Abstract
The quantitative analysis of organosulfur compounds is important for the quality control of various garlic products along with studying their molecular functionality and nutraceutical properties. In this study, a liquid chromatography-tandem mass spectrometry-selected reaction monitoring (LC-MS/MS-SRM) method with electrospray ionization detection was developed and validated for the rapid, simultaneous quantification of four representative organosulfur compounds in garlic: alliin, S-allyl-L-cysteine, γ-glutamyl-S-allyl-L-cysteine, and allicin. Stable SRM transitions were achieved for these compounds under optimized conditions, and the linear range extended from 1 to 2000 ng/mL. The limits of detection and quantification ranged from 0.003 to 0.058 ng/mL and from 0.01 to 0.19 ng/mL, respectively. Excellent recovery and reproducibility at different spiking levels were achieved. The method was successfully applied to the simultaneous quantification of organosulfur compounds in fresh garlic samples. This highly selective and sensitive LC-MS/MS-SRM method is expected to be a useful tool for studying molecular functionality and the quality control of garlic products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Garlic (Allium sativum L.), a well-known vegetable, has been used as not only a flavor but also a medical herb and a source of nutritional supplements worldwide. Garlic has been reported to exhibit extensive biological activities (Butt et al. 2009; Santhosha et al. 2013; Suleria et al. 2015), including antioxidant (Capasso 2013), antifatigue (Morihara et al. 2007), anticancer (Nicastro et al. 2015), and antimicrobial (Lanzotti et al. 2014) activities. Garlic may also have a positive role in immunonutrition (Nantz et al. 2012) and help prevent cardiovascular diseases (Castro et al. 2010; Rahman 2007a). The main bioactive components in garlic are organosulfur compounds (Yun et al. 2014). Allicin, which is responsible for garlic’s strong odor, is the most powerful medicinal compound derived from garlic. Allicin is converted from its precursor alliin by the enzyme alliinase when tissue damage occurs, and it readily breaks down into a series of sulfur-containing metabolites (Block 1985). S-allyl-L-cysteine (SAC) is another major bioactive compound in garlic, which is converted from γ-glutamyl-S-allyl-L-cysteine (GSAC) by γ-glutamyl transpeptidase (Ichikawa et al. 2006a). Due to its relative stability (Kodera et al. 2002), SAC has been used as a compliance marker in clinical studies involving garlic consumption (Amagase et al. 2001). Evidence has also suggested that SAC can be converted to alliin (Hughes et al. 2005; Jones et al. 2007). Therefore, alliin, SAC, GSAC, and allicin represent the organosulfur compounds of garlic and can be used to monitor the quality of garlic and its products along with their potential biological activities.
In addition to raw garlic, various garlic supplements are available in the market, including garlic powders, garlic oils, garlic oil macerates, aged garlic extracts, and black garlic. The content of organosulfur compounds in garlic and garlic products is usually low and varies with the methods used to process and store the product (Beato et al. 2012; Gorinstein et al. 2009; Ichikawa et al. 2006a; Liang et al. 2015). Moreover, in the era of molecular nutrition, quantitative analysis of active component is indispensable for the in-depth study of their functionalities. Therefore, the development of a highly sensitive and selective method for quantifying representative organosulfur compounds is important for the quality control and molecular functionality study of garlic products.
Currently, methods based on high-performance liquid chromatography (HPLC) are most commonly used for the quantification of organosulfur compounds in garlic (Arnault et al. 2003; Beato et al. 2012; Ichikawa et al. 2006a; Ichikawa et al. 2006b). However, HPLC often shows relatively low resolving power, and the quantification of organosulfur compounds using HPLC methods is complicated because of interference from other compounds, particularly for the analysis of garlic products with complex matrices. Although gas chromatography–mass spectrometry (GC-MS) has been employed to analyze garlic sulfur compounds directly or after derivatization (Kubec et al. 1999), the degradation of compounds such as allicin during GC limit this method’s applicability (Mondy et al. 2001). Moreover, our early attempts to develop a quantification assay for these organosulfur compounds revealed that alliin, SAC, and GSAC cannot be volatilized under normal GC conditions without sample derivatization.
When considering the specificity, sensitivity, and stability of samples during analysis, liquid chromatography-tandem mass spectrometry-selected reaction monitoring (LC-MS/MS-SRM), which has been used to quantify trace-level analytes in complex matrices, shows promise for the analysis of organosulfur compounds in garlic (Diana Di Mavungu et al. 2009; Gianazza et al. 2014; Guo et al. 2012; Kitteringham et al. 2009). Among the organosulfur compounds of garlic, only SAC has been analyzed with LC-MS/MS-SRM to date (Lee et al. 2015; Lee et al. 2014); the LC-MS/MS-SRM analyses of alliin, GSAC, and allicin, and most importantly, the simultaneous analysis of all four of these garlic compounds have not yet been reported.
In this study, a rapid, sensitive, and specific LC-MS/MS-SRM method was developed for the simultaneous quantification of alliin, SAC, GSAC, and allicin in garlic products. The method was validated with respect to the specificity, linearity, limits of detection and quantification, and accuracy. As a proof of concept, two fresh garlic samples were analyzed using the new method. This method is expected to be used in the quality control of garlic products during their production as well as in studies on the metabolism and bioavailability of garlic. It will also be useful to study the molecular functionality of garlic products.
Material and Methods
Chemicals and Reagents
The reference standards of L-(+)-alliin (CAS No. 556-27-4) and allicin (CAS No. 539-86-6) were purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). SAC (CAS No. 21593-77-1) and GSAC (CAS No. 91216-95-4) were purchased from Sigma-Aldrich (Munich, Germany) and Pharmacopeial Convention, Inc. (Rockville, MD, USA), respectively. The purities of all compounds were greater than 98 %. LCMS-grade acetonitrile was obtained from Wako (Tokyo, Japan), and water was supplied by a Milli-Q Ultrapure water system (Millipore Corporation, Bedford, Mass., USA). A 0.01 M aqueous solution of hydrochloric acid (HCl) was used for sample preparation.
Sample Preparation
Mixtures of the four standards in serial concentrations were prepared in a 0.01 M HCl solution. Two fresh garlic samples were obtained from the local supermarket (Lumiere, Fukuoka, Japan): sample no. 1 was cultivated in Aomori Prefecture, Japan, and sample no. 2 was cultivated in Shandong Province, China. The fresh garlic cloves (10–15 g) were crushed using a Millser IFM-620DG (Iwatani, Osaka, Japan). The crushed garlic was then extracted with 30 mL of 0.01 M HCl solution at 40 °C for 25 min with the assistance of a sonicator (550 W, Elmasonic S 100H, New Jersey, USA). After centrifugation at 3500 rpm for 5 min, the supernatant (extract 1) was collected, and the pellet was again extracted with 15 mL of 0.01 M HCl for 5 min. The supernatant (extract 2) was collected and combined with extract 1. The supernatant was diluted to 50 mL with the HCl solution and centrifuged again at 3500 rpm for 5 min. The final supernatant was collected and used for the LC-MS/MS analysis. All the standards and samples were filtered through 0.2-μm membrane filters before analysis and kept at 4 °C before and during analysis.
LC-MS/MS Analysis
LC-MS/MS analyses were performed using an Agilent 1260 Infinity LC system coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The separation was conducted on a SUPELCO Discovery HS F5 column (3 μm, 3 × 150 mm; Sigma-Aldrich, Germany) using a binary mobile phase composed of A (water supplemented with 0.1 % formic acid) and B (acetonitrile). The mobile phase program was as follows: 0–15 min, B 0–100 %; 15–25 min, B 100 %. The flow rate was 0.4 mL/min, and the column oven was maintained at 30 °C. The injection volume was 5 μL.
The mass spectrometer was operated using an Agilent Jet Stream electrospray ionization (ESI) source in positive ion mode. The optimal MS parameters were as follows: capillary voltage, 3.5 kV; nebulizer gas pressure, 50 psi; dry gas temperature, 280 °C; dry gas flow rate, 10 L/min; sheath gas temperature, 350 °C; and sheath gas flow rate, 12 L/min. Quantitative analysis was performed in SRM mode.
Results and Discussion
Optimization of Analysis Conditions
Optimal separation is a critical factor in the simultaneous detection of multiple targets in complex matrices. Alliin, SAC, GSAC, and allicin have different polarities. The high polarity (XLogP3 = −3.5, PubChem) of alliin usually complicates the retention by common reverse-phase LC columns. To appropriately separate these four organosulfur compounds and obtain the relatively long retention time necessary to distinguish alliin from the void volume peak, several reverse-phase LC columns have been tested (data not shown). An octadecyl column (3.0 × 150 mm, 1.8 μm) and a pentafluorophenylpropyl (PFPP) column (3.0 × 150 mm, 3 μm) were found to be able to achieve the abovementioned two goals. The latter column exhibited a longer retention time for alliin (3.53 vs. 3.33 min) and was thus chosen for this study. PFPP modified silica columns usually have longer retention for polarity compounds compared to octadecyl columns, which might relate to the strong Lewis acid property of PFPP (Marin and Barbas 2006).
The SRM transitions for these compounds were optimized by single-MS full-scan mode followed by product ion scan mode using the standard compounds. ESI in positive ion mode was found to have the best performance for the mobile phase conditions investigated. Agilent Jet Stream thermal gradient focusing technology can enhance the sensitivity of ESI-MS by improving the desolvation and spatial focusing of ions (Mordehai and Fjeldsted 2009). Herein, an Agilent Jet Stream electrospray ionization (ESI) source was applied to ensure the sensitivity of detection for these four garlic compounds. The precursor ions were [M+H]+. The predominant stable fragment from the precursor ion in MS/MS was selected as the product ion. The precursor–product ion pairs (quantification transitions) for the compounds were found to be m/z 178.1-88.1 (alliin), m/z 162.1-73.1 (SAC), m/z 291.1-145.0 (GSAC), and m/z 163.0-41.2 (allicin; Fig. 1). The corresponding fragmentation patterns for these four compounds are also illustrated in Fig. 1. The optimal collision energies and fragmentor voltages for the quantification transitions of alliin, SAC, GSAC, and allicin were 4 and 70 eV, 12 and 70 eV, 12 and 100 eV, and 16 and 50 eV, respectively. The transitions m/z 178.1 > m/z 42.0 (alliin), m/z 162.1 > m/z 41.3 (SAC), m/z 291.1 > m/z 73.0 (GSAC) and m/z 163.0 > m/z 73.0 (allicin) were used for qualification. The corresponding collision energies for the qualification transitions were 10, 20, 20, and 20 eV, respectively. The intensity ratios of the qualification ion to the quantification ion were 34.3% (alliin), 34.2% (SAC), 37.5% (GSAC), and 24.3% (allicin), which were also satisfactorily reproduced between the standards and testing samples (data not shown). Those optimized MS/MS parameters were automatically obtained by Agilent MassHunter Optimizer software.
The stability of garlic organosulfur compounds is always an important issue for their quantification. In particular, allicin is extremely unstable due to the presence of a thiol group (Fujisawa et al. 2008; Monosulphide and Monosulphide 2001; Prati et al. 2014), which is rapidly metabolized into diallyl sulfide, diallyl disulfide, ajoene, etc. (Rahman 2007b). Low pH has been found to inhibit the activity of alliinase and slow the decomposition of alliin and allicin (Lawson and Hughes 1992; Monosulphide and Monosulphide 2001). Here, we used HCl aqueous solution (0.01 M, pH 2.0) as the solvent to prepare the samples (Ichikawa et al. 2006b), and the samples were kept at 4 °C. All four studied compounds were stable under these conditions for at least 72 h (data not shown).
Method Validation
To evaluate the potential for the practical application of the proposed method, five critical parameters were studied: linearity, limit of detection (LOD), limit of quantification (LOQ), apparent recovery, and repeatability. The linearity was determined using 8–12 levels of mixed standard solution (1–5000 ng/mL). A good SRM chromatographic resolution was achieved (Fig. 2a). The calibration curves for all analytes were linear over a wide concentration range with correlation coefficients (r) greater than 0.99 (Fig. 2b and Table 1). The injected 0.1 ng/mL of alliin, SAC, GSAC, and allicin generated signal-to-noise ratios (S/N) of 35, 64.9, 106.8, and 5.2, respectively. The LOD and LOQ were determined as the concentrations with S/N = 3 and S/N = 10, respectively. The LODs for these compounds ranged from 0.003 to 0.058 ng/mL, while the LOQs ranged from 0.01 to 0.19 ng/mL (Table 1).
The apparent recovery and repeatability were evaluated at five spiked levels (Table 1). Different amounts of these four compounds were spiked with the sample extraction solution. The apparent recoveries were determined in duplicate to sextuplicate basing on the calibration curves. The apparent recoveries for alliin, SAC, GSAC, and allicin ranged from 95.7 to 103.1 %, 101.3 to 108.7 %, 93.7 to 109.1 %, and 97.6 to 110.8 %, respectively. The relative standard deviation (RSD) for these compounds at different spiked levels ranged from 0.14 to 6.9 %, which suggests that the repeatability of the method is satisfactory. Furthermore, the retention times were conservative in the intra- and inter-day tests (Table 1). These results indicate that the proposed method is satisfactory in terms of linearity, sensitivity, and precision.
Method Application
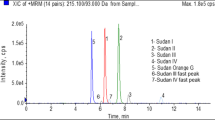
To demonstrate the applicability of the LC-MS-SRM method to real garlic samples, the method was applied to two fresh garlic samples, No.1 and No.2, which were cultivated in Japan and China, respectively. The TICC (total ion current chromatogram) and SRM chromatograms obtained from 100- to 500-fold diluted fresh garlic extracts are shown in Fig. 3. Although the garlic matrix is complex, clear SRM peaks were observed for alliin, SAC, GSAC, and allicin at the expected retention times (Fig. 3c, d).
The method was successfully applied to quantify the representative organosulfur compounds in the fresh garlic samples (Table 2). The extracts of the samples were diluted 10-fold and 500-fold for the quantitation of alliin and other three compounds, respectively. Alliin was detected at a very low concentration (0.14 and 0.19 μg/g fresh weigh for No.1 and No.2, respectively), whereas high concentrations of allicin were detected (2322.9 and 2601.5 μg/g fresh weigh in No.1 and No.2, respectively), suggesting that most of the alliin in the samples was enzymatically converted to allicin. The concentrations of all four compounds were slightly higher in sample No.2 than in sample No.1. The variation in the concentrations of these compounds between the two garlic samples might be related to the differences among the cultivars, planting area, and maturity. The contents of alliin, GSAC, and allicin in fresh garlic were previously determined by HPLC to be in the range of 5400–14500 μg/g, 1900–8200 μg/g, and 2500–5000 μg/g, respectively (Iberl et al. 1990; Saito, 2008), while the content of SAC in raw garlic was reported as 22.73 μg/g (Bae et al. 2012). Herein, the contents of SAC, GSAC, and allicin were close to the reported ranges determined by HPLC, whereas the concentration of alliin was not in agreement with the HPLC result. In other two previous studies with HPLC quantification, the content of alliin was also found to be high in the dry garlic (Yoo et al. 2014; Montano et al. 2011). The relatively high alliin content previously reported may be because alliin was determined before being converted to allicin. Another possible explanation is that quantification was affected by the peak overlap caused by the solvent or another component. Our proposed LC-MS/MS-SRM method eliminates the effects of overlapping peaks.
When compared to usual HPLC quantification methods, our proposed LC-MS/MS-SRM method has significant advantages on specificity and sensitivity. Because in the LC-MS/MS-SRM method, the quantification was based on a more sensitive ion signal and the qualification was based on the retention time plus highly selective MS/MS ion pairs. When compared to the existing LC-MS/MS-SRM method for SAC (Lee et al. 2015; Lee et al. 2014), the advantages of our method is the optimized condition not only for quantifying SAC but also for quantifying other three representative garlic compounds simultaneously. These advantages give the method great potential to be used for the quality control of various garlic products that might have trace-level of organosulfur compounds or contain complex matrices.
Conclusion
An LC-MS/MS-SRM method for the rapid, simultaneous quantification of alliin, SAC, GSAC, and allicin in garlic products was developed. The method showed good linearity over a wide concentration range with very low LOD and LQD. Excellent recovery and reproducibility at different spiking levels were achieved. In a proof-of-concept study, this method was successfully applied to the simultaneous detection of four organosulfur compounds in fresh garlic. This highly selective and sensitive LC-MS/MS-SRM method for the simultaneous quantification of alliin, SAC, GSAC, and allicin will be a useful tool for the study of molecular functionality and for the reliable quality control of garlic products.
References
Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Intake of garlic and its bioactive components. J Nutr 131:955s–962s
Arnault I, Christides JP, Mandon N, Haffner T, Kahane R, Auger J (2003) High-performance ion-pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J Chromatogr A 991:69–75
Bae SE, Cho SY, Won YD, Lee SH, Park HJ (2012) A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC. LWT-Food Science and Technology 46:532–535
Beato VM, Sanchez AH, de Castro A, Montano A (2012) Effect of processing and storage time on the contents of organosulfur compounds in pickled blanched garlic. J Agric Food Chem 60:3485–3491. doi:10.1021/jf3002075
Block E (1985) The chemistry of garlic and onions. SciAm 252:114
Butt MS, Sultan MT, Butt MS, Iqbal J (2009) Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr 49:538–551. doi:10.1080/10408390802145344
Capasso A (2013) Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 18:690–700. doi:10.3390/molecules18010690
Castro C, Lorenzo AG, Gonzalez A, Cruzado M (2010) Garlic components inhibit angiotensin II-induced cell-cycle progression and migration: Involvement of cell-cycle inhibitor p27(Kip1) and mitogen-activated protein kinase. Mol Nutr Food Res 54:781–787. doi:10.1002/mnfr.200900108
Diana Di Mavungu J et al (2009) LC-MS/MS multi-analyte method for mycotoxin determination in food supplements food additives and contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 26:885–895. doi:10.1080/02652030902774649
Fujisawa H, Suma K, Origuchi K, Kumagai H, Seki T, Ariga T (2008) Biological and chemical stability of garlic-derived allicin. J Agric Food Chem 56:4229–4235
Gianazza E, Tremoli E, Banfi C (2014) The selected reaction monitoring/multiple reaction monitoring-based mass spectrometry approach for the accurate quantitation of proteins: clinical applications in the cardiovascular diseases. Expert Rev Proteomics 11:771–788. doi:10.1586/14789450.2014.947966
Gorinstein S et al (2009) Comparative control of the bioactivity of some frequently consumed vegetables subjected to different processing conditions. Food Control 20:407–413
Guo B, Chen B, Liu AM, Zhu WT, Yao SZ (2012) Liquid chromatography-mass spectrometric multiple reaction monitoring-based strategies for expanding targeted profiling towards quantitative metabolomics. Curr Drug Metab 13:1226–1243
Hughes J, Tregova A, Tomsett AB, Jones MG, Cosstick R, Collin HA (2005) Synthesis of the flavour precursor, alliin, in garlic tissue cultures. Phytochemistry 66:187–194. doi:10.1016/j.phytochem.2004.11.009
Iberl B, Winkler G, Müller B, Knobloch K (1990) Quantitative determination of allicin and alliin from garlic by HPLC. Planta Med 56:320–326
Ichikawa M, Ide N, Ono K (2006a) Changes in organosulfur compounds in garlic cloves during storage. J Agric Food Chem 54:4849–4854. doi:10.1021/jf060083o
Ichikawa M, Ide N, Yoshida J, Yamaguchi H, Ono K (2006b) Determination of seven organosulfur compounds in garlic by high-performance liquid chromatography. J Agric Food Chem 54:1535–1540. doi:10.1021/jf051742k
Jones M et al (2007) The biochemical and physiological genesis of alliin in garlic. Medicinal and Aromatic Plant Science and Biotechnology 1:21–24
Kitteringham NR, Jenkins RE, Lane CS, Elliott VL, Park BK (2009) Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J Chromatogr B 877:1229–1239. doi:10.1016/j.jchromb.2008.11.013
Kodera Y et al (2002) Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J Agric Food Chem 50:622–632. doi:10.1021/jf0106648
Kubec R, Svobodova M, Velisek J (1999) Gas chromatographic determination of S-alk(en)ylcysteine sulfoxides. J Chromatogr A 862:85–94
Lanzotti V, Scala F, Bonanomi G (2014) Compounds from allium species with cytotoxic and antimicrobial activity. Phytochem Rev 13:769–791. doi:10.1007/s11101-014-9366-0
Lawson LD, Hughes BG (1992) Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med 58:345–350
Lee S, Yoo M, Kim S, Shin D (2014) Identification and quantification of S-allyl-l-cysteine in heated garlic juice by HPLC with ultraviolet and mass spectrometry detection. LWT Food Sci Technol 57:516–521. doi:10.1016/j.lwt.2014.02.002
Lee S, Chang NI, Yoo M, Choi JH, Shin D (2015) Development and validation of S-allyl-L-cysteine in rat plasma using a mixed-mode reversed-phase and cation-exchange LC-ESI-MS/MS method: application to pharmacokinetic studies. J Chromatogr Sci 53:54–59. doi:10.1093/chromsci/bmu013
Liang T, Wei F, Lu Y, Kodani Y, Nakada M, Miyakawa T, Tanokura M (2015) Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J Agric Food Chem 63:683–691. doi:10.1021/jf504836d
Marin A, Barbas C (2006) Systematic comparison of different functionality columns for a classical pharmaceutical problem. J Pharm Biomed Anal 40:262–270
Mondy N, Naudin A, Christides J, Mandon N, Auger J (2001) Comparison of GC-MS and HPLC for the analysis of allium volatiles. Chromatographia 53:S356–S360
Monosulphide D, Monosulphide DT (2001) Stability of allicin in garlic—a kinetic study. Indian journal of chemical technology 8:195–199
Montano A, Beato VM, Mansilla F, Orgaz F (2011) Effect of genetic characteristics and environmental factors on organosulfur compounds in garlic (Allium sativum L.) grown in Andalusia, Spain. J Agric Food Chem 59(4):1301–1307
Mordehai A, Fjeldsted J (2009) Agilent jet stream thermal gradient focusing technology agilent technologies technical note., publication number 5990 3494
Morihara N, Nishihama T, Ushijima M, Ide N, Takeda H, Hayama M (2007) Garlic as an anti-fatigue agent. Mol Nutr Food Res 51:1329–1334. doi:10.1002/mnfr.200700062
Nantz MIP, Rowe CA, Muller CE, Creasy RA, Stanilka JM, Percival SS (2012) Supplementation with aged garlic extract improves both NK and gamma delta-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr 31:337–344. doi:10.1016/j.clnu.2011.11.019
Nicastro HL, Ross SA, Milner JA (2015) Garlic and onions: their cancer prevention properties. Cancer Prev Res 8:181–189. doi:10.1158/1940-6207.capr-14-0172
Prati P, Henrique CM, Souza AS, Silva VSN, Pacheco MTB (2014) Evaluation of allicin stability in processed garlic of different cultivars. Food Science and Technology (Campinas) 34:623–628
Rahman K (2007a) Effects of garlic on platelet biochemistry and physiology. Mol Nutr Food Res 51:1335–1344. doi:10.1002/mnfr.200700058
Rahman MS (2007b) Allicin and other functional active components in garlic: health benefits and bioavailability international. Journal of Food Properties 10:245–268
Saito Y (2008) Garlic science: chemistry of garlic. Asakura Publishing Co, Ltd., p 96
Santhosha SG, Jamuna P, Prabhavathi SN (2013) Bioactive components of garlic and their physiological role in health maintenance: a review. Food Bioscience 3:59–74. doi:10.1016/j.fbio.2013.07.001
Suleria HAR, Butt MS, Khalid N, Sultan S, Raza A, Aleem M, Abbas M (2015) Garlic (Allium sativum): diet based therapy of 21st century—a review. Asian Pacific Journal of Tropical Disease 5:271–278. doi:10.1016/S2222-1808(14)60782-9
Yoo M, Lee S, Kim S, Hwang JB, Choe J, Shin D (2014) Composition of organosulfur compounds from cool-and warm-type garlic (Allium sativum L.) in Korea. Food Sci Biotechnol 23(2):337–344
Yun HM, Ban JO, Park KR, Lee CK, Jeong HS, Han SB, Hong JT (2014) Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol Ther 142:183–195. doi:10.1016/j.pharmthera.2013.12.005
Acknowledgments
We thank Temahimado Co., Ltd. (Kagoshima, Japan) for providing the standard compounds. We thank the Center for Advanced Instrumental and Educational Supports (Faculty of Agriculture, Kyushu University) for providing the LCMS-IT-TOF instrument for exploring the ionization conditions in the initial stage of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Qinchang Zhu declares that he has no conflict of interest. Kenichi Kakino declares that he has no conflict of interest. Chika Nogami declares that he has no conflict of interest. Koichiro Ohnuki declares that he has no conflict of interest. Kuniyoshi Shimizu declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Zhu, Q., Kakino, K., Nogami, C. et al. An LC-MS/MS-SRM Method for Simultaneous Quantification of Four Representative Organosulfur Compounds in Garlic Products. Food Anal. Methods 9, 3378–3384 (2016). https://doi.org/10.1007/s12161-016-0535-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0535-1