Abstract
Background
Herpes simplex virus type 2 (HSV-2) genital lesion recurrence is modulated by psychological factors, but no such link with viral shedding (and thus asymptomatic transmission) has been observed in humans.
Purpose
The moderating effects of average psychological distress, emotional stability, and emotion regulation on HSV-2 recurrence were tested.
Methods
Nineteen HSV-2 seropositive women were followed over 22 weeks. Daily measures of HSV-2 recurrence and psychological distress were collected. HSV-2 lesions and viral shedding were modeled as linear oscillator systems, with psychological distress moderating the periodicity of each process.
Results
High levels of distress, more labile moods, and less ability to regulate emotional states were associated with fewer days elapsed between the onset of lesion episodes. Viral shedding showed the same pattern.
Conclusions
Results are consistent with research indicating that psychological distress suppresses immune system functioning, and provide new evidence that genital HSV-2 viral shedding is related to, and regulated by, psychological distress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpes simplex virus type 2 (HSV-2) genital infections are very common in North America. As many as 25 % of women and 12.5 % of men are currently infected with HSV-2 [1], although only about 20 % of these individuals are aware of their infection [2]. Rates have remained stable over the last decade [2], yet concern about rising rates remains due to the ease with which the virus is spread. The correlation between latent recurrence and lesion presence is not perfect; thus, HSV-2 can be spread in the presence or absence of lesions, or even by individuals who are unaware they are infected. HSV-2 infections will remain a significant health concern until an effective vaccine is developed, and will remain a health concern thereafter for those who are already seropositive.
Herpes recurrences can be painful both physically and psychologically. Most symptomatic individuals experience recurrent infections within the first year of primary infection [3], and the median HSV-2 recurrence rate is estimated to be four to six times per year [4]. Recurrences are associated with painful itching and/or tingling/burning sensations, fever, headache, malaise, vaginal/urethral discharge, tender lymph nodes, and, in extreme cases, necrosis (in immunosuppressed patients). HSV-2 infections are also associated with increased risk for contracting human immunodeficiency virus (HIV) [5, 6]. Having herpes carries tremendous stigma, and sufferers may experience significant depression, anxiety, guilt, and shame related to their infection; increased risk for illicit drug use; altered sexual interest and behavior; and difficulty forging and maintaining relationships [7, 8].
Despite the wide spectrum of clinical disease and virologic severity among otherwise healthy persons, little is known about the mechanisms of HSV-2 disease variability. A growing body of psychoneuroimmunological research suggests that HSV-2 recurrence is influenced by inter- and intraindividual differences in personality characteristics and psychological distress. A recent meta-analysis [9] suggests a small but robust relationship between psychosocial stress (e.g., psychological distress, personality types or coping styles, stressful life events) and cutaneous HSV-1 or HSV-2 recurrence. This relationship was strongest for psychological distress, and for oral herpes compared with genital herpes [9]. This comprehensive meta-analysis and more recent empirical studies [10] suggest that HSV infections should, to some extent, be considered a psychosomatic illness. However, despite evidence for HSV recurrence malleability associated with psychological factors (e.g., [9, 10]), the relation between psychosocial stress and illness severity is not fully understood [10]. A clearer understanding of this link would have implications for the treatment and control of HSV-2 infection.
Viral shedding is an important component of recurrent herpetic disease. Experiments using animal models have consistently demonstrated a role of stress in reactivating latent HSV infection (e.g., [11–13]), yet no such relationship has been observed in humans. Some human research, however, has observed an association between psychosocial stress and other indicators of latent HSV-1 and HSV-2 reactivation, such as decreased HSV-specific T-cell response [14, 15] and increased HSV antibody levels [15, 16]. Considering evidence that genital herpes lesions are associated with more frequent HSV-2 viral shedding [17], and that research has demonstrated a relation between psychosocial stress and cutaneous HSV infection, it is surprising that similar effects in terms of HSV-2 viral shedding have not been observed.
HSV-2 lesion recurrence and psychosocial stress are related, and perhaps mutually influential (e.g., [9, 10]), but the effect sizes observed to date may be somewhat attenuated, or (as may be the case with viral shedding) may not be observed at all. Two bases for the supposition that the HSV recurrence–psychological distress link is stronger than is generally observed in research are noted. First, many research designs have had an insufficient number of measurement occasions, and permit too much time to elapse between measurement occasions, to observe the relation between psychosocial stress and HSV recurrence. Full specification of this relationship between psychosocial stress and HSV-2 recurrence requires objective measures of disease recurrence collected with sufficient duration and frequency to capture periodicity, complex system dynamics, and bi-directional relationships. Few of the prospective studies in a recent comprehensive meta-analysis [9] collected daily distress ratings [18–20]; two of the three studies which did [18, 19; and later 10] demonstrated robust temporal effects of psychological distress on herpes recurrence above and beyond prodromal symptoms. The data used in the present study were collected daily, and should yield enough sensitivity to detect even subtle associations.
The second basis for this claim is that time series require innovative analytic strategies that capture the wealth of information contained in intensively repeated data. HSV-2 reactivation is a dynamic, cyclic process [21], and the use of traditional linear regression or fixed effects models may misinterpret meaningful, dynamic variation instead as measurement error. If that is indeed the case, psychological distress may have a greater influence on HSV-2 reactivation—both lesion outbreaks and viral load—than has been previously demonstrated in research. Recent developments in quantitative methods may help to overcome this problem.
Using contemporary models of dynamic change, the present study analyzes intensively repeated data of psychological distress, viral shedding, and lesion occurrence collected from 19 HSV-2 seropositive women. Genital HSV-2 recurrence was modeled as an oscillatory process using latent differential equation models [22], and the extent to which individual differences in psychological distress influence the periodicity of recurrent herpetic disease was examined. We predicted that higher levels of distress, emotion dysregulation, and emotional instability would be associated with faster cycling of HSV-2 genital lesions and viral shedding.
Methods
Sample
The sample consisted of 19 healthy HSV-2 seropositive women enrolled in a randomized, double-blind, placebo-controlled crossover trial of acyclovir as a treatment for HSV-2 infection. The women were recruited from a prior sample of 26 women who participated in a study of subclinical HSV-2 shedding [23]. Briefly, in the original study women were recruited through newspaper advertisements and information distributed to local health care providers. Women were excluded if they were immunocompromised, seropositive for HSV-1, or planning pregnancy [23]. Approximately 2 years elapsed between the initial study and the present follow-up study [10]. The median age of women was 28.8 years (range = 23.9–52.9 years), and the median time since self-reported primary infection was 3.1 years (range = 1.9–4.5 years).
Procedure
The crossover trial consisted of two study arms, each lasting approximately 10 weeks and separated by a 2-week washout period (Medianplacebo = 70 days, Medianacyclovir = 71 days). Women were randomly assigned to receive either acyclovir 400 mg twice daily (the standard suppressive dose) during the first study arm followed by placebo during the second study arm, or placebo followed by acyclovir. Every 2 weeks, women returned to the University of Washington Virology Research Clinic in Seattle for clinic visits with a physician.
At study enrollment, women were trained how to swab their genital area to obtain cervicovaginal, vulvar, and perianal HSV-2 secretion samples for laboratory processing via polymerase chain reaction (PCR). Except during the washout period, women swabbed their genital area daily, and placed swabs in separate vials containing 1 mL PCR digestive buffer. Women refrigerated biospecimens after collection, and returned the specimens to the clinic during their bi-weekly visits. Women also maintained a daily diary in which they reported the presence of genital herpes lesions.
Measures
Psychological Distress
Except during the washout period, women reported daily levels of anxiety, depression, and stress using single-item visual analog scales. Scales are 10 cm long and have anchors of “No [psychological distress construct]” and “Most intense [psychological distress construct] imaginable.” A line is drawn along the scale at the point that best represents the level of each construct over the course of the entire day. Visual analog scales generally correlate highly with other measures of anxiety and depression [24–26], demonstrate strong face validity, and are easily completed on a daily basis [10]. Similar measures have been used previously in HSV research [10, 18, 20, 27].
A woman’s mean score across all days within a study arm was used as an index of her trait level of anxiety/depression, or her average stress appraisal (higher mean values represent higher trait level distress). The frequency of oscillation around a woman’s trait level distress (discussed below) within each study arm was used as a proxy for emotion regulation (higher frequencies represent better ability to maintain a baseline condition). Finally, emotional reactivity was operationalized as the magnitude of a woman’s fluctuations in distress, and was calculated using the standard deviation of a woman’s psychological distress scores across all days within a study arm (higher standard deviations represent greater emotional reactivity). All moderators were standardized between women prior to model fitting.Footnote 1 Correlations among moderator variables can be found in Table 1.
HSV-2 Recurrence
HSV-2 recurrence was measured in two ways: (a) the presence of at least one genital lesion, determined from diary entries and/or clinician inspection during bi-weekly clinic visits; and (b) the presence of viral shedding, determined by detection of HSV-2 on swabs of the genital mucosa by DNA PCR procedures (a binary variable was created to indicate shedding versus no shedding).
Statistical Analyses
Statistical Software
To test the hypotheses that psychological distress is related to HSV-2 symptom recurrence, a series of structural equation models was fit to the data using OpenMx v. 1.3.2 [28]. OpenMx is run inside the R statistical programming environment, and all analyses were conducted using R v. 2.15.2 [29]. Models were fit using full information maximum likelihood (FIML) estimation procedures, which are highly efficient and are carried out on raw data observations rather than a covariance matrix, and assume observations to be missing at random. Models fit using FIML thus have the advantage of allowing individuals with any data present to be included in analyses, and produce unbiased estimates and smaller standard errors when compared with other methods of handling missingness under MAR or MCAR assumptions [30, 31].
Dynamical Systems Analysis
Time series data are not independent samplings of behavior. Rather, the present state of a behavior is dependent upon its previous states. Dynamical systems analysis (DSA) models time dependent data observations as linear oscillator systems [32–34]:
where x is the zero-order derivative or displacement from equilibrium at time i, \( \dot{x} \) is the first derivative or change in displacement, \( \ddot{x} \) is the second derivative or rate of change in displacement, e is the measurement error, η is the stiffness parameter of the oscillating system (related to the frequency of the oscillation), and ζ is the damping parameter of the system (related to changes in the amplitude of oscillation) [35]. A system oscillates when η < 0 and the amplitude of its oscillations dampens when ζ < 0, amplifies when ζ > 0, or remains constant when ζ = 0 [34]. The period, or length of oscillation (i.e., the inverse of the frequency), is then calculated as
The dynamics of a process may change as a function of the level of another variable [34, 35]. In a moderated system, an exogenous variable affects the process of self-regulation exhibited by a system. This is represented mathematically as
where z j is the moderator (which may be continuous or discrete) for person j. The term (η 0 + η 1 z) represents how the frequency of a process changes as a function of the level of moderator z. Similarly, the term (ζ 0 + ζ 1 z) indicates how the damping of a process varies as a function of the moderator. At high levels of moderator z, slower frequency of oscillation and slower return to equilibrium is observed. At low levels of z, the process exhibits faster frequency and faster return to equilibrium.Footnote 2
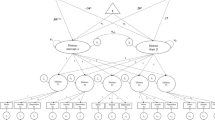
The present study used structural equation modeling (SEM) to estimate the frequency and damping parameters in Eq. 3. Data were first time-delay embedded [36] then fit by moderated latent differential equation (LDE) models [34, 35]. A path diagram of a moderated LDE model is presented in Fig. 1.
Moderated latent differential equation (LDE) model. An LDE model is a special case of a latent growth curve model. In these models, the latent variables (denoted in the diagram by circles) represent the x (intercept), \( \dot{x} \) (slope), and \( \ddot{x} \) (curvature) variables in Eqs. 1 and 3; the manifest variables (denoted as squares in the diagram) are successive observations of a process and are indicators of the underlying latent growth curve factors. The single-headed arrows at the latent level (labeled η 0,1 and ζ 0,1) are the regression coefficients from Eq. 3
Dynamical systems analysis offers an important advantage over traditional linear regression models: distinguishing random measurement error from dynamic, meaningful variation. In a dynamic system, fluctuations around an equilibrium point are a valid characteristic of the system, and DSA is capable of modeling these fluctuations. Meaningful variation in a dynamic or cyclic process cannot be captured by traditional linear regression and is instead assumed to be random measurement error [33]. Therefore, DSA is more sensitive to dynamic relationships between two variables than traditional statistical methods for time series analysis. DSA is also not as sensitive as linear regression to power issues arising from small sample sizes. Like other person-centered or person-specific approaches to data analysis [37], DSA derives its power from the number of measurement occasions, rather than the number of persons—as few as just one individual can be used to estimate a process.
Current Approach
To account for the random assignment of conditions and the possibility that treatment with acyclovir affected the dynamic process of HSV-2 recurrence, the time series data were divided into two subsets: placebo (no treatment) and acyclovir (treatment) arms of the clinical trial. Multiple-group SEM analyses were conducted using these subsets of data.
Due to computational limitations associated with the use of binary outcome data in structural equation models, lesion recurrence (0 = no lesion; 1 = lesion) and viral shedding (0 = no shedding; 1 = shedding) were treated as continuous variables. To account for the resulting skew (approximately 15 % of days in the placebo arm and approximately 5 % in the treatment arm were positive for a genital lesion; in the placebo and acyclovir arms, approximately 24 % and 8 % of days, respectively, were positive for viral shedding), the data were balanced by creating identical (but opposite) data observations. This results in a time-delay embedded dataset with twice as many rows of data, but with exactly 50 % of observations reflecting the presence of a lesion. The rationale for “mirroring” the data is that the periodicity of lesions or shedding should not be affected by whether the data are coded 0 or 1, but rather by the length of time between changes from no lesion/shedding to lesion/shedding (or vice versa). Data simulations suggest that this method recovers LDE parameter estimates with high precision [38].
Prior to model fitting, a number of preliminary analyses were conducted. First, the time dependency of data observations was examined by looking at auto-correlation function plots [39] (ACFs; see Fig. 2). Treatment arm ACFs for each woman demonstrated a correlation pattern that fluctuated as a function of the time elapsed between measurement occasions, consistent with the presence of oscillation in the HSV-2 recurrence data. Similar analyses using time-shuffled observations did not demonstrate oscillation in the correlation patterns of the shuffled data, providing evidence for the time dependency of data observations. The treatment arm for most women suggested no oscillation of HSV-2 recurrence; however, because a small minority of women (5/19) did have ACFs that suggested periodicity of HSV-2 recurrence, treatment arm data were modeled as well.
Example auto-correlation plots from one woman during the placebo study arm. Solid lines are the sample-estimated correlations, dotted lines are 95 % confidence intervals for time-shuffled data observations, and thus represent the null hypothesis that the data are not time dependent. Correlations are considered statistically significant if they fall outside of the dotted lines
The next step prior to model fitting was to determine an appropriate embedding dimension [36]. It is recommended that at least four observations (i.e., dimensions), with the duration between measurements being no longer than 1/8 of an oscillation period, be used in order to adequately capture cycling [22]. To identify an appropriate number of dimensions, for each of six through 14 embedding dimensions, the zero-order, first, and second derivatives of the data observations were calculated using a smoothing process (generalized local linear approximation, or GLLA [40]), and multiple regression was used to estimate the η and ζ parameters of each study arm. The η and ζ estimates were then plotted against embedding dimension to determine the point at which these estimates stabilize, a method that has been used previously to identify optimal embedding dimensions [41]. Plots suggested ten embedding dimensions were optimal. The ideal number of embedding dimensions for frequency parameters used to approximate distress variability was computed using this same process.
Two-group moderated latent differential equation models [34, 35] were fit to the data. In multiple-group structural equation models, parameters are estimated simultaneously for each group of the parent model, and may be freely estimated or constrained to be equal across groups. Model comparisons were used to test the equivalence across groups of the frequency and damping parameters. Variances and covariances were freely estimated across the study arms.
Results
HSV-2 Genital Lesions
A moderated LDE model was fit to the time-delay embedded genital lesion data. Model comparisons were used to determine the best-fitting model and to test the equivalence of parameter estimates across study arms. Estimates for the best-fitting models for each moderator of lesion presence are presented in the Lesions columns of Table 2.
The negative frequency parameter (η 0) estimates support the preliminary findings that genital lesion presence is a cyclic process, one that is suppressed greatly by treatment with acyclovir. Using Eq. 2 and the frequency (η 0) and damping (ζ 0) parameter estimates to calculate the frequency of oscillation, it was estimated that the cycle of genital lesion presence repeated approximately every 42 days while a woman was in the placebo arm of the study, and was non-oscillating while on acyclovir (oscillatory periods were estimated to be longer than the study period [70 days], suggesting no periodicity; this was consistent with what we observed in women’s ACF plots for the acyclovir arm). The lack of periodicity in the treatment arm suggests that there are insufficient measurement occasions to accurately predict periodicity or differences in periodicity as a function of distress, and that the results for this study arm should be interpreted with caution. Therefore, we present only results for the placebo arm in this report.
Evidence that psychological distress moderates a woman’s genital lesion recurrence was found. Trait level depressive affect moderated the frequency of oscillation of genital lesion recurrence (η 1 = − .006, SE = .001): When untreated with antiviral medication, lesion onset becomes more frequent as a woman’s trait level of depressive symptoms increases. To illustrate, a woman exhibiting average depressive affect (relative to the sample) will experience lesion onset every 41.7 days, whereas a woman who averages one standard deviation unit below the sample mean of depressive affect will experience onset every 49.1 days; a woman who averages one SD unit above the sample mean experiences lesion onset every 36.9 days. Similar results were observed for trait anxiety (η 1 = − .003, SE = .001). Mean stress levels showed the opposite effect (η 1 = .002, SE = .001), although the effects were not as pronounced as for depressive affect and anxiety.
Emotion regulation also was a significant moderator of HSV-2 genital lesion recurrence. Greater dysregulation in depressive affect was associated with faster lesion cycling (η 1 = − .003, SE = .001). Similar effects were observed for anxiety dysregulation (η 1 = − .005, SE = .000) and stress appraisal dysregulation (η 1 = − .006, SE = .001). Emotional reactivity similarly moderated HSV-2 genital lesion recurrence. More extreme shifts in depressive affect was associated with faster cycling (η 1 = − .005, SE = .001), as were shifts in symptoms of anxiety (η 1 = −.003, SE = .001) and appraisals of stress (η 1 = −.003, SE = .001). For example, a woman displaying average reactivity with respect to depressive affect experiences lesion onset every 41.7 days, whereas a woman experiencing one SD unit less reactivity has a cycle that repeats every 47.7 days; a woman experiencing one SD unit more reactivity experiences lesion onset every 37.5 days.
To illustrate some of these results, non-zero estimates from Table 2 were substituted into Eq. 3 and numerical integration (using the lsoda() function in the R package ‘deSolve’ [42]) was used to generate simulated time series data conditional on a set of starting values. The illustration in Fig. 3 is an interaction plot, and demonstrates how the untreated genital lesion process over a 70-day period varies as a function of trait level distress (we used depressive affect in this example). The solid line represents the genital lesion system at the average levels of trait depressive affect (z score = 0), the short-dashed line represents the same system at high levels of depressive affect (one standard deviation unit above average, or z score = 1), and the long-dashed line represents the system at low levels of depressive affect (one standard deviation unit below average, or z score = −1). The plot demonstrates how a hypothetical woman’s trajectory of untreated lesion recurrence might look based on what level of trait depressive affect she exhibits relative to other individuals.
To illustrate these results more empirically, we plotted each study participant’s lesion recurrence over the duration of the placebo arm (dotted lines in Electronic Supplementary Material Figs. 1a, b). We then simulated data (as described above) using each woman’s z score for a given moderator, and superimposed the resulting model-predicted lesion recurrence trajectory onto her actual data observations and empirical trajectory (which was calculated using loess smoothing). We present two well-predicted and two poorly-predicted study participants. The various colors represent the particular moderator used to generate the model-predicted trajectories.
HSV-2 Viral Shedding
Cycling was detected in HSV-2 viral shedding, the frequency of which was suppressed greatly by treatment with acyclovir. A shedding episode is initiated approximately every 41½ days while a woman was in the placebo arm of the study; no oscillation was detected in the treatment arm, in part due to the extensive damping of the HSV-2 viral shedding process. Once again, we present results from the placebo study arm only (see Viral Shedding columns of Table 2).
The frequency of oscillation of viral shedding was significantly moderated by trait depressive affect (η 1 =−.008, SE = .001). A woman experiencing average depressive affect has a shedding cycle that repeats every 41.1 days, whereas a woman exhibiting one SD unit less trait depressive affect experiences shedding onset every 50.7 days; a woman experiencing one SD unit greater average depressive affect has a cycle lasting 35.9 days. Similar (though less marked) results were observed for trait level anxiety (η 1 =−.003, SE = .001) and average daily stress (η 1 =−.003, SE = .001).
Emotion regulation also moderated the HSV-2 viral shedding process. Poor regulation of affect and stress levels was associated with faster cycling (depressive affect regulation: η 1 =−.011, SE = .001; anxiety regulation: η 1 =−.011, SE = .001; stress regulation: η 1 = − .012, SE = .001). Emotional reactivity also significantly moderated HSV-2 viral shedding episode onset frequency. Extreme changes in depressive affect were associated with increased frequency of viral shedding (η 1 = − .004, SE = .001), whereas extreme changes in anxiety was associated with decreased frequency of viral shedding (η 1 = .002, SE = .001). Shifts in daily stress appraisals had no effect on viral shedding cycling (η 1 = .000, SE = .001). The interaction plot in Fig. 4 illustrates how emotion regulation (as measured by stability in anxiousness) influences the untreated cycling of HSV-2 viral load. Electronic Supplementary Material Figs. 2a, b demonstrate how the model-predicted trajectories compare with empirically-derived trajectories.
Effect Size and Power
Effect sizes for each best-fitting LDE model are presented in Table 2. The ratio of the curvature of the alternative model to that of the null model was subtracted from one to produce a pseudo-R 2. This represents the percent reduction in error that the linear oscillator model produces compared with a model without causal pathways. These pseudo-R 2 values ranged from 15–18 %.
To estimate power to detect our effects, 1,000 samples for 70 observations on 19 subjects were simulated using numerical integration as described above. Data were simulated using non-zero parameter estimates from best-fitting models and varying signal-to-noise ratios (SNRs); data for a null model were similarly generated. Data were time-delay embedded, and 0th, 1st, and 2nd derivatives were calculated for each process using GLLA [40], and parameter estimates were derived using linear multiple regression. These simulation conditions suggest that we had approximately 20–74 % power to detect the smallest effects presented in this report (these were calculated using more and less conservative SNRs, respectively; the ratio of variance in displacement of each process to measurement error suggests that the less conservative SNR ratio, which corresponds to the larger power estimate, is more appropriate).
Discussion
Recurrence of HSV-2 is related to certain psychological factors, including psychosocial distress, personality factors, and the experience of stressful events [9]. In past research, recurrence has been defined by the appearance of a lesion, although latent reactivation—viral shedding in the presence or absence of lesions—occurs more frequently [43]. To better capture HSV-2 recurrence, Strachan et al. [10] used both viral shedding and genital lesion presence as indicators of disease severity, and tested for effects that psychological factors (i.e., anxiety, depression, and stress) may exert on disease recurrence. In that report, psychological distress predicted lesion onset 5 days later, but no relationship between distress and viral shedding was observed. The present report analyzes data from that study using contemporary models of dynamic cyclical change, which are capable of separating dynamic variation from measurement error in order to detect cyclic processes.
A series of moderated LDE models [34, 35] showed that higher levels of psychological distress were associated with shorter time durations between onsets of both lesion and shedding episodes. The same pattern was observed for emotion regulation and emotional reactivity: Women with a greater capacity to maintain their baseline level of emotion or who experienced less extreme mood states tended to have a greater length of time between onsets of recurrence episodes. Overall, our results suggest that HSV-2 seropositive women who have low trait levels of distress, are better able to regulate their affect or stress levels, or who respond less intensely to daily stressors may be better equipped to regulate both cutaneous recurrence and asymptomatic reactivation of HSV-2.
Associations between psychological distress and more frequent herpes lesions are reliably observed in the literature [9], but the viral shedding–distress association we observed represents a novel contribution—to our knowledge, this is the first such report of this phenomenon in humans. Our finding is consistent with a wide body of immunology research documenting the immunosuppressive effects of psychological distress [44]; is validated by research using animal models that has demonstrated similar effects [11–13]; and expands on human research that has observed this association using indirect measures [45–47] of asymptomatic latent HSV reactivation (e.g., antibody production, T-cell response [14–16]) by demonstrating a direct psychophysiological link. We suggest that replication studies utilize dynamical systems analysis or other analytic methods capable of detecting periodicity in HSV-2 recurrence; past research using traditional analytic methods—which mistake “error” arising from periodicity for error resulting from measurement imprecision—has not observed a distress–shedding link [10]. Indeed, examining how psychological distress (or other important moderating factors) predicts deviations from the average recurrence trajectory of the sample may be a more sensitive, fruitful line of research to pursue moving forward.
The current study also offers a unique, applied perspective to the HSV literature. A particular strength of the model we use is that it is predictive of HSV-2 recurrence patterns over a 70-day period of time; most prior research has focused on explanatory models of HSV-2 recurrence as a function of psychological distress in just the few days prior to a lesion outbreak or shedding episode. Both explanatory and predictive models are important and necessary to understand HSV-2 recurrence, but dynamic models may have particular utility for developing psychological interventions as an adjunct treatment for individuals with genital herpes.
Limitations and Future Directions
Several limitations of the present study are noted. The generalizability of our results to HSV-2 seropositive men is limited. Although men and women experience comparable rates of subclinical HSV-2 recurrence [48], there is some evidence that men may experience more frequent lesions than women [49]. Men and women also differ in terms of depressive affect [50], symptoms of anxiety [51], and responsiveness to stress [52]. Our sample also excluded women who had HSV-1 antibodies. This may limit the generalizability of our findings to women seropositive for both HSV-1 and HSV-2, although research suggests that rates of symptomatic and asymptomatic recurrence are comparable among women who are seropositive for types 1 and 2 versus type 2 alone [53].
A limitation of linear oscillator models is that they can estimate the number of days that elapse between the onset of lesion or shedding episodes, but not the length of recurrence episodes or length of lesion- or shedding-free days between episodes. Different analysis approaches that do not involve smoothing methods are required to answer these questions, and would complement information provided through the analysis of HSV-2 recurrence from a dynamical systems perspective. Still, DSA may be useful for predicting times when instances of cutaneous HSV-2 recurrence and/or asymptomatic reactivation of latent HSV-2 infection may cluster.
Although higher distress, poorer emotion regulation, and greater emotional reactivity was associated with decreased time between the onset of lesion or shedding episodes was observed, there were a few deviations from this pattern (stress and lesion recurrence, anxiety reactivity and latent reactivation). The anxiety reactivity and shedding association was in fact nonsignificant in the fully saturated model (i.e., a model which included the interaction of treatment arm), suggesting that this may be a chance finding. All of the other findings were significant in both the saturated and the nested, reduced models. The stress level and lesion association finding was surprising, particularly since we observed a protective effect of low average stress levels on HSV-2 viral shedding frequency. Replication of this association using other samples will be necessary to determine whether this finding also represents a random deviation from an overall distress–periodicity pattern, or whether there is something particular about average daily stress levels that produces this paradoxical finding.
Although we exercise caution in interpreting this unexpected finding, one possible explanation is that stress—as opposed to symptoms of anxiety or depression—results in more frequent shedding of virus but does not reduce the host’s ability to clear virus from mucosal membranes. It is widely accepted that chronic stress suppresses and acute stress boosts immune functioning [44], and research suggests that perceived stress or affective reactivity to stress are the salient factors in the stress–immunity relationship rather than the stressful events themselves ([54–56]; but see [57]). Women in the current study indicated how much stress they experienced daily, but without data regarding the nature of the stressors they encountered (e.g., objective vs. subjective, acute vs. chronic), it is difficult to speculate why average stress levels did not follow the same pattern we observed for the remaining moderators. Past research suggests that persistent versus acute stress is an important distinction in HSV-2 research [27], and future research that differentiates between these types of stress may prove important for understanding why a protective effect of average stress on HSV-2 lesion recurrence emerged in the present study.
The model used in the present report was unable to detect periodicity in the treatment arm of the study. This could be because acyclovir was highly effective at reducing the recurrence of HSV-2 genital lesions and viral shedding, but it could also be due to too few measurement occasions in the treatment arm to detect a drastically reduced frequency of HSV-2 recurrence, or to some combination of these two factors. The current analyses suggest that the study of HSV-2 recurrence periodicity as a function of antiviral treatment (in the presence or absence of individual differences in psychological distress) requires following individuals prospectively for an extended length of time—longer than the time frame used (70 days per study arm). Some research measuring viral shedding multiple times daily suggests that HSV viral shedding episodes may occur more frequently than once daily [58]; it is also possible that daily swabbing was not frequent enough to detect viral shedding of short duration in both study arms, resulting in an over-smoothing of the process (and thus underestimating the frequency of viral shedding). Future research may also need to increase the frequency with which biospecimen samples are collected.
Although the current study considered the effect of individual differences in psychological distress, the role that other psychological processes (such as personality factors) may play in HSV-2 recurrence patterns was not considered. Past research has not observed an effect of personality factors on genital herpes recurrence [9, 10], but the dynamical systems approach taken in this paper may illuminate an association between personality factors and HSV-2 recurrence periodicity. Non-psychological moderators that may explain a significant proportion of variation in recurrence periodicity also were not examined. Aging (associated with greater deficiencies in immunity [59]), and time elapsed since primary infection (associated with reduced frequency of recurrence [4]) may be important factors to consider in future research.
Past research suggests that psychological distress may be both a cause and a consequence of HSV-2 recurrence [10]. Rather than considering individual differences in the periodicity of HSV-2 recurrence, future research may model HSV-2 recurrence and psychological distress instead as coupled processes [31, 60]. Such models may be an alternative to linear regression or auto-regressive models to understand temporal relationships between, for example, increases in anxiety and the onset of a lesion or shedding episode. Future research may also focus on how the coupling between genital lesion recurrence and viral shedding varies as a function of psychological distress or emotion regulation; theoretically, individuals under less distress will have weaker coupling of genital lesion recurrence to viral shedding episodes. Our group is currently investigating these questions for both recurrent HSV-2 genital herpes and recurrent HSV-1 cold sores.
Treatment Implications
The results in this report have implications for the psychological management (secondary to treatment with antiviral medication) of genital herpes infections. Cognitive behavioral therapy, with a focus on cognitive restructuring, stress management, relaxation training, imagery, and disease education has gained empirical support for reducing genital herpes recurrence rates [61–64]. The current findings suggest that interventions targeted at reducing overall distress levels and improving response to distress (e.g., enhancing emotion regulation skills) may also be helpful for reducing the recurrence rate for individuals with HSV-2 genital herpes. The viral shedding results have particular implications for disease control. For example, stress management interventions might be developed for HSV-2 serodiscordant couples to test whether such an intervention reduces the risk of transmission to the uninfected partner. Psychoeducation and stress management techniques may also be provided by physicians diagnosing new cases of HSV-2 genital herpes to help reduce the spread of the disease.
Conclusions
HSV-2 recurrence is a dynamic process that varies as a function of psychological distress, emotional stability, and emotion regulation. Onset of cutaneous HSV-2 recurrence and reactivation of latent infection were more frequent in women with higher average distress levels, more labile moods, and less effective emotion regulation. The results presented here are consistent with research indicating that psychological distress suppresses immune system functioning, and this report provides new evidence that HSV-2 viral shedding is related to, and regulated by, psychological distress. Dynamical systems analysis will prove useful for observing HSV-2 recurrence processes, and others like them, in the future.
Notes
Note that after standardization, low z-scores for emotion regulation in fact reflect better regulation, because oscillation is faster at more negative values.
This is the case when η 1 and/or ζ 1 are positive; the reverse occurs when these estimates are negative.
References
Centers for Disease Control and Prevention (CDC). Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years—United States, 2005–2008. Morb Mortal Wkly Rep. 2010; 59: 456-459.
Centers for Disease Control and Prevention (CDC). Press release (2010, March 8): CDC study finds U.S. herpes rates remain high. Available at http://www.cdc.gov/nchhstp/newsroom/hsv2pressrelease.html. Accessibility verified August 24, 2013.
Schiffer JT, Corey L. Herpes simplex virus. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practices of Infectious Diseases. 7th ed. Philadelphia: Churchill Livingstone; 2009: 1943-1962.
Benedetti JK, Zeh J, Corey L. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med. 1999; 131: 14-20.
Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004; 35: 435-445.
Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: A meta-analysis. J Infect Dis. 2002; 185: 45-52.
VanderPlate C, Aral SO. Psychosocial aspects of genital herpes virus infection. Health Psychol. 1987; 6: 57-72.
Swanson JM, Remy L, Chenitz C, et al. Illicit drug use among young adults with genital herpes. Public Health Nurs. 1993; 10: 197-203.
Chida Y, Mao X. Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun. 2009; 23: 917-925.
Strachan E, Saracino M, Selke S, Magaret A, Buchwald D, Wald A. The effects of daily distress and personality on genital HSV shedding and lesions in a randomized, double-blind, placebo-controlled, crossover trial of acyclovir in HSV-2 seropositive women. Brain Behav Immun. 2011; 25: 1475-1481.
Blondeau JM, Aoki FY, Glavin GB. Stress-induced reactivation of latent herpes simplex virus infection in rat lumbar dorsal root ganglia. J Psychosom Res. 1993; 37: 843-849.
Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007; 179: 322-328.
Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. PNAS. 1998; 95: 7231-7235.
Schmidt DD, Schmidt PM, Crabtree BF, Hyun J, Anderson P, Smith C. The temporal relationship of psychosocial stress to cellular immunity and herpes labialis recurrences. Fam Med. 1991; 23: 594-599.
Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type I. Ann Behav Med. 1997; 19: 78-82.
Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985; 8: 249-260.
Tronstein E, Johnston C, Huang M-L, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011; 305: 1441-1449.
Dalkvist J, Whalin T-BR, Bartsch E, Forsbeck M. Herpes simplex and mood: A prospective study. Psychosom Med. 1995; 57: 127-137.
McLarnon LD, Kaloupek GG. Psychological investigation of genital herpes recurrence: Prospective assessment and cognitive-behavioral intervention for a chronic physical disorder. Health Psychol. 1988; 7: 231-249.
Rand KH, Hoon EF, Massey JK, Johnson JH. Daily stress and recurrence of genital herpes simplex. Arch Intern Med. 1990; 150: 1889-1893.
Schiffer JT, Abu-Raddad L, Mark KE, et al. Frequent release of low amounts of herpes simplex virus from neurons: Results of a mathematical model. Sci Transl Med. 2009; 1: 1-9.
Boker SM, Neale MC, Rausch JR. Latent differential equation modeling with multivariate multi-occasion indicators. In: van Montfort HOK, Satorra A, eds. Recent Developments on Structural Equation Models: Theory and Applications. Amsterdam: Kluwer; 2004: 151-174.
Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of sub-clinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996; 124: 8-15.
Ahearn EP. The use of visual analog scales in mood disorders: A critical review. J Psychiatr Res. 1997; 31: 569-579.
Lesage F-X, Berjot S, Deschamps F. Clinical stress assessment using a visual analogue scale. Occup Med (Lond). 2012; 62: 600-605.
Williams VSL, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual Life Outcome. 2010; 8: 57.
Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med. 1999; 159: 2430-2436.
Boker SM, Neale M, Maes H, et al. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011; 76: 306-317.
R: A Language and Environment for Statistical Computing [computer program]. Version 2.15.2. Vienna, Austria: R Foundation for Statistical Computing; 2012.
Newman DA. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organ Res Methods. 2003; 6: 238-362.
Schlomer GL, Bauman S, Card NA. Best practices for missing data management in counseling psychology. J Couns Psychol. 2010; 57: 1-10.
Boker SM, Montpetit MA, Hunter MD, Bergeman CS. Modeling resilience with differential equations. In: Molenaar P, Newell K, eds. Learning and Development: Individual Pathways of Change. Washington, DC: American Psychological Association; 2010: 183-206.
Boker SM, Nesselroade JR. A method for modeling the intrinsic dynamics of intraindividual variability: Recovering the parameters of simulated oscillators in multi-wave panel data. Multivar Behav Res. 2002; 37: 127-160.
Hu Y, Boker S, Neale M, Klump K. Coupled latent differential equation with moderators: Simulation and application [published online ahead of print May 6 2013]. Psychol Methods. http://psycnet.apa.org.proxy.its.virginia.edu/psycarticles/2013-15547-001.pdf.
Staples AD, Boker SM, Bergeman CS. Moderated latent differential equations. Multivar Behav Res. [under review].
Noakes L. The Takens embedding theorem. Int J Bifurc Chaos. 1991; 1: 867-872.
Ram N, Brose A, Molenaar PCM. Dynamic factor analysis: Modeling person-specific process. In: Little TD, ed. The Oxford Handbook of Quantitative Methods in Psychology: Vol. 2: Statistical Analysis. New York: Oxford University Press; 2013: 441-457.
Hu Y. Dynamic Process for Ordinal Data: Simulation and Application [doctoral dissertation]. Charlottesville, VA: University of Virginia; 2013.
Box GEP, Jenkins GM, Reinsel GC. Time Series Analysis: Forecasting and Control. 4th ed. Hoboken, NJ: Wiley; 2008.
Boker SM, Deboeck PR, Edler C, Keel PK. Generalized local linear approximation of derivatives from time series. In: Chow SM, Ferrar E, eds. Statistical Methods for Modeling Human Dynamics: An Interdisciplinary Dialogue. Boca Raton, FL: Taylor & Francis; 2009: 161-178.
Deboeck PR, Boker SM, Bergeman C. Modeling individual damped linear oscillator processes with differential equations: Using surrogate data analysis to estimate the smoothing parameter. Multivar Behav Res. 2008; 43: 497-523.
Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: Package deSolve. J Stat Soft. 2010; 33: 1-25.
Mostad SB, Kreiss JK, Ryncarz A, et al. Cervical shedding of herpes simplex virus and cytomegalovirus throughout the menstrual cycle in women infected with human immunodeficiency virus type 1. Am J Obstet Gynecol. 2000; 183: 948-955.
Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004; 130: 601-630.
Mbopi-Kéou F-X, Bélec L, Dalessio J, et al. Cervicovaginal neutralizing antibodies to herpes simplex virus (HSV) in women seropositive for HSV types 1 and 2. Clin Diagn Lab Immunol. 2003; 10: 388-393.
Reeves WC, Corey L, Adams HG, Vonter LA, Holmes KK. Risk of recurrence after first episodes of genital herpes: Relation to HSV type and antibody response. N Engl J Med. 1981; 305: 315-319.
Schiffer JT. Mucosal HSV-2 specific CD8+ T-cells represent containment of prior viral shedding rather than a correlate of future protection. Front Immunol. 2013; 4: 209.
Wald A, Zeh J, Selke S, Warren T, Ashley R, Corey L. Genital shedding of herpes simplex virus among men. J Infect Dis. 2002; 186(Suppl 1): S34-S39.
Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994; 121: 847-854.
Nolan-Hoeksema S. Gender differences in depression. Curr Dir Psychol Sci. 2001; 10: 173-176.
Armstrong KA, Khawaja NG. Gender differences in anxiety: An investigation of the symptoms, cognitions, and sensitivity towards anxiety in a nonclinical population. Behav Cogn Psychother. 2002; 30: 227-231.
Matud MP. Gender differences in stress and coping styles. Personal Individ Differ. 2004; 37: 1401-1415.
Wald A, Zeh J, Selke S, Ashley R, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995; 333: 770-775.
Burns VE, Drayson M, Ring C, Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosom Med. 2002; 64: 963-970.
Taylor SE, Kemeny ME, Reed GM, Bower JE, Gruenewald TL. Psychological resources, positive illusions, and health. Am Psychol. 2000; 55: 99-109.
van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary control. Psychosom Med. 1996; 58: 447-458.
Cohen S, Tyrrel DAJ, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol. 1993; 64: 131-140.
Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008; 198: 1141-1149.
Rymkiewisz PD, Heng YX, Vasudev A, Larbi A. The immune system in the aging human. Immunol Res. 2012; 53: 235-250.
Boker SM, Laurenceau JP. Coupled dynamics and mutually adaptive context. In: Little TD, Bovaird JA, Card NA, eds. Modeling Ecological and Contextual Effects in Longitudinal Studies of Human Development. Mahwah, NJ: Lawrence Erlbaum; 2007: 299-324.
Cruess S, Antoni M, Cruess D, et al. Reductions in herpes simplex virus type 2 antibody titers after cognitive behavioral stress management and relationships with neuroendocrine function, relaxation skills, and social support in HIV-positive men. Psychosom Med. 2000; 62: 828-837.
Longo DJ, Clum GA, Yaeger NJ. Psychosocial treatment for genital herpes. J Consult Clin Psychol. 1988; 56: 61-66.
Lutgendorf SK, Antoni MH, Ironson G, et al. Cognitive-behavioral stress management decreases dysphoric mood and herpes simplex virus-type 2 antibody titers in symptomatic HIV-positive gay men. J Consult Clin Psychol. 1997; 65: 31-43.
McLarnon LD, Kaloupek DG. Psychological investigation of genital herpes recurrence: Prospective assessment and cognitive-behavioral intervention for a chronic disorder. Health Psychol. 1988; 7: 231-249.
Acknowledgments
We gratefully acknowledge Steven M. Boker and Yueqin Hu for the consultation they provided throughout the preparation of this article. We also thank Anna Wald, Stacy Selke, and Amalia Magaret for providing the data used in this report and for their helpful comments on earlier versions of this article.
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards
Erin E. Horn, Eric Turkheimer, and Eric D. Strachan declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(DOCX 270 kb)
Supplementary Figure 2
(DOCX 263 kb)
About this article
Cite this article
Horn, E.E., Turkheimer, E. & Strachan, E. Psychological Distress, Emotional Stability, and Emotion Regulation Moderate Dynamics of Herpes Simplex Virus Type 2 Recurrence. ann. behav. med. 49, 187–198 (2015). https://doi.org/10.1007/s12160-014-9640-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-014-9640-9