baby bottles by stir bar sorptive extraction-thermal desorption-capillary GC-MS. J Sep Sci 32:3829–3836
Gao R, Mu X, Hao Y, Zhang L, Zhang J, Tang Y (2014) Combination of surface imprinting and immobilized template techniques for preparation of core–shell molecularly imprinted polymers based on directly amino-modified Fe3O4 nanoparticles for specific recognition of bovine hemoglobin. J Mater Chem B 2:1733–1741
Griffete N, Li H, Lamouri A, Redeuilh C, Chen K, Dong Z, Nowak S, Ammar S, Mangeney C (2012) Magnetic nanocrystals coated by molecularly imprinted polymers for the recognition of bisphenol A. J Mater Chem 22:1807–1811
Guo W, Hu W, Pan J, Zhou H, Guan W, Wang X, Dai J, Xu L (2011) Selective adsorption and separation of BPA from aqueous solution using novel molecularly imprinted polymers based on kaolinite/Fe3O4 composites. Chem Eng J 171:603–611
Hiroi H, Tsutsumi O, Momoeda M, Takai Y, Osuga Y, Taketani Y (1999) Differential interactions of biphenol A and 17β-estradiol α with estrogen receptor α (ERα) and ERβ. Endocr J 46:773–778
Hu Y, Li J, Hu Y, Li G (2010) Development of selective and chemically stable coating for stir bar sorptive extraction by molecularly imprinted technique. Talanta 82:464–470
Hu XL, Wu X, Yang FF, Wang Q, He CY, Liu SR (2016) Novel surface dummy molecularly imprinted silica as sorbent for solid-phase extraction of bisphenol A from water samples. Talanta 148:29–36
Ji Y, Yin J, Xu Z, Zhao C, Huang H, Zhang H, Wang C (2009) Preparation of magnetic molecularly imprinted polymer for rapid determination of bisphenol A in environmental water and milk samples. Anal Bioanal Chem 395:1125–1133
Jiang X, Tian W, Zhao C, Zhang H, Liu M (2007) A novel sol-gel-material prepared by a surface imprinting technique for the selective solid-phase extraction of bisphenol A. Talanta 2:119–125
Li Y, Dong C, Chu J, Qi J, Li X (2011) Surface molecular imprinting onto fluorescein-coated magnetic nanoparticles via reversible addition fragmentation chain transfer polymerization: a facile three-in-one system for recognition and separation of endocrine disrupting chemicals. Nano 3:280–287
Li J, Zhang X, Liu Y, Tong H, Xu Y, Liu S (2016) Preparation of a hollow porous molecularly imprinted polymer usingtetrabromobisphenol A as a dummy template and its application as SPE sorbent for determination of bisphenol A in tap water. Talanta 117:281–287
Lin Z, Cheng W, Li Y, Liu Z, Chen X, Huang C (2012) A novel superparamagnetic surface molecularly imprinted nanoparticle adopting dummy template: an efficient solid-phase extraction adsorbent for bisphenol A. Anal Chim Acta 720:71–76
Muhammad HR, Noorfatimah Y, Bahruddin S, Sazlinda K, Nor MH (2017) Rapid ultrasound assisted emulsification micro-solid phase extraction based on molecularly imprinted polymer for HPLC-DAD determination of bisphenol A in aqueous matrices. Talanta 171:242–249
Reyes-Gallardo EM, Lucena R, Cárdenas S, Valcárcel M (2016) Dispersive micro-solid phase extraction of bisphenol A from milk using magnetic nylon 6 composite and its final determination by HPLC-UV. Microchem J 124:751–756
Rodriguez-Mozaz S, López de Alda J, Barceló D (2004) Monitoring of estrogens, pesticides and bisphenol A in natural waters and drinking water treatment plants by solid-phase extraction–liquid chromatography–mass spectrometry. J Chromatogr A 1045:85–92
Tan F, Zhao HX, Li XN, Quan X, Chen JW, XiangXM ZX (2009) Preparation and evaluation of molecularly imprinted solid-phase microextraction fibers for selective extraction of bisphenol A in complex samples. J Chromatogr A 1216:5647–5654
Vandenberg N, Hauser R, Marcus M, Olea N, Welshons V (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177
Wei FD, Liu XP, Zhai MJ, Cai Z, Xu GH, Yang J, Du SH, Hu Q (2013) Molecularly imprinted nanosilica solid-phase extraction for bisphenol A in fish samples. Food Anal Methods 6:415–420
Xu Z, Ding L, Long Y, Xu L, Wang L, Xu C (2011) Preparation and evaluation of superparamagnetic surface molecularly imprinted polymer nanoparticles for selective extraction of bisphenol A in packed food. Anal Methods 3:1737–1744
Xu L, Pan J, Dai J, Li X, Hang H, Cao Z, Yan Y (2012) Preparation of thermal-responsive magnetic molecularly imprinted polymers for selective removal of antibiotics from aqueous solution. J Hazard Mater 233-234:48–56
Xu ZG, Yang ZL, Liu ZM (2014) Development of dual-templates molecularly imprinted stir bar sorptive extraction and its application for the analysis of environmental estrogens in water and plastic samples. J Chromatogr A 1358:52–59
Yang Y, Yu J, Yin J, Shao B, Zhang J (2014) Molecularly imprinted solid-phase extraction for selective extraction of bisphenol analogues in beverages and canned food. J Agric Food Chem 62:11130–11137
Yu D, Hu XL, Wei ST, Wang Q, He CY, Liu SR (2015) Dummy molecularly imprinted mesoporous silica prepared by hybrid imprinting method for solid-phase extraction of bisphenol A. J Chromatogr A 1396:17–24
Yuan YH, Liu Y, Teng WD, Tan JA, Liang Y, Tang YW (2016) Preparation of core-shell magnetic molecular imprinted polymer with binary monomer for the fast and selective extraction of bisphenol A from milk. J Chromatogr A 1462:2–7
Zhang H, Xue M, Lu Y, Dai Z, Wang H (2010) Microwave-assisted extraction for the simultaneous determination of Novolac glycidyl ethers, bisphenol A diglycidyl ether, and its derivatives in canned food using HPLC with fluorescence detection. J Sep Sci 33:235–243
Zhang XB, Tong HW, Yong GP, Liu SM, Guan YF (2013) An improved stober method towards uniform and monodisperse Fe3O4@C nanospheres. J Mater Chem A 1:7488–7493
Zhu R, Zhao W, Zhai M, Wei F, Cai Z, Sheng N, Hu Q (2010) Molecularly imprinted layer-coated silica nanoparticles for selective solid-phase extraction of bisphenol A from chemical cleansing and cosmetics samples. Ana Chem Acta 658:209–216
Acknowledgements
We gratefully acknowledge the Commonwealth Scientific Foundation for Industry of Chinese Inspection and Quarantine (No. 201210071) of the Ministry of National Science and Technology of China and China Tobacco Chuanyu Industrial Corporation (No. 2013-164).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that this manuscript corresponds to an original research article neither published before nor presently submitted for publication elsewhere.
Conflict of Interest
Tingting Chang declares that she has no conflict of interest. Xiangyang Yan declares that she has no conflict of interest. Shaomin Liu declares that he has no conflict of interest. Yuxin Liu declares that he has no conflict of interest.
Ethics Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Chang, T., Yan, X., Liu, S. et al. Magnetic Dummy Template Silica Sol–Gel Molecularly Imprinted Polymer Nanospheres as Magnetic Solid-Phase Extraction Material for the Selective and Sensitive Determination of Bisphenol A in Plastic Bottled Beverages. Food Anal. Methods 10, 3980–3990 (2017). https://doi.org/10.1007/s12161-017-0969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0969-0