Abstract
Lettuce pigments play important physiological functions, such as photosynthetic processes and light stress defense. Spectral indices are widely used for predicting vegetable pigments in a non-destructive way. This work was carried out to propose and validate mathematical models based on spectral indices, capable of predicting carotenoid and anthocyanin contents in different lettuce cultivars, grown under organic, hydroponic and alternative farming systems. Standardized Difference Vegetation Index, Simple Ratio Index, and Difference Spectral Index were modified and combined with linear, exponential, power, and logarithm regressions during the modeling process. The most accurate models presented d and r values greater than 0.7 and 0.6 for the external validation, respectively. When estimating carotenoid and anthocyanin contents, the root-mean-square error did not exceed 0.14 and 0.41 mg kg−1, respectively. The maximum mean bias error values were −0.07 mg kg−1 for carotenoids and 0.08 mg kg−1for the anthocyanins. Models based on the Difference Spectral Index with exponential fitting performed better, but linear fittings combined to the other spectral indices also confirmed the feasibility of using the proposed models for estimating lettuce pigment contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lettuce (Lactuca sativa L.) is one of the most grown and consumed salad vegetables in the world, with production of 24,896,115.89 t in 2013 (Faostat 2013) and average daily consumption of 22.5 g/person in Europe (Pérez-López et al. 2015). Lettuce is a rich font of vitamins (A, B1, B2, C, E), bioactive composites, and minerals as iron and calcium. Additionally, it contains high amount of water in its tissues, as well as reduced lipid and energetic contents (Nicolle et al. 2004).
Carotenoids and anthocyanins are important pigments found in lettuce cultivars, which perform essential physiological functions in plants such as the photosynthetic processes and light stress defense (Gross 1991). Carotenoids are pigments that protect the chlorophyll molecule from photooxidation under excessive light (Rodriguez-Amaya 2001), also having antioxidant action and presenting provitamin A activity (Rodriguez-Amaya et al. 2008). Anthocyanins are pigments produced by the plant secondary metabolism, playing an important role in the human healthy due to their antioxidant activity (Chon et al. 2012).
Chemical analysis of food is increasingly sophisticated, searching for more accurate quantifications, reduced labor time, and greater safety in the laboratory activities. In this sense, spectrometry has shown itself an efficient technique for this purpose (Richardson et al. 2002). The spectral analysis is a non-destructive way to extract a large amount of information about a sample (Cozzolino et al. 2011). Based on the plant or food spectral signature, it is possible to quantify pigments (Gitelson et al. 2006; Schlemmer et al. 2013; Dobrota et al. 2015), water content (Féret et al. 2011; Rallo et al. 2014), nutrient content (Li et al. 2014; Nigon et al. 2015), quality parameters (Stagakis et al. 2012; Jha et al. 2014), and presence of diseases and injuries (Cao et al. 2013; Mahlein et al. 2013), among other important agricultural product properties. Specifically for lettuces, the visible and near infrared (VIS/NIR) spectroscopy has been proven effective for estimating storage period (Jacobs et al. 2016), classifying different cultivars (Moura et al. 2016), estimating nitrogen content (Mao et al. 2015), and determining changes occurred as function of nutrient deficiencies (Pacumbaba and Beyl 2011). Steidle Neto et al. (2016) developed partial least squares models to predict the lettuce chlorophyll, carotenoid, and anthocyanin contents based on the full leaf spectrum.
The spectral indices are a less complex alternative to estimate plant pigments and are widely used for extracting information about biological products by combining two or more wavelengths which are representative of the studied food or plant property (Féret et al. 2011). Among several spectral indices, the Standardized Difference Vegetation Index (SDVI), Simple Ratio Index (SRI), and Difference Spectral Index (DSI) are the most used for predictions of pigment content (Sims and Gamon 2002; Garriga et al. 2014; Dobrota et al. 2015), nutrient concentration (Nigon et al. 2015; Schlemmer et al. 2013), and water content (Rallo et al. 2014).
Despite the several studies about plant pigment predictions at leaf and canopy levels based on spectral indices, estimation of leaf carotenoid and anthocyanin contents from reflectance is pointed as a challenge (Fassnacht et al. 2015; Jacobs et al. 2016). According to Sims and Gamon (2002), this probably occurs because chlorophyll has absorption peaks (around 550 nm) overlapping with the ones of carotenoid and anthocyanin. Further, the higher concentration of chlorophyll than carotenoid in most leaves can affect the predictions. Merzlyak et al. (2003) also affirmed that leaf carotenoid and anthocyanin analysis by spectrometry is complicated due the occurrence of several xanthophylls whose pools undergo disproportionate changes during leaf ontogeny and upon adaptation of leaves to variable light conditions. But, depending on the studied food or plant property, further improvement in the spectral indices based models is possible by combining different wavelengths or by varying the equation fittings.
Specifically for the lettuces of distinct cultivars and grown under different farming systems, this may contribute for improving the pigment content predictions, increasing the accuracies of the models and allowing in-field non-destructive measurements. This work was carried out to propose and validate mathematical models, based on spectral indices, capable of predicting carotenoid and anthocyanin contents in different lettuce cultivars, grown under organic, hydroponic, and alternative farming systems.
Methodology
Lettuce Growing
The lettuce cultivars Crystal (green crinkled leaves), Regina 2000 (plain green leaves), and Mimosa (slightly red crinkled leaves) were cultivated under hydroponic, organic, and alternative conditions in Capim Branco city, Minas Gerais State, Brazil (19° 32′ S, 44° 07′ W; 757 m). Growing different lettuce cultivars in different farming systems was important to obtain representative datasets for calibrating and validating the proposed models, accurately estimating the pigment contents and allowing the use of this approach to a wide range of situations.
A Nutrient Film Technique (NFT) hydroponic system was installed in a greenhouse covered with a new low-density polyethylene (150 μm). An aluminized mesh (50% light interference) was also stretched under the cover and partially blocked the solar radiation input. Seeds were planted in phenolic foam boards and seedlings were transplanted to growing channels after 15 days. The electrical conductivity of the nutrient solution was 1.5 dS m−1. It was composed by water, monoammonium phosphate, potassium nitrate, calcium nitrate, monopotassium phosphate, iron, and the micronutrients manganese, zinc, and boron. Plants were watered every 15 min to prevent their drying.
The organic system was mounted under a low-density polyethylene (150 μm) cover with 2 years use time. Seeds were planted in polypropylene trays containing vegetable humus and cattle manure as substrate. Seedlings were transplanted to field after 45 days. Soil was mixed with organic compound from cattle manure and vegetable biomass. Fertilizing based on syrup containing lime sulfur (1%) was applied 10 and 20 days after transplanting. Irrigation schedule and other growing practices followed the crop requirements.
The alternative system was installed without cover. Chemical pesticides were not applied to the plants, but synthetic fertilizers were used. Seeds were directly planted in the field, which was fertilized with organic compound from cattle manure and vegetable biomass, and with mineral granulated fertilizer NPK 4-14-8. The irrigation schedule and crop practices followed the crop requirements.
The experimental parcels were disposed in casualized blocks with three repetitions. Each block corresponded to a farming system and was composed by six cultivation lines containing each one five plants. Thus, there were 30 plants in each block (10 of each cultivar). The number of plants cultivated was greater than the required for the laboratory measurements, since it was considered the selection of the healthy, vigorous and well-formed lettuces. For each farming system, nine plants of each cultivar were harvested and transported in thermal boxes to the Agricultural Engineering Laboratory, located at the Federal University of São João del-Rei, Campus Sete Lagoas (19° 48′ S, 44° 17′ W; 732 m), where were cleaned.
Spectral Reflectance Measurements
At the laboratory, spectrometric data acquisition was performed on three leaves of each lettuce, which were randomly selected. Three separate measurements on adaxial surface were made in each leaf, avoiding its central vein and boundaries, resulting in a total of 81 spectra.
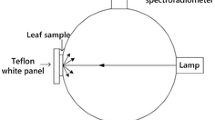
A portable spectrometer (JAZ-EL350, Ocean Optics, Dunedin, Florida, USA) was used for obtaining the spectral signatures of each lettuce leaf. This equipment was coupled to a computer by USB cable and to an integrating sphere with a built-in tungsten-halogen light source (ISP-REF, Ocean Optics, Dunedin, Florida, USA) by a 300-μm optical fiber. Since lettuce leaves are thick, an opaque black surface was used aiming at avoiding reflection contributions of light after its transmission through the leaf. The leaf was located between the black surface and the integrating sphere during the reflectance measurements. The spectrometer was preconfigured to acquire and store reflectance data in Vis/NIR wavelength range (500–1023 nm), with a spectral resolution of 0.36 nm. Data were collected by the SpectraSuite software (Ocean Optics, Dunedin, Florida, USA). The lettuce spectral reflectance was expressed as a relative percentage of standard minimum and maximum reflectances, as described by Steidle Neto et al. (2016) and Xing and Baerdemaeker (2005):
where \( {R}_{\lambda}^{\mathrm{cal}} \) is the calibrated spectral reflectance from the leaf (%), \( {R}_{\lambda}^{\mathrm{leaf}} \) is the direct spectral reflectance from the leaf (dimensionless), \( {R}_{\lambda}^{\mathrm{dark}} \) is the minimal spectral reflectance (dimensionless), and \( {R}_{\lambda}^{\mathrm{ref}} \) is the maximum spectral reflectance (dimensionless).
The minimal spectral reflectance was obtained in the absence of light, when sample port aperture of the integrating sphere was obstructed and the light source was turned off. The maximum spectral reflectance referred to the value measured in a diffuse reflectance standard (WS-1-SL, Ocean Optics, Dunedin, Florida, USA) with Spectralon™, after the warmed up of the light source (≈10 min) was waited.
Determination of Pigment Contents by Laboratory Techniques
Total carotenoid content was measured by macerating 5 g of each fresh leaf in a mortar with acetone. This homogenate was filtered under vacuum through a Büchner funnel, progressively adding acetone until no more color can be extracted. The filtered extract was separated by adding 100% pure petroleum ether and distilled water. The extract absorbance was taken at 450 nm in a spectrophotometer (700S, FEMTO, São Paulo, SP, Brazil) calibrated with acetone. The total carotenoid content was calculated by equation proposed by Rodriguez-Amaya (2001) and presented by Carvalho et al. (2012):
where Car is the total carotenoid content (mg kg−1), A450 is the sample absorbance at 450 nm, E is the absorption coefficient of β-carotene in petroleum ether (2592), V is the total volume of extract (mL), and W is the sample fresh weight (g).
Anthocyanins were estimated by pH-differential method, as proposed by Giusti and Wrolstad (2001) and Deylami et al. (2016). These pigments were expressed as cyanidin-3-glucoside equivalents in weight. Extracts were obtained by adding acidified methanol (0.1% HCL) to 1 mL of each sample. Before centrifugation, extracts were separately diluted in potassium chloride buffer (pH 1.0) and sodium acetate buffer (pH 4.5). Absorbances were measured simultaneously at 510 and 700 nm in the same spectrophotometer used for quantifying the carotenoid contents, 30 min after dilution of extracts. The anthocyanin content was calculated as:
where Ant is the anthocyanin content (mg kg−1), A510 and A700 are the sample absorbances at 510 and 700 nm, M is the cyanidin molecular weight (449.2 g mol−1), K is the cyanidin molar extinction coefficient (26,900 L cm−1 mol−1), and F is the dilution factor (26.67).
Spectral Indices and Mathematical Models
Regression models correlating different spectral indices with pigment measured in laboratory were evaluated with the purpose of estimating lettuce carotenoid and anthocyanin. For this, reflectance data of the 81 lettuce samples were divided into calibration and external validation sets, both of them containing representative data from lettuce samples. The calibration set was composed by 70% of the data (54 spectra and pigment values) and was used for developing the models. From the 54 spectra, there were 18 of each farming system and for each farming system there were six spectra of each cultivar. The external validation set corresponded to 30% of the data (27 spectra and pigment values) and was performed for assessing the model predictive performances from independent samples. From the 27 spectra, there were nine of each farming system and for each farming system, there were three spectra of each cultivar. This sampling plan followed the suggested by Kramer (1998), who affirmed that the number of samples in the calibration set should be more than 10 times the variable components in the experiment. In this study, the farming system and the cultivars were considered as variable components in the experiment, representing independent sources of significant variation in the data.
During the calibration, Standardized Difference Vegetation Index (SDVI—Eq. (4)), Simple Ratio Index (SRI—Eq. (5)) and Difference Spectral Index (DSI—Eq. (6)) were modified considering different combinations of two different wavelengths, and combined with linear, exponential, power and logarithm regressions for each response variable. During this process, the spectral index was the independent variable and the pigment content was the dependent one.
where R is the spectral reflectance (%), λ1 and λ2 are different wavelengths (nm).
Regressions were performed in the SPECTRA software, which was written in C++ programming language, specifically for this study. During the software execution, the spectral reflectance data were grouped two by two with an interval of 10 nm, totalizing 42,480 models (3540 wavelength combinations × 4 regression types × 3 spectral indices) for each pigment. The SPECTRA output showed all equations with their respective coefficients of determination.
Three models with greater coefficients of determination for each spectral index and regression type were selected for performing the external validation process, totalizing 36 models for each pigment. The external validation was done on an electronic spreadsheet, where each model was applied to the independent dataset and statistical indicators were calculated to evaluate the calibration and validation results. These statistical indicators were the mean bias error (BIAS—Eq. (7)), root-mean-square error (RMSE—Eq. (8)), and Willmott’s index of agreement (d—Eq. (9)). The standard error of laboratory (SEL—Eq. (10)) and the standard error of prediction (SEP—Eq. (11)) were also calculated for evaluating the predictive capacities of the proposed models.
where y r. is the pigment content measured in laboratory (mg kg−1), y p is the pigment predicted by the model (mg kg−1), n is the number of samples (dimensionless), \( \overline{y_{\mathrm{r}}} \) is the average pigment content measured in laboratory (mg kg−1), y 1 and y 2 are duplicate pigment contents measured in laboratory (mg kg−1).
For each spectral index, the models indicated for predicting the lettuce carotenoid and anthocyanin contents were those with the higher coefficients of determination in the calibration process, higher index of agreement in the external validation, as well as lower BIAS and RMSE in the external validation. The BIAS represents the difference between the mean of observations and the mean of estimates, with values close to zero indicating low systematic error between the measured and predicted values. The lower the RMSE is, the more precise the model is and less scattered the dataset is. Both BIAS and RMSE are related with the unit in which the evaluated property is expressed, as well as with the dataset range of values. On the other hand, the Willmott’s index of agreement is dimensionless and bounded by 0 and 1, with greater values indicating more accurate predictions. SEL is related to the data reproducibility and is an indirect indicator of the calibration model suitability. SEP is generally used to assess the calibration accuracy, indicating how well the calibrated equation will perform in other analysis. Ideally, SEP and SEL should be similar for calibration, indicating that variability of the prediction is close to that of the conventional method.
Results and Discussion
Figure 1 presents the average spectral signatures of the studied lettuce cultivars under the different farming systems. It was found that lettuce reflectances exhibited similar patterns, agreeing with those obtained by Xue and Yang (2009), Li et al. (2006), and Jacobs et al. (2016), when evaluating the storage period and nutritional composition of lettuces by spectrometry.
A slight variation was observed in the visible wavelengths when comparing the average spectra of lettuces grown under hydroponic, organic, and alternative farming systems. In general, average lettuce reflectances were smaller than 20% from 500 to 690 nm, with a peak at about 550 nn (green region). The average lettuce reflectances increase to about 40% in the near-infrared range (700–1000 nm), probably due to their internal cellular structure. According to Knipling (1970), little of the radiation in the near-infrared wavelengths is absorbed internally by leaves, but about 40 to 60% of it is scattered upward through the surface of incidence and is designated reflected radiation.
Table 1 shows the most efficient models for predicting lettuce pigments based on spectral indices and their respective coefficients of determination for calibration. Among the tested regressions, the exponential and linear fittings presented the greater regression coefficients, which varied from 0.40 to 0.60 and can be considered as moderate. This means that there are other factors, which were not discussed in this study, but influence the pigment contents in lettuces. Despite this, the proposed models represented well the relationship between the spectral indices and the pigment contents for the calibration dataset, as shown by SEL, SEP, and RMSE. These statistical indicators were low and very similar for the three selected models for each evaluated pigment, confirming their predictive abilities. The SEL, SEP, and RMSE values revealed a greater variability in the anthocyanin measurements and predictions during the calibration process. However, this did not affect the performance of the models.
The more sensitive wavelengths for lettuce carotenoid predictions were 500, 567, 577, and 698 nm, which are close to results reported previously for other food and plants (Gitelson et al. 2006; Merzlyak et al. 2003; Zarco-Tejada et al. 2013). Reflectances at 510, 529, 539, and 548 nm were the most useful for lettuce anthocyanin predictions, also agreeing with previous studies (Merzlyak et al. 2008; Gitelson et al. 2006).
The wavelengths verified as appropriate for predicting the lettuce carotenoid and anthocyanin contents are also presented in Fig. 2 with the spectral signatures used during the calibration and validation of the models. As reported by Moura et al. (2016), from 500 to 650 nm reflectances of the red lettuces cultivated under the alternative system were always smaller than the green ones, as well as than the green and red lettuces cultivated under hydroponic and organic systems. These differences probably occurred due to the different farming system cover types, which resulted in different radiation intensities during the cultivations and affected the lettuce pigment synthesis. Marin et al. (2015) verified that radiation can be correlated to the content of phenolic acids and flavonoids, influencing in the color of red lettuce cultivars.
More wavelengths near 550 nm were associated with the anthocyanin contents, probably because the absorption maximum in vivo of this pigment is near 550 nm, inhibiting the carotenoid contribution (Merzlyak et al. 2003). Further, the red lettuces presented higher anthocyanin contents, which can be associated to the decrease of the reflectance peak around 550 nm and with its flattening.
Table 2 presents the most efficient models for predicting lettuce pigments based on spectral indices and their respective validation statistical indicators. All selected models presented good and very similar accuracies.
The spectral index R 500 − R698 exhibited thebest validation results when estimating lettuce carotenoids. Gitelson et al. (2006) indicated spectral indices based on wavelengths around 510 as most sensitive to the carotenoids in higher plant leaves. In addition, these authors presented the near infrared range as useful for adjusting the variability in leaf structure and thickness, as well as removing the contribution of chlorophyll absorption from reflectance around 550 nm. Merzlyak et al. (2003) also reported that the spectral indices based on 520 and 700 nm can be used as an accurate measure of carotenoid content in food.
When estimating lettuce anthocyanins, the spectral index R 529 − R 548 presented best validation results. Gitelson et al. (2001) also related close linear correlations between spectral index based on 550 nm and anthocyanin contents for different tree leaves. The 500–550 nm waveband is also reported by Landi et al. (2015) as sensitive for leaf anthocyanins.
External validation presented larger r 2 values for both studied pigments, but similar RMSE values. The coefficients of determination from 0.42 to 0.56 for carotenoids and from 0.86 to 0.98 for anthocyanins indicate that 42–56% of changes in the lettuce carotenoid contents were explained by the proposed spectral indices during the external validation, while 86–96% of changes in the lettuce anthocyanins contents were explained by the proposed spectral indices. The RMSE expresses the mathematical model precision, supplying information about the data scatter. The values of this statistical indicator varied from 0.73 to 0.74 mg kg−1 in a dataset with chlorophyll contents between 5 and 8.5 mg kg−1. Thus, a scattering of around 21% of the observed chlorophyll range was stated for the pigment prediction. These results agree with the reported by Xiaobo et al. (2011), who verified RMSE values corresponding to 17, 18, and 20% of the observed range of cucumber leaf chlorophyll when applying the SRI, SDVI, and DSI spectral indices, respectively. The RMSE values for carotenoid contents were 0.13 and 0.14 mg kg−1, and the observed values of this pigment ranged from 0.9 to 1.8 mg kg−1. Therefore, a scattering of around 15% was verified when predicting the lettuce carotenoid contents, which is very similar to that found by Yi et al. (2014) who observed a scattering of 14% of the observed carotenoid range for cotton canopy when using the SDVI spectral index. When evaluating the lettuce anthocyanin predictions, RMSE values were between 0.34 and 0.41 mg kg−1, corresponding to scatterings from 11 to 14% of this pigment range (1–40 mg kg−1). Steele et al. (2009) predicted the anthocyanin contents of grapevine leaves by using spectral indices and found RMSE values corresponding to 19% of the observed pigment range.
The BIAS represents the systematic error among the predicted and measured values, with results close to zero indicating more accurate models, negative values representing underfitting, and positive values corresponding to overfitting. For all selected models, BIAS were close to zero, indicating low systematic errors during the validation process. For both lettuce chlorophyll and carotenoid predictions, the BIAS indicated a slight underfitting, while for anthocyanins, the models slightly overestimated the real values. The greater BIAS of chlorophyll models are justified by the greater values observed for this pigment when compared with the carotenoid and anthocyanin ones.
The Willmott’s index of agreement (d) also represents the model accuracy, varying from 0 to 100% with the maximum percentage reflecting a perfect agreement between observed and predicted data. This is an important statistical indicator since it is a measure of the degree to which the predictions are error free. Further, it is a standardized measure in which cross-comparisons for a variety of models, regardless of units, can be made.
Based on the presented results, the proposed models were considered suitable for non-destructive and in-field lettuce carotenoid and anthocyanin measurements. The proposed spectral indices and equation fittings were capable of well represent the main spectral features of these pigments in green and red lettuces grown under different farming systems.
Conclusions
Results obtained in this study suggest the applicability of using spectral indices for non-destructive analysis of carotenoid and anthocyanins in different lettuce cultivars grown under organic, hydroponic, and alternative farming systems. Since a reduced number of samples were used during the analyses, this can be considered a feasibility study. Models based on the modified DSI with exponential fitting performed better, but linear fittings combined to the other spectral indices also confirmed the possibility of using the proposed models for estimating lettuce pigment contents.
References
Cao X, Luo Y, Zhou Y, Duan X, Cheng D (2013) Detection of powdery mildew in two winter wheat cultivars using canopy hyperspectral reflectance. Crop Prot 45:124–131
Carvalho LMJ, Gomes PB, Godoy RLO, Pacheco S, Monte PHF, Carvalho JLV, Nutti MR, Neves ACL, Vieira ACRA, Ramos SRR (2012) Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): a preliminary study. Food Res Int 47(2):337–340
Chon S, Boo H, Heo B, Gorinstein S (2012) Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int J Food Sci Nutr 63(1):45–48
Cozzolino D, Cynkar WU, Shah N, Smith P (2011) Multivariate data analysis applied to spectroscopy: potential application to juice and fruit quality. Food Res Int 44:1888–1896
Deylami MZ, Rahman RA, Tan CP, Bakar J, Olusegun L (2016) Effect of blanching on enzyme activity, color change, anthocyanin stability and extractability of mangosteen pericarp: a kinetic study. J Food Eng 178:12–19
Dobrota C, Lazar L, Baciu C (2015) Assessment of physiological state of Betula pendula and Carpinus betulus through leaf reflectance measurements. Flora 216:26–34
Faostat (2013). Food and agriculture organization of the United Nations: Statistics Division. Retrieved March 16, 2016 from: http://faostat3.fao.org/browse/Q/QC/E. Accessed 16 March 2016
Fassnacht FE, Stenzel S, Gitelson AA (2015) Non-destructive estimation of foliar carotenoid content of tree species using merged vegetation indices. J Plant Physiol 176:210–217
Féret JB, François C, Gitelson AA, Barry KM, Panigada C, Richardson AD, Jacquemoud S (2011) Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens Environ 115:2742–2750
Garriga M, Retamales JB, Romero-Bravo S, Caligari PDS, Lobos GA (2014) Chlorophyll, anthocyanin, and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J Integr Plant Biol 56(5):505–515
Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol 74(1):38–45
Gitelson AA, Keydan GP, Merzlyak MN (2006) Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys Res Lett 33:1–5
Giusti MM, Wrolstad RE (2001) Anthocyanins: characterization and measurement with UV-visible spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. Wiley, New York
Gross J (1991) Pigments in vegetables: chlorophylls and carotenoids. Van Nostrand Reinhold, New York
Jacobs BAJG, Verlinden BE, Bobelyn E, Decombel A, Bleyaert P, Van Lommel J, Vandevelde I, Saeys W, Nicolai BM (2016) Estimation of the prior storage period of lambs’s lettuce based on visible/near infrared reflectance spectroscopy. Postharvest Biol Technol 113:95–105
Jha SN, Narsaiah K, Jaiswal P, Bhardwaj R, Gupta M, Kumar R, Sharma R (2014) Nondestructive prediction of maturity of mango using near infrared spectroscopy. J Food Eng 124:152–157
Knipling EB (1970) Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens Environ 1:155–159
Kramer R (1998) Chemometric techniques for quantitative analysis. Marcel Dekker Inc., CRC Press, New York
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17
Li B, Liew OW, Asundo AK (2006) Pre-visual detection of iron and phosphorus deficiency by transformed reflectance spectra. Journal of Photochemistry Photobiology 85:131–139
Li F, Mistele B, Hu Y, Chen X, Schmidhalter U (2014) Reflectance estimation of canopy nitrogen content in winter wheat using optimized hyperspectral spectral índices and partial least squares regression. Eur J Agron 52:198–209
Mahlein AK, Rumpf T, Welke P, Dehne HW, Plümer L, Steiner U, Oerke EC (2013) Development of spectral indices for detecting and identifying plant diseases. Remote Sens Environ 128:21–30
Mao H, Gao H, Zhang X, Kumi F (2015) Nondestructive measurement of total nitrogen in lettuce by integrating spectroscopy and computer vision. Sci Hortic 184:1–7
Marin A, Ferreres F, Barberá GG, Gil MI (2015) Weather variability influences color and phenolic content of pigmented baby leaf lettuces throughout the season. J Agric Food Chemistry 63(6):1673–1681
Merzlyak MN, Gitelson AA, Chivkunova OB, Solovchenko AE, Pogosyan SI (2003) Application of reflectance spectroscopy for analysis of higher plant pigments. Russ J Plant Physiol 50(5):704–710
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59(14):3903–3911
Moura LO, Lopes DC, Steidle Neto AJ, Ferraz LCL, Carlos LA, Martins LM (2016) Evaluation of techniques for automatic classification of lettuce based on spectral reflectance. Food Anal Methods 9:1799–1806
Nicolle C, Cardinault N, Gueu E, Jaffrelo L, Rock E, Mazur A, Amouroux P, Rémésy C (2004) Health effect of vegetable-based diet: lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin Nutr 23:605–614
Nigon TJ, Mulla DJ, Rosen CJ, Cohen Y, Alchanatis V, Knight J, Rud R (2015) Hyperspectral aerial imagery for detecting nitrogen stress in two potato cultivars. Comput Electron Agric 112:36–46
Pacumbaba RO Jr, Beyl CA (2011) Changes in hyperspectral reflectance signatures of lettuce leaves in response to macronutrients deficiencies. Adv Space Res 48:32–42
Pérez-López U, Miranda-Apodaca J, Lacuesta M, Mena-Petite A, Muñoz-Rueda A (2015) Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Science Horticulturae 195:56–66
Rallo G, Minacapilli M, Ciraolo G, Provenzano G (2014) Detecting crop water status in mature olive groves using vegetation spectral measurements. Biosyst Eng 128:52–68
Richardson AD, Duigan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Rodriguez-Amaya, D.B. (2001). A guide to carotenoid analysis in foods 71p Campinas: UNICAMP.
Rodriguez-Amaya DB, Kimura M, Amaya-Farfan J (2008) Fontes brasileiras de carotenóides: tabela brasileira de composição de carotenóides em alimentos. 100p. MMA/SBF, Brasília
Schlemmer M, Gitelson A, Schepers J, Ferguson R, Peng Y (2013) Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. International Journal of Applied Earth Observation 25:47–54
Sims DA, Gamon JA (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens Environ 81:337–354
Stagakis S, González-Dugo V, Cid P, Guillén-Climent ML, Zarco-Tejada PJ (2012) Monitoring water stress and fruit quality in an orange orchard under regulated deficit irrigation using narrow-band structural and physiological remote sensing índices. ISPRS J Photogramm Remote Sens 71:47–61
Steele MR, Gitelson AA, Rundquist DC, Merzlyak MN (2009) Nondestructive estimation of anthocyanin content in grapevine leaves. Am J Enol Vitic 60(1):87–92
Steidle Neto AJ, Moura LO, Lopes DC, Carlos LA, Martins LM, Ferraz LCL (2016) Non-destructive prediction of pigment content in lettuce based on visible-NIR spectroscopy. J Sci Food Agric. doi:10.1002/jsfa.8002
Xiaobo Z, Jiyong S, Limin H, Jiewen Z, Hanpin M, Zhenwei C, Yanxiao L, Holmes M (2011) In vivo noninvasive detection of chlorophyll distribution in cucumber (Cucumis sativus) leaves by indices based on hyperspectral imaging. Anal Chim Acta 706:105–112
Xing J, Baerdemaeker JD (2005) Bruise detection on ‘Jonagold’ apples using hyperspectral imaging. Postharvest Biol Technol 37:152–162
Xue L, Yang L (2009) Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. Journal of Photogrammetry and Remote Sensing 64:97–106
Yi Q, Jiapaer G, Chen J, Bao A, Wang F (2014) Different units of measurement of carotenoids estimation in cotton using hyperspectral indices and partial least square regression. ISPRS J Photogramm Remote Sens 91:72–84
Zarco-Tejada PJ, Gullén-Climent ML, Hernández-Clemente R, Catalina A, González MR, Martin P (2013) Estimating leaf carotenoid content in vineyards using high resolution hyperspectral imagery acquired from an unmanned aerial vehicle (UAV). Agric For Meteorol 171-172:281–294
Acknowledgments
The authors acknowledge the Federal University of São João del-Rei, the Agroecology and Organic Production Technological Vocational Center (Guayi—CNPq research project 487727/2013-4), the CAPES foundation, the organic farm Vista Alegre, the hydroponic farm Recanto da Lua, and the alternative farm Represa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—grant number 487727/2013-4).
Conflict of Interest
Lorena de Oliveira Moura declares that she has no conflict of interest.
Daniela de Carvalho Lopes declares that she has no conflict of interest.
Antonio José Steidle Neto declares that he has no conflict of interest.
Leila de Castro Louback Ferraz declares that she has no conflict of interest.
Lanamar de Almeida Carlos declares that she has no conflict of interest.
Luma Moreira Martins declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Lopes, D.d., Moura, L.d., Steidle Neto, A.J. et al. Spectral Indices for Non-destructive Determination of Lettuce Pigments. Food Anal. Methods 10, 2807–2814 (2017). https://doi.org/10.1007/s12161-017-0853-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0853-y