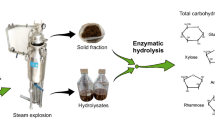
Abstract
Agave bagasse (AB) is a lignocellulosic biomass with potential to obtain high value-added products such as lignin, microcrystalline cellulose, and polysaccharides that can be used for biofuel production. A Central Composite Design (CCD) was used to evaluate the effect of temperature, thermal treatment time, ethanol concentration, solid/liquid ratio, size particle, and sodium hydroxide concentration on delignification and lignin recovery from AB. In addition, the influence of delignification on the enzymatic hydrolysis using commercial enzymes was determined. This organosolv process allowed a delignification of 91.49% and 76% of the lignin recovery, at 150 °C, 10 min, 40% (v/v) ethanol, solid/liquid ratio of 1:10 g/mL, particle size of 0.5 mm – 1 mm, and 5.5% (w/v) sodium hydroxide. The maximum specific yield of enzymatic hydrolysis (SYEH), under the best conditions for delignification, was of 844.40 ± 9.70 mg total sugars per g of bagasse pretreated with organosolv (BPO), which was 4.42 times higher than the yield obtained from bagasse without pretreatment (BWP). Additionally, the enzymatic hydrolysate of BPO produced 303.62 ± 10.62 NmLCH4/g BPO. Furthermore, an energy recovery efficiency of 64.13 ± 0.9% was obtained for BPO hydrolysate which is 2.60 times higher than that of BWP hydrolysate (24.88 ± 0.92%). In a biorefinery concept, alkaline organosolv pretreatment of AB was highly efficient for lignin production, and improved the enzymatic hydrolysis of polysaccharides, which allowed a higher energy recovery.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
AB is a lignocellulosic biomass generated during the production of tequila. A production of 550,304 tons of AB was reported for the period 2017–2021 [1]. This sector often struggles to properly dispose the large amount of AB that accumulates in the distilleries, becoming an environmental and financial burden [2]. The AB has gained attention as a potential feedstock to produce biofuels and value-added by-products in a biorefinery scheme with impact in the circular bioeconomy to deal with the massive production of AB, avoid environmental problems related to its improper disposal (which is associated with leachates, odor generation, and atmospheric pollution), and improve the recovery of its economic value [3]. AB is a lignocellulosic biomass, composed mainly of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose can be hydrolyzed to produce monomeric sugars that can be used in biofuels production. However, lignin is one of the most important factors affecting the biodegradability of lignocellulosic materials [4]. Lignin can be removed and recovered to produce high value-added products such as hydrogels, resins, binding, dispersing, and emulsifying agents, offering a remarkable platform for valorization of lignocellulosic biomass through lignin-based biorefineries [5, 6]. Therefore, it is important to investigate delignification treatments to maximize lignin removal. It is also important to investigate the best conditions for acid precipitation of lignin to maximize its recovery.
Several pretreatment methods have been described for the removal of lignin from lignocellulosic biomass, including alkaline hydrogen peroxide and ionic liquids [6, 7]. Particularly, the organosolv process involves the use of solvents such as methanol, ethanol, and other organic solvents to solubilize lignin. Among these solvents, ethanol-based organosolv pretreatment has attracted more attention due to its eco-friendliness and low toxicity, as well as the possibility to recycle the solvents [8]. This process produces a liquor containing solubilized lignin and a delignified biomass. Lignin can be acid precipitated to obtain a high purity organosolv lignin. The delignified biomass can be enzymatically hydrolyzed to solubilized sugars for further biocombustible production (ethanol, biogas, and biohydrogen) [9].
There are some reports on lignin removal from AB using organosolv pretreatment. Fernández-Rodríguez et al. [10] achieved recovery lignin yields of approximately 9.49% using organosolv pretreatment. Caspeta et al. [11] used acid catalyzed ethanosolv pretreatment to investigate the effect of temperature, thermal treatment time, sulfuric acid concentrations, and ethanol concentrations parameters on the lignin removal and on the enzymatic hydrolysis of AB and reported a recovery of 100% of total lignin.
On the other hand, the alkali catalyzed organosolv pretreatment is one of the leading technologies for solubilization of lignin and alteration of cellulose matrix to increase cellulose digestibility [12]. In an alkaline medium, hydrolysis of α-aryl-ether bonds in the lignin structure occurs in a lesser extent when compared with acid catalyzed organosolv delignification [13]. Consequently, the breakage of β-aryl-ether bonds between lignin units is the predominant reaction mechanism in alkaline medium. This is due to the reaction between NaOH and the hydroxyl group of lignin, where the oxygen from the hydroxyl group of NaOH acts as a nucleophile, forming an ether bond with the lignin [13]. Since, β-aryl ether bonds constitute 40 to 65% of the total linkages in lignin [14], a more efficient lignin removal would be expected under alkaline conditions. On the other hand, the remaining biomass after lignin removal are enriched in polysaccharides, such as cellulose and hemicellulose. Hydrolysis using appropriate enzymes is the most effective method to release simple sugars from these polysaccharides. Some studies reported that delignification increases the release of sugars from cellulose and hemicellulose [15, 16].
Methane production has been investigated using enzymatic hydrolysates from untreated AB [17,18,19]. Additionally, methane production using hydrolysates from different pretreatments of AB has also been investigated [15, 20]. However, methane production from enzymatic hydrolysates of alkaline organosolv delignified AB has not been evaluated.
Therefore, the objective of this work was to determinate the best conditions for delignification and recovery of lignin from AB using alkaline organosolv pretreatment, as well as to evaluate the effect of delignification on enzymatic hydrolysis and further methane production using the enzymatic hydrolysate.
Materials and Methods
Agave Bagasse
AB was obtained from Casa Herradura (Amatitán, Jalisco, Mexico). The bagasse was sun-dried and grinded by means of an agricultural shredder (0.5 mm – 3.35 mm); afterwards, it was washed four times with distilled water to eliminate residual sugars from the tequila production. Then, it was sun-dried again and sieved through a mesh-sieve with a particle size of 0.5 – 1 mm.
Alkaline Organosolv Delignification
AB was treated using the alkaline organosolv method according to Fernández-Rodríguez et al. [10], with modifications as described below. To remove lignin, biomass was mixed with a solution of ethanol (JT Baker, Madrid), distilled water, and sodium hydroxide (Macron Fine Chemicals, Pennsylvania) according to the experimental design described below. Cooking process was carried out in a stainless-steel batch pressurized reactor with a PID temperature controller designed by the Biorefinery Group ( http://www.Biorefinerygroup.com) [21] with a total volume of 190 mL s (50-mL working volume), and heated with an electrical resistance. According to Table 1, two temperatures were used during the screening with the Placket Burman design, 150 °C and 170 °C. Heating times to reach each temperature were 29 and 33 min respectively. The reactor was maintained isothermally at the set temperatures for the times shown in Table 1. Thereafter, the reactor was cooled with a cold-water bath. Solid and liquid were separated by a plastic colander with 1.19-mm sieve. To recover lignin, the pH of the liquid was lowered to pH 1.5 with hydrochloric acid (Fermont, Canada) 6 M to precipitate the lignin. Then, the precipitate was washed with distilled water until neutral pH and dried at 60 °C [10].
The effect of alkaline organosolv parameters on the delignification (percent of lignin removal based on untreated agave bagasse) and lignin recovery (percent of lignin recovery based on lignin removed) was determined by means of response surface methodology, using Design Expert 11.0. An initial screening using a Plackett-Burman experimental design was employed to define the most significant parameters for the organosolv pretreatment (Table 1). Dependent variables were lignin removal and lignin recovery. Independent variables and levels were as follow: temperature of 150 and 170 °C, ethanol concentration of 10 and 40% (v/v), solid/liquid ratio of 1:10 and 1:15 (w/v), size particle < 1 mm and > 3.35 mm, sodium hydroxide concentrations of 0% and 1% (w/v), thermal treatment time of 10 and 50 min. Once the most significant parameters were defined, a central composite design was employed to optimize the lignin removal and recovery (Table 2). To obtain a higher amount of delignified fiber, AB was treated in a greater reactor with a volume of 832 mL (400-mL working volume) under the best conditions found previously using the 100-mL reactor.
To monitor the impact of pretreatment (such as temperature, time, and pH) on AB, the severity factor (indexed as a scaling factor) was calculated as [log (R′o)]. This factor was calculated as follows:
where R′o is a combined severity factor proposed by Pedersen et al. [22], Ro is the severity factor proposed by Shiva et al. [23], tmax is the time (min) needed to attain the maximum alkaline organosolv temperature, ctrl and ctrf are the time (min) needed for the heating-cooling stages, respectively. The value 100 is the temperature of reference, T(t) and T′(t) (°C) are the temperature profiles in the heating and the cooling stages respectively, and ω is an empirical parameter (value of 14.75) related to the activation energy.
Analytical Characterization
Content of ligning and sugars (glucose and xilose) from structural carbohydrates of the AB and the solid fiber samples obtained after the experimental treatments of both designs, (Plackett-Burman and CCD), were determined by the method reported by the National Renewable Energy Laboratory (NREL protocol) [24]. A biomass sample of 300 mg was mixed with 3 mL of 72% H2SO4 in a Hatch tube and incubated at 30 °C for 1 h. The sample was mixed with a vortex every 5 min. Subsequently, the sample was transferred to a Schott flask, and 84 mL of distilled water was added to dilute the acid to a concentration of 4% (v/v). Then, the flask was placed in the autoclave at 121 °C for 1 h. The flask was cooled, and then the solution was filtered in a previously tared Gooch crucible. Gooch crucible was dried at 105 °C for 12 h, and then it was weighed. The solid contained in the crucible corresponds to the acid insoluble lignin (AIL). On the other hand, to determine the acid soluble lignin (ASL), the filtered liquid was analyzed in a UV spectrophotometer at 205 nm [10]. The sugar content in the filtered liquid (glucose and xylose) was determined by means of a High-Performance Liquid Chromatography (HPLC) using an Agilent Technologies 1260 Infinity II equipment with a Metacarb 87H column (Varian, USA) and a refractive index detector. A sample of 20 μL was analyzed with 50 mM H2SO4 as mobile phase at a flow rate of 0.7 mL/min at 60°C.
The crystallinity index of the bagasse fibers was determined using a Bruker D8 Advance X-Ray Diffractometer (XRD) (Tokyo, Japan), with monochromatic Cu Kα radiation (λ = 1.5818 Å), with a voltage of 44 keV and a current of 25 mA. The fiber fractions were analyzed in a 2𝜃 range of 5–70° [25]. The results were processed in the Magic plot program using the deconvolution method.
The morphology of AB fibers was characterized using a ESEM FEI-QUANTA 200 Scanning Electron Microscope (SEM) (Holland) [25]. Surface morphological analysis was carried out under low vacuum.
Enzymatic Hydrolysis
The effect of AB delignification by alkaline organosolv pretreatment on enzymatic hydrolysis was conducted using a commercial cellulose enzyme (Cellic-CTec2 with an initial enzymatic activity of 109.65 FPU/mL and 6631.17 XYU/mL). The hydrolysis conditions for the enzyme preparation were 25 g of biomass in 250 mL of acetate buffer 50 mM at pH 5.5 and enzyme concentration of 15 FPU/g biomass. The hydrolysis was conducted at 50 °C, 120 rpm, and 24 h [26]. The hydrolysates were characterized regarding the content of Chemical Oxygen Demand (COD) following the Standard Methods [27] and total sugars according to the Dubois assay [28]. To evaluate the contribution of the enzymatic preparation to the COD and the total sugars to the agave hydrolysates, enzymatic controls were performed with the same conditions as for the hydrolysates, but biomass addition was excluded.
Methane Production from Enzymatic Hydrolysate
Methane production experiments were carried out in an Automatic Methane Potential Test System (AMPTS II) (Bioprocess Control, Lund, Sweden) using a BMP protocol reported previously [17]. In brief, glass bottles of 600 mL, with a working volume of 360 mL, were used. Enzymatic hydrolysates of bagasse without pretreatment and hydrolysates of bagasse pretreated with alkaline organosolv were used as substrates. Also, three controls were evaluated, an enzyme control (assay with buffer and enzyme as substrate), a positive control (assay with glucose as model substrate), and a negative control or endogenous (assay without substrate). Methane produced by the enzyme and negative controls were subtracted from the methane produced by the hydrolysates. The positive control was used to validate the results of the BMP assay according to Tapia-Rodríguez et al. [17]. All experiments were evaluated by triplicate. Each bottle was filled with an appropriate volume of mineral medium that included 1 g NH4Cl/L, 0.1 g NaCl/L, 0.1 g MgCl2ꞏ6H2O/L, 0.05 g/CaCl2ꞏ2H2O/L, 0.4 g K2HPO4ꞏ3H2O/L. Hydrolysates and glucose were added at a concentration of 5 g COD/g of substrate. An inoculum with a concentration of 10 g Total Volatile Solid (TVS)/L was used. The inoculum was an anaerobic granular sludge from an anaerobic digester of a vinasse-wastewater treatment plant at Casa Herradura, a tequila distillery located in Amatitán, Jalisco, Mexico. Additionally, sodium bicarbonate was added to adjust the final alkalinity of the mixture to 3 g CaCO3/L, while maintaining a pH range between 6.8 and 7.2. Headspace was initially purged with N2 gas for 60 s. All experiments were conducted at 37 °C and 120 rpm.
Energy Recovery Efficiency
where, ERE: energy recovery efficiency in percentage; COD hydrolysate (g COD/L); BMP hydrolysate (LNCH4/g CODadd); biomass: initial dry weight of AB used in the enzymatic hydrolysis (g/L); methane energy equivalent (35.8 kJ/L CH4); calorific value of AB (16.35 kJ/g bagasse) which is the heat produced by the complete combustion of AB.
Results
Agave Bagasse Composition
AB composition was determined to evaluate its potential use as feedstock for lignin and methane production. AB contains 43.65 ± 1.84% and 19.09 ± 0.1% of glucans and xylan respectively, which are feasible substrates for methane production. The total content of lignin was 19.11 ± 0.80%, while 4.60 ± 0.74% and 5.44 ± 0.65% correspond to extractives and ash, respectively. These results are slightly higher than those reported by Caspeta et al. [11], 38.60%, 13.60%, and 16% for glucans, xylan, and lignin, respectively. The observed differences in composition are due to different factors, such as agave species, growing regions, climates, and age of the plant [29].
Alkaline Organosolv Pretreatment
To the best of our knowledge, there are no reports on delignification of AB by alkaline organosolv pretreatment. Therefore, a Placket Burman design was first performed to determinate the most significant independent variables that maximize two response variables, delignification of AB and lignin recovery. The results are shown in Table 1. Overall, delignification values from 8.93 to 51.73% and lignin recovery values from 0 to 100% were achieved with the alkaline organosolv pretreatment. Table 1 shows that experiment 8 was the best condition for lignin removal with a 51.73% of delignification. On the other hand, the highest value of lignin recovery, 100%, was observed in experiment 2.
Results of the Plackett Burman design were represented in a Pareto chart (data not shown). According to this chart and analyses of variance (ANOVAs) (data not shown), the most significant variables were determined at a 95% confidence level (p <0.05) for both response variables. In the case of delignification, the concentration of sodium hydroxide was the only significant factor. For the other response variable, lignin recovery, the concentration of sodium hydroxide and the S/L ratio were significant. Therefore, these two variables were included in the CCD experiments. On the other hand, temperature, size particle, reaction time, and concentration of ethanol were non-significant for both response variables.
Because one of the aims of this work was to achieve a high lignin recovery yield, this yield was considered the main response parameter to fix the non-significant variables for the CCD experiments. Therefore, conditions for the non-significant response variables, defined by experiment 2, which showed the highest lignin recovery were selected for the CCD experiments. Thus, a temperature of 150 °C, an ethanol concentration of 40% (v/v), and size particle less than 1 mm were fixed variables in the CCD experiments. Regarding the reaction time, the highest lignin recovery, according to experiment 2, was obtained at a time of 50 min. However, because reaction time is a non-significant variable a shorter reaction time of 10 min was selected, because it produces less energy demand than a longer time of 50 min.
To define the pretreatment conditions for maximizing delignification and lignin recovery, a CCD experimental design was performed varying both the NaOH concentration and the S/L ratio. The other parameters were fixed as explained previously. The experimental design indicated that the NaOH concentration contributed 46.14% and 20.23% to delignification and lignin recovery, respectively, while the S/L ratio contributed 5.03% and 2.64% to delignification and lignin recovery. Therefore, NaOH concentration was the factor with the greater influence on both response variables. As previously mentioned, the Pareto diagram showed that NaOH concentration has a positive effect, that is, the higher the NaOH concentration the higher the delignification and recovery of lignin. Table 2 shows that the highest delignification value (94.42%) was observed in experiment 4, while the highest lignin recovery value (75.52%) was observed in experiment 7. However, experiment 11 showed high delignification as well as lignin recovery (91.49% and 72.16% respectively), and both values are very similar to the values observed in experiment 4 (for delignification) and experiment 7 (for lignin recovery). For this reason, the conditions of experiment 11, as well as the conditions defined for the non-significant variables by the Plackett Burman design, were selected to carry out the alkaline organosolv pretreatment on a larger scale.
The response surfaces of delignification and lignin recovery as function of the significant factors are shown in Fig. 1. ANOVA of the CCD indicated the statistical model was significant and showed no lack of fit. The adjusted R2 statistic explains 99.67% and 95.44% of the variability for delignification and lignin recovery model. According to Gutiérrez-Pulido et al. [30] models with adjusted R2 values greater than 70% are considered to have a good prediction quality. Therefore, both models were accepted. It can be seen from the figures that the higher delignification percentages were obtained for high sodium hydroxide concentrations and that the influence of S/L ratio was weak. It can be also noticed from Fig. 1 that the higher lignin recovery percentages were obtained for high sodium hydroxide concentrations and S/L ratios.
According to the analysis of the CCD data, the following quadratic equations for each response variable were obtained:
where Y1: delignification (%) and Y2: lignin recovery (%); X1: sodium hydroxide concentration and X2: S/L ratio.
The optimal conditions for delignification and lignin recovery predicted by these equations were NaOH concentration of 9.95% and 1:7.75 g/mL of S/L ratio with a predicted 95.04% of delignification and 67.41% of lignin recovery. Although the optimal conditions defined by the surface response methodology were slightly different to the ones obtained with experiment 11, the delignification and lignin recovery values were similar to those obtained with experiment 11. To obtain enough pretreated biomass for its characterization and the mass balance described below in Sect. 3.3 and 3.6, the alkaline organosolv pretreatment was carried out in an 832-mL reactor. Although the same conditions found for experiment 11, during the optimization process in a 190-mL reactor, were used for the 832-mL reactor, lower values for delignification of 84.56% and for lignin recovery of 42.60% were obtained. This difference could be attributed to the scaling up of the volume reactor. To evaluate this possibility the severity factor was calculated. The 832-mL reactor had a severity factor of 10.71, whereas the smaller 190-mL reactor presented a slightly lower severity of 10.63. Therefore, it is unlikely that the severity factor could account for the lower effectiveness of the larger reactor. Another difference between both reactors is the mixing regime. The larger reactor has a propeller system, whereas the smaller reactor does not have one. Thus, it would be expected that the larger reactor would be more effective which is the opposite to the observed results. Therefore, additional factors may be at play, and further investigation is needed to understand the underlying causes of these observations.
Other authors have pretreated AB using the acid-catalyzed organosolv method achieving lower percentages of delignification as compared to delignification values reported in this work. Pérez-Pimienta et al. [31] used a solution with water, ethanol, and sulfuric acid and reached 45% of delignification after pretreatment at 160 °C, 10 min, and 1:10 (w/v) solid/liquid ratio. Caspeta et al. [11] used sulfuric acid as catalyst in a solution containing water and ethanol, 160 °C during 10 min and reached 65.61% delignification. Delignification value obtained in the present study is 2.03 and 1.53 times higher than values for acid-catalyzed organosolv reported by Pérez-Pimienta et al. [31] and Caspeta et al. [11], respectively. The increased levels of delignification observed in the alkaline organosolv pretreatment could be attributed to the high content of β-aryl-ether bonds in lignin and the fact that breakage of β-aryl-ether bonds is predominant in the alkaline medium as mentioned in the introduction. Furthermore, the delignification percentage achieved in the present study in the 832-mL reactor (84.56%) was 1.8 times higher than the value of 47.87% reported by Pérez-Pimienta et al. [32] for AB pretreated with ionic liquids in a 1-L reactor (Table 3).
On the contrary, the delignification percentage obtained in this work in the 832-mL reactor was lower than the 97% reported by Galindo-Hernández et al. [15] for AB pretreated with alkaline hydrogen peroxide. This last difference could be attributed to the different methodologies used to determine the composition of AB. In the case of Galindo-Hernández et al. [15] thermogravimetric analysis was used, while the NREL protocol was used in this work which is a more precise method for lignocellulosic biomass characterization.
Regarding lignin recovery, Fernández-Rodríguez et al. [10] reported a 9.49% of lignin recovery with a non-catalyzed organosolv pretreatment using a mixture of ethanol/water (70:30 v/v) at 200°C during 90 min and a solid/liquid ratio of 1:15 (w/v). This lignin recovery percentage was lower than the one obtained in this study (12.62%, Table 3), but under more severe conditions of temperature, ethanol concentration, and reaction time. On the other hand, Caspeta et al. [11] reported 100% of lignin recovery with an acid-catalyzed organosolv, which is higher than the lignin recovery yield obtained in the present study (72.16%, Table 3). The present study differs from that of Caspeta et al. [11] in the way of measuring lignin. In the case of Caspeta et al. [11] the fibers were washed with ethanol and the ethanol washes were combined with the liquor containing lignin, whereas in the present study the fiber was washed with water and the washing water was discarded. Therefore, some lignin was lost in the washing water. Overall, lignin removal by alkaline organosolv pretreatment was higher than the acid-catalyzed organosolv, and the ionic liquid delignification. Regarding lignin recovery, the alkaline organosolv pretreatment was higher than the one reported for non-catalyzed organosolv, but lower than the recovery reported for acid-catalyzed organosolv.
Crystallinity and Morphological Characterization
Effect of Pretreatment on Biomass Crystallinity Index
Crystallinity of cellulose is acknowledged as an important characteristic that affects cellulose enzymatic hydrolysis efficiency.
The CrI of bagasse without pretreatment (BWP) and pretreated with alkaline organosolv (BPO) was calculated based on the X-ray diffraction (XRD) results (Fig. 2). The diffractograms of BWP, BPO, and standard cellulose (Avicel) show characteristic peaks for microcrystalline cellulose at 2θ of 15°, 16.5°, 22.8°, and 34.6°. In addition to cellulose signals, there are distinctive and prominent peaks (lines) at 2θ of 14.8°, 24.2°, 30.0°, and 38° corresponding to calcium oxalate as previously reported by Pérez-Pimienta et al. [33]. The CrI for BWP was 56%. This value is similar to the CrI value of 51.2% reported by López-Gutiérrez [25] for untreated AB. The difference in the CrI values is due to natural variability of agave crops. Also, Pérez-Pimienta et al. [31] determined the CrI for untreated AB obtaining a lower value of 39%. In the case of Pérez-Pimienta et al. [31] the observed difference of the CrI value is likely due to the different methodologies used to determine CrI. In the present study the crystallinity index was calculated using the deconvolution method, which allows separation of the amorphous from the crystalline signals (considering the four peaks of crystalline cellulose) by curve fitting, assuming Gaussian functions for each peak [34]. In contrast, the method used by Perez Pimienta et al. [31] considered only the scattered intensity in the main peak. After alkaline organosolv pretreatment, the CrI increased to 59.04 %. This increment was attributed to the elimination of amorphous hemicellulose and lignin which enriched the content of crystalline cellulose in BPO [8, 35]. This enrichment is consistent with the cellulose content present in the delignified fiber measured by NREL. Pérez-Pimienta et al. [31] also reported an increase of the CrI after organosolv pretreatment on AB, which increased to 44.6%
Effect of Pretreatment on Biomass Morphology
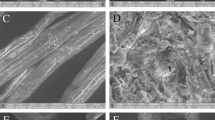
Structural modification between BWP and BPO are illustrated in Fig. 3. The surface of the AB without pretreatment (Fig. 3a) is mainly constituted by the sclerenchyma tissue (support tissue formed by non-living cells with a lignified secondary wall; its main function is of a mechanical nature) and parenchyma tissue (fundamental tissue made up of living cells with a primary wall, rich in hemicellulose, pectic, and soluble substances) [36]. Additionally, calcium oxalates are present in raphide and styloid forms (Fig. 3a). Comparison of Fig. 3a, b, shows that some tissues such as the parenchyma and the sclerenchyma were removed after alkaline organosolv pretreatment. This can be attributed to the delignification process. Additionally, Fig. 3a presents well-defined calcium oxalate crystals. However, after pretreatment (Fig. 3b), the micrographs show that AB fibers have a low amount of calcium oxalate crystals due to the partial removal of them. The removal of the sclerenchyma and parenchyma was reflected in the XRD analyses. Lignin and hemicellulose are amorphous polymers; therefore, their removal causes an increase in CrI. Furthermore, this is supported by the NREL analysis, which showed that 84.60% of lignin and 76.50% of xylan were removed, whereas a 45.60% of glucans were removed during the pretreatment process.
Micrographs of agave bagasse with different treatments: a bagasse without pretreatment (BWP); b bagasse pretreated with alkaline organosolv under the best conditions (BPO); c and d bagasse pretreated with alkaline hydrogen peroxide (BAHP). All micrographs were obtained at a magnification of 250× except for micrograph d, which was obtained at 100×
Figure 3b also shows that structural changes after alkaline organosolv pretreatment are mainly on the surface of bagasse fibers, suggesting that other lignified structures such as xylem and phloem that are internal structures are not affected. Figure 3c, d corresponds to AB fibers after alkaline hydrogen peroxide pretreatment [37]. In this case, the figure shows two micrographs with different degrees of delignification due to pretreatment. The changes on the fiber structure are not homogenous along the fiber. For example, Fig. 3c shows mainly changes on the surface of the fiber, while Fig. 3d shows severe internal degradation of the fiber. In fact, Fig. 3d shows vascular bundles that are internal lignified structures (xylem and phloem) that cannot be observed after alkaline organosolv pretreatment, supporting the idea that alkaline organosolv pretreatment does not affect these internal structures.
Enzymatic Hydrolysis
The effect of alkaline organosolv treatment on the enzymatic hydrolysis of AB was evaluated. Enzymatic hydrolysates of BWP and BPO were obtained using the commercial enzyme preparation Cellic-CTec2. Results of the characterization of the hydrolysates and the specific yield of enzymatic hydrolysis (SYEH) are shown in Table 4. Results show a positive effect of the alkaline organosolv pretreatment on the COD and total sugar concentrations of the enzymatic hydrolysates. After pretreatment, COD concentration in the enzymatic hydrolysate increased approximately 3 times, and total sugar concentration in the enzymatic hydrolysate increased 4.5 times as compared to the values obtained from the enzymatic hydrolysate obtained from untreated bagasse. Observed increases in COD and total sugar concentrations are because the alkaline organosolv treatment removed 84.60% of lignin and 76.50% of xylan from the bagasse. Therefore, enzymatic hydrolysis of remaining holocellulose (mainly cellulose and a small percentage of hemicellulose) in the BPO fiber was more effective. Xylan removal during organosolv treatment has been previously reported for agave bagasse. In this sense, Caspeta et al. [11] and Pérez-Pimienta et al. [32] reported xylan removal efficiencies of 81.80% and 50.60%, respectively, using acid-catalyzed organosolv.
Table 4 also shows that the SYEH of the BPO hydrolysate was 4.42 times higher than the SYEH of the BWP hydrolysate. Overall, these results indicate that alkaline organosolv pretreatment improved enzymatic hydrolysis by modifying the recalcitrant structure of bagasse during the delignification process enabling the access of the enzymes to holocellulose. Similar results have been observed in previous studies that reported increases in total sugar concentration and SYEH of AB hydrolysates obtained with different pretreatments, including organosolv [12, 15, 31].
In this work, the SYEH obtained using Cellic CTec2 for the hydrolysis of BPO was 844.40 ± 9.70 mg TS/g bagasse, which is significatively higher than the SYEH values of 733 mg TS/g Bagasse and 477 mg glucose/g bagasse reported by Pérez-Pimienta et al. [33] and Caspeta et al. [11] respectively for AB pretreated with acid organosolv (Table 3). Pérez-Pimienta et al. [31] and Galindo-Hernandez et al. [15] also reported SYEH values of 507 mg TS/g bagasse and 297 mg TS/g bagasse for AB pretreated with ionic liquids and alkaline hydrogen peroxide, respectively. Compared to these pretreatments, the SYEH values obtained in this study were 1.67 and 2.84 times higher, respectively. Overall, these results show that alkaline organosolv pretreatment was more effective for enhancing enzymatic hydrolysis of agave bagasse than the acid-catalyzed organosolv, ionic liquid, and alkaline hydrogen peroxide pretreatments due to the high removal of lignin and xylan from the bagasse leading to a more effective enzymatic hydrolysis of the BPO fiber.
Methane Production
The potential for methane production of BWP and BPO hydrolysates was evaluated. The values of biochemical methane potential (BMP), biodegradability, specific methane yield (SMY), and ERE obtained for BWP and BPO hydrolysates are shown in Table 5. Glucose control produced 308.24 ± 2.13 NmLCH4/g CODadd, which correspond to 88.1% of the theoretical value, result that validates the BMP assays [17]. BPO hydrolysate presented a higher BMP (311.02 ± 4.83 NmLCH4/g DQO) as compared with BWP hydrolysate (275.33 ± 0.94 NmLCH4/g DQO). Considering that both assays were performed at a normalized COD of 5 g/L, the observed differences in BMPs are due to different hydrolysate biodegradability. The biodegradability percentage is estimated considering the BMP in terms of mL CH4/g CODadd and the theoretical value of 350 mL CH4/g COD [38]. Table 5 shows that the BPO hydrolysate has a biodegradability of 88.90 ± 1.40% while the BWP hydrolysate has a biodegradability of 78.67 ± 0.30%. Such differences in biodegradability could be related with the presence of oligomers of carbohydrates associated with lignin in the BWP hydrolysate, which are not easily transformed to methane under the BMP experimental conditions. On the contrary, in the case of BPO hydrolysate, there would be a lower content of carbohydrate-lignin complexes since a high percentage of the lignin was removed by the pretreatment before enzymatic hydrolysis. These results indicate that the alkaline organosolv pretreatment improves the biodegradability of the enzymatic hydrolysate. According to Table 5, the SMY value for the BPO hydrolysate was 2.73 times higher compared with BWP hydrolysate, indicating a higher potential for methane production. This result shows the importance of the higher biodegradability of BPO hydrolysate, which is due to the presence of more readily biodegradable organic compounds.
Moreover, the SMY value of the BPO hydrolysate (303.62 ± 10.62 mL CH4/g bagasse) was 23% lower than that reported for AB pretreated with alkaline hydrogen peroxide (393.40 ± 13 mL CH4/g bagasse) by Galindo-Hernández et al. [15], but 2.62 times higher than that reported for AB pretreated with ozone by Valdez-Vazquez et al. [20]. These results show that the type of pretreatment has different effect on the enhancement of the biodegradability and methane production potential from lignocellulosic substrates.
Table 5 presents the ERE values for the hydrolysates of BWP and BPO, which express the percentage of the energy contained in the bagasse (16.35 kJ/g bagasse) that was recovered. Table 5 shows that ERE value for BPO hydrolysate (64.13 ± 0.99%) was 2.60 times higher than that of BWP hydrolysate (24.88 ± 0.92%). This substantial increment in ERE can be attributed to the delignification process that enhances the biodegradability of the BPO hydrolysate.
The ERE value achieved in this study for the BPO hydrolysate was 64.13% which is lower than the value of 86.14% achieved by Galindo-Hernández et al. [15], who used alkaline peroxide hydrogen in AB, but 2.54 times higher than the value of 25.20% reported by Valdez-Vazquez et al. [20] for AB pretreated with ozone.
Overall, these results show that alkaline organosolv pretreatment allowed a more efficient energy recovery, higher than the obtained with the ozone pretreatment and lower than the one obtained with the alkaline hydrogen peroxide pretreatment.
Mass Balance
The mass balance for the pretreatment at the best conditions for the alkaline organsolv pretreatment and for the enzymatic hydrolysis is presented in Fig. 4. This figure shows that 40.10% of AB biomass was recovered after alkaline organosolv treatment, mainly due to the high delignification (84.56%) and high holocellulose removal (76.50% of hemicellulose and 45.60% of cellulose) during the delignification process. A lignin fraction of 2.06 g of lignin was recovered which represents 36.15% of initial lignin. Therefore, alkaline organosolv is not a selective pretreatment since not only removes lignin but polysaccharides, too.
The solid obtained after pretreatment was subject to enzymatic hydrolysis because its high content of holocellulose, 8.21 g. After enzymatic hydrolysis, 16.40 g of the total sugar was obtained in the hydrolysate in the form of monomeric sugars. Subsequently monomeric sugars available from the enzymatic hydrolysate were used to produce methane. The final methane production was 2.60 g CH4 per 29.80 g BWP. After enzymatic hydrolysis, the solid residue was composed of the non-hydrolyzed polysaccharides (0.57 g glucan and 0.47 g xylan), 0.70 g of lignin and 0.43 g of ash. Potentially, this solid enriched in lignin could be used to generate composite films or phenolic derivates in a biorefinery concept.
In a biorefinery scheme, alkaline organosolv process produces a lignin rich stream that corresponds to the liquid fraction after organosolv pretreatment. This liquid fraction is commonly called “black liquor.” In this stream, 4.82 g lignin per 29.80 g of BWP was present. After acid precipitation of lignin, 2.06 g is recovered as precipitated lignin with a composition of 74.83% of lignin and 21.83% of holocellulose. The high content of lignin in the precipitate is due to the high hemicellulose removal during the alkaline organosolv pretreatment.
This solid fraction has the potential to produce high value-added products such as binding agents, dispersing agents, emulsifying agents, sequestrant agents, hydrogels, micro and nano capsules, as well as a raw material for synthetic polymers such as phenol–formaldehyde resin, epoxy resin, polyurethane resin, and polyester [5, 6]. Delignified black liquor contain the non-precipitated lignin (2.76 g/29.8 g of BWP). Also, it contains a fraction of holocellulose (10.11 g/29.8 g of BWP). The black liquor was not chemically characterized. However, it is expected that in addition to lignin, monomers and oligomers of cellulose and hemicellulose are present, although the relative proportion of each type of compound is unknown. A complete valorization of the black liquor requires a further process to separate the remaining lignin. This delignified black liquor could be used to produce more methane, but the low pH of the black liquor probably will not allow anaerobic digestion.
Alkaline organosolv pretreatment investigated in this study presented better operational conditions to remove lignin as compared to acid catalyze and non-catalyzed organosolv, such as a shorter time and lower temperature process. Overall, from a biorefinery processing perspective, the obtained results are very important due to lignin recovery and the recovery of energy in the form of methane.
Conclusions
The present study showed that alkaline organosolv pretreatment is a highly efficient process to delignify the AB. Also, a fiber enriched in cellulose and in less proportion in hemicellulose was obtained for enzymatic hydrolysis. Indeed, the SYEH of the alkaline organosolv pretreated fiber increased 4.43 times as compared to the AB without delignification. In terms of methane production from the enzymatic hydrolysate, the use of alkaline organosolv pretreatment yields a more biodegradable enzymatic hydrolysate, which allowed a substantial increase in ERE, 2.6 times higher than in the absence of the pretreatment. These results demonstrate the effectiveness of the alkaline organosolv pretreatment in improving lignin removal and recovery, and energy recovery from delignified bagasse under more environmentally friendly conditions, since it does not require strong oxidizing agents such as ozone or hydrogen peroxide, and ethanol can be recovered and reused. It also demonstrates that alkaline organosolv pretreatment of agave bagasse has a high potential for a biorefinery approach.
Data Availability
Data supporting the results are available on request from the authors.
References
Tequila Regulatory Council (2022) URL: https://www.crt.org.mx/EstadisticasCRTweb/. Accessed 5Sept 2023
Huerta-Cardoso O, Durazo-Cardenas I, Marchante-Rodriguez V, Longhurst P, Coulon F, Encinas-Oropesa A (2020) Up-cycling of agave tequilana bagasse-fibres: a study on the effect of fibre-surface treatments on interfacial bonding and mechanical properties. Results in Materials 8:100158. https://doi.org/10.1016/j.rinma.2020.100158
Palomo-Briones R, López-Gutiérrez I, Islas-Lugo F, Galindo-Hernández KL, Munguía-Aguilar D, Rincón-Pérez JA, Cortés-Carmona MÁ, Alatriste-Mondragón F, Razo-Flores E (2018) Agave bagasse biorefinery: processing and perspectives. Clean Techn Environ Policy 20:1423–1441. https://doi.org/10.1007/s10098-017-1421-2
Monlau F, Barakat A, Trably E, Dumas C, Steyer J-P, Carrère H (2013) Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit Rev Environ Sci Technol 43:260–322. https://doi.org/10.1080/10643389.2011.604258
Stewart D (2008) Lignin as a base material for materials applications: chemistry, application and economics. Ind Crop Prod 27:202–207. https://doi.org/10.1016/j.indcrop.2007.07.008
Ponnusamy VK, Nguyen DD, Dharmaraja J, Shobana S, Banu JR, Saratale RG, Chang SW, Kumar G (2019) A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol 271:462–472. https://doi.org/10.1016/j.biortech.2018.09.070
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Lin H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206. https://doi.org/10.1016/j.fuproc.2016.12.007
Zhang H, Zhang J, Xie J, Qin Y (2020) Effects of NaOH-catalyzed organosolv pretreatment and surfactant on the sugar production from sugarcane bagasse. Bioresour Technol 312:123601. https://doi.org/10.1016/j.biortech.2020.123601
Zhao X, Li S, Wu R, Liu D (2017) Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymaticsaccharification: chemistry, kinetics, and substrate structures. Biofuels Bioprod Biorefin 11:567–590. https://doi.org/10.1002/bbb.1768
Fernández-Rodríguez J, Gordobil O, Robles E, González-Alriols M, Labidi J (2017) Lignin valorization from side-streams produced during agricultural waste pulping and total chlorine free bleaching. J Clean Prod 142:2609–2617. https://doi.org/10.1016/j.jclepro.2016.10.198
Caspeta L, Caro-Bermúdez MA, Ponce-Noyola T, Martinez A (2014) Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Appl Energy 113:277–286. https://doi.org/10.1016/j.apenergy.2013.07.036
Yuan W, Gong Z, Wang G, Zhou W, Liu Y, Wang X, Zhao M (2018) Alkaline organosolv pretreatment of corn stover for enhancing the enzymatic digestibility. Bioresour Technol 265:464–470. https://doi.org/10.1016/j.biortech.2018.06.038
Rabelo SC, Nakasu PYS, Scopel E, Araújo MF, Cardoso LH, da Costa AC (2023) Organosolv pretreatment for biorefineries: current status, perspectives, and challenges. Bioresour Technol 369:128331. https://doi.org/10.1016/j.biortech.2022.128331
Zhou Z, Lei F, Li P, Jiang J (2018) Lignocellulosic biomass to biofuels and biochemicals: a comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol Bioeng 115:2683–2702. https://doi.org/10.1002/bit.26788
Galindo-Hernández KL, Tapia-Rodríguez A, Alatriste-Mondragón F, Celis LB, Arreola-Vargas J, Razo-Flores E (2018) Enhancing saccharification of Agave tequilana bagasse by oxidative delignification and enzymatic synergism for the production of hydrogen and methane. Int J Hydrog Energy 43:22116–22125. https://doi.org/10.1016/j.ijhydene.2018.10.071
Velázquez-Valadez U, Farías-Sánchez JC, Vargas-Santillán A, Castro-Montoya AJ (2016) Tequilana weber agave bagasse enzymatic hydrolysis for the production of fermentable sugars: oxidative-alkaline pretreatment and kinetic modeling. Bioenergy Res 9:998–1004. https://doi.org/10.1007/s12155-016-9757-8
Tapia-Rodríguez A, Ibarra-Faz E, Razo-Flores E (2019) Hydrogen and methane production potential of agave bagasse enzymatic hydrolysates and comparative technoeconomic feasibility implications. Int J Hydrog Energy 44:17792–17801. https://doi.org/10.1016/j.ijhydene.2019.05.087
López-Gutiérrez I, Montiel-Corona V, Calderón-Soto LF, Palomo-Briones R, Méndez-Acosta HO, Razo-Flores E, Ontiveros-Valencia A, Alatriste-Mondragón F (2021) Evaluation of the continuous methane production from an enzymatic agave bagasse hydrolysate in suspended (CSTR) and granular biomass systems (UASB). Fuel 304:121406. https://doi.org/10.1016/j.fuel.2021.121406
Arreola-Vargas J, Flores-Larios A, González-Álvarez V, Corona-González RI, Méndez-Acosta HO (2016) Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int J Hydrog Energy 41:897–904. https://doi.org/10.1016/j.ijhydene.2015.11.016
Valdez-Vazquez I, Alatriste-Mondragón F, Arreola-Vargas J, Buitrón G, Carrillo-Reyes J, León-Becerril E, Mendez-Acosta HO, Ortíz I, Weber B (2020) A comparison of biological, enzymatic, chemical and hydrothermal pretreatments for producing biomethane from Agave bagasse. Ind Crop Prod 145:112160. https://doi.org/10.1016/j.indcrop.2020.112160
Aguilar DL, Rodríguez-Jasso RM, Zanuso E, de Rodríguez DJ, Amaya-Delgado L, Sanchez A, Ruiz HA (2018) Scale-up and evaluation of hydrothermal pretreatment in isothermal and non-isothermal regimen for bioethanol production using agave bagasse. Bioresour Technol 263:112–119. https://doi.org/10.1016/j.biortech.2018.04.100
Pedersen M, Meyer AS (2010) Lignocellulose pretreatment severity—relating pH to biomatrix opening. New Biotechnol 27:739–750. https://doi.org/10.1016/j.nbt.2010.05.003
Shiva R-JRM, Rosero-Chasoy G, López-Sandin I, Morais ARC, Ruiz HA (2023) Enzymatic hydrolysis, kinetic modeling of hemicellulose fraction, and energy efficiency of autohydrolysis pretreatment using agave bagasse. Bioenergy Res 16:75–87. https://doi.org/10.1007/s12155-022-10442-0
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. Technical Report NREL/TP-510-42618. Laboratory Analytical Procedure (LAP). https://www.nrel.gov/docs/gen/fy13/42618.pdf
López-Gutiérrez I (2015) Producción de hidrógeno a partir de hidrolizados de bagazo de Agave tequilana Weber var. azul: efecto del procesamiento de la piña y de la sacarificación del bagazo. M.Sc. Thesis. Instituto Potosino de Investigación Científica y Tecnológica, AC. 98 pages . https://repositorio.ipicyt.edu.mx/bitstream/handle/11627/2951/TMIPICYTL6P72015.pdf?sequence=1&isAllowed=y . Accessed 5 Sept 2023
López-Gutiérrez I, Razo-Flores E, Méndez-Acosta HO, Amaya-Delgado L, Alatriste-Mondragón F (2021) Optimization by response surface methodology of the enzymatic hydrolysis of non-pretreated agave bagasse with binary mixtures of commercial enzymatic preparations. Biomass Convers Biorefin 11:2923–2935. https://doi.org/10.1007/s13399-020-00698-x
APHA (2012) Standard Methods for the Examination of Water and Waste Water, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Laura Bridgewater
Michel DB, Gilles KA, Hamilton JK, Rebers PA, Fred S (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Abreu Sherrer J (2013) Aprovechamiento de bagazo de Agave tequilana Weber para la producción de bio-hidrógeno. M.Sc. Thesis. Instituto Potosino de Investigación Científica y Tecnológica, AC. 1–111 pages. https://repositorio.ipicyt.edu.mx/bitstream/handle/11627/86/AbreuSherrer.pdf?sequence=1&isAllowed=y . Accessed 5 Sept 2023
Gutiérrez Pulido H, De la Vara SR (2008) Análisis y diseño de experimentos, Segunda edn. McGraw-Hill Interamericana, México
Pérez-Pimienta JA, Vargas-Tah A, López-Ortega KM, Medina-López YN, Mendoza-Pérez JA, Avila S, Singh S, Simmons BA, Loaces I, Martinez A (2017) Sequential enzymatic saccharification and fermentation of ionic liquid and organosolv pretreated agave bagasse for ethanol production. Bioresour Technol 225:191–198. https://doi.org/10.1016/j.biortech.2016.11.064
Perez-Pimienta JA, Flores-Gómez CA, Ruiz HA, Sathitsuksanoh N, Balan V, da Costa SL, Dale BE, Singh S, Simmons BA (2016) Evaluation of agave bagasse recalcitrance using AFEXTM, autohydrolysis, and ionic liquid pretreatments. Bioresour Technol 211:216–223. https://doi.org/10.1016/j.biortech.2016.03.103
Perez-Pimienta JA, Poggi-Varaldo HM, Ponce-Noyola T, Ramos-Valdivia AC, Chavez-Carvayar JA, Stavila V, Simmons BA (2016) Fractional pretreatment of raw and calcium oxalate-extracted agave bagasse using ionic liquid and alkaline hydrogen peroxide. Biomass Bioenergy 91:48–55. https://doi.org/10.1016/j.biombioe.2016.05.001
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10. https://doi.org/10.1186/1754-6834-3-10
Liu KX, Li HQ, Zhang J, Zhang ZG, Xu J (2016) The effect of non-structural components and lignin on hemicellulose extraction. Bioresour Technol 214:755–760. https://doi.org/10.1016/j.biortech.2016.05.036
Esau K,(1985) Anatomia Vegetal. 3era Edición. Ediciones OMEGA, S.A., Barcelona. ISBN: 84-282-0169-2, p 168–320.
Meza-Maytorena LC (2018) Biorefinería del bagazo de Agave tequilana: extracción de lignina por diferentes pretratamientos y producción de metano. M.Sc: Thesis. Instituto Potosino de Investigación Científica y Tecnológica A.C. 1-100 pp. https://repositorio.ipicyt.edu.mx/bitstream/handle/11627/4779/TMIPICYTM4B52018.pdf?sequence=4&isAllowed=y. Accessed 5 Sept 2023
Buitrón G, Hernández-Juárez A, Hernández-Ramírez MD, Sánchez A (2019) Biochemical methane potential from lignocellulosic wastes hydrothermally pretreated. Ind Crop Prod 139:111555. https://doi.org/10.1016/j.indcrop.2019.111555
Acknowledgements
The National Council of Science and Technology awarded an academic scholarship (762620) to Lilia Chizelt Meza-Maytorena. Ana Iris Peña Maldonado carried out the SEM analysis at the National Laboratory for Nanosciences and Nanotechnology Research. Beatriz Adriana Rivera Escoto carried out the X-ray diffraction analysis at the National Laboratory for Nanoscience and Nanotechnology Research.
Funding
“This work was supported by the CONACYT-SENER-Energy Sustainability Sector Fund, CEMIE-Bio, Gaseous Biofuels Cluster 247006”. The Mexican Science and Technology Council (CONACYT, Mexico) is acknowledged for the Infrastructure Project—FOP02-2021-04 (Ref. 317250).
Author information
Authors and Affiliations
Contributions
LCMM: conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization. HAR: conceptualization, methodology, formal analysis, resources, writing—review and editing, supervision, funding acquisition. CND: formal analysis, writing—review and editing. VAEB: formal analysis, writing—review and editing. FPR: formal analysis, editing. FAM: conceptualization, methodology; formal analysis, resources, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 377 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meza-Maytorena, L.C., Ruiz, H.A., Nieto-Delgado, C. et al. Revalorization of Agave Bagasse by Alkaline Organosolv Treatment and the Effect of Delignification on Enzymatic Saccharification and Methane Production. Bioenerg. Res. 17, 245–258 (2024). https://doi.org/10.1007/s12155-023-10663-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10663-x