Abstract
Depleting fossil fuel resources such as crude oil and coal are responsible for greenhouse gas emissions and global warming. Hence, converting biogenic waste such as animal waste and agricultural and industrial residues to biogas (CH4 and CO2) using anaerobic digestion (AD) is a sustainable, renewable, and environmentally friendly way of producing fuel. This nature-derived bioconversion process provides energy security and additional environmental services such as waste management. Furthermore, the liquid and solid waste generated through the AD process could be used as soil amendments to increase fertility. However, natural recalcitrance of biomass pertaining to the intricate network of polysaccharides and lignin, high crystallinity of cellulose, and reduced accessible surface area are some of the major bottlenecks to utilizing these resources as received. Pretreatment helps open up the plant cell wall by disrupting the lignin carbohydrate complex, de-lignifying the biomass, aiding the enzymes to access the polysaccharides due to higher surface area efficiently, and hydrolyze them into simple sugars with the help of bacterial consortium during AD process. This review gives an overview of physical, chemical, biological, and combinatorial pretreatment methods of lignocellulosic substrates and their effect on AD process. Biological pretreatment has emerged as a more desirable pretreatment method in terms of environment safety and efficiency for lignin degradation. Though the higher pretreatment duration has been observed as the most significant challenge that need to be addressed for its adoption on commercial scale. Therefore, research is required to either explore the naturally occurring or prepare the genetically engineered microbes for selective degradation of lignin at faster rates and high tolerance for variation in environment factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global energy demands are continuously increasing. With the limited available fossil fuels resources, alternate sustainable renewable sources of energy are being explored [1]. Such energy includes wind, solar, hydro, geothermal, and biomass. In the previous decade, the installed capacity of renewable energy resources has increased continuously and reached 2,537 GW globally by the end of 2019 as per International Renewable Energy Agency (IRENA) (Fig. 1) [2]. Among biomass resources, lignocellulosic biomass has particularly garnered research interest in the past two decades as a potential renewable energy source due to its affordability and abundance in nature [3,4,5]. Furthermore, utilizing lignocellulosic biomass substrates for energy generation may increase energy security and sustainable economic growth and benefit the environment by efficiently managing the waste materials [6, 7].
Biogas is a mixture of gases, primarily consisting of methane and carbon dioxide can be generated from lignocellulosic biomass through the anaerobic digestion (AD) process. In this process, organic matter present in biomass is converted into biogas using a consortium of bacteria, including methanogens [8,9,10]. Utilizing the biogas generated from lignocellulosic waste is more advantageous since this process offers waste management and ecological and environmental benefits. Recently, agriculture residues like wheat straw (WS), rice straw (RS), corn stover (CS), sorghum, and millet have gained immense attention for energy production due to their abundant availability [11]. Though some of the agricultural residues, including straw, are primarily used as animal feed and as bedding material for livestock, most of them are allowed to burn in an open environment contributing to increased particulate matter in the air and smog. Particularly in India, around 500–550 million tons (Mt) of crop remains are accumulated annually, opening up an opportunity to produce biogas that could offer sustainable renewable energy sources [12].
Lignocellulosic biomass has three key chemical constituents: cellulose, hemicellulose, and lignin [8, 11]. Out of the three constituents, lignin is the most resistant for enzymatic degradation, which serves as a barrier for the hydrolytic enzymes of anaerobic microorganisms during AD to access the cellulose. Therefore, a pretreatment step before AD helps overcome this problem by making the biomass more readily accessible by hydrolytic enzymes to simple sugars [13]. The present review is a comprehensive compilation of multidisciplinary pretreatment strategies for augmenting biogas production, emphasizing biological pretreatment. This review will outline the structural units of lignocellulosic biomass and the challenges imposed by these components during biomass conversion to methane. The pretreatment section reviews different types of pretreatments adopted in the past decade, including physical, chemical, and biological methods. Later, the biological pretreatment and how an enzyme helps overcome some of the biomass recalcitrance have been discussed. Additionally, the review also highlights the role of genetic engineering of some fungi to overexpress ligninolytic enzymes in the secretome to raise the efficacy of bio-delignification to overcome biomass recalcitrance while producing biogas.
Composition and Structure of Lignocellulosic Biomass
Cellulose, hemicellulose, and lignin are the three main chemical compositional units of lignocellulosic biomass, while other minor components include pectins and proteins extractives, and ash. Figure 2 shows the structural units of cellulose, hemicellulose, and lignin. Celluloses is a homopolymer consisting of only hexose monomers (Glucose). On the other hand, hemicellulose is a heteropolymer which can be comprised of mixture of hexose and pentose monomers. Lignin is also a heteropolymer consisting of three types of monomers: coniferyl, coumaryl, and sinapyl alcohol (Fig. 2C). Cellulose is a polysaccharide comprised of d-glucose monomer units connected through β-(1–4) glycosidic bonds and bundled together to form microfibrils. Cellulose characteristics and recalcitrance are governed by the chain length, crystallinity, and extent of polymerization. Majority of cellulose in biomass is organized as a crystalline structure, while a small portion is arranged in an irregular amorphous structure. Amorphous cellulose is more easily degradable when compared to the crystalline counterpart due to cellulose's high hydrophilic properties, which aids its interaction with water molecules [14].
The backbone chain of hemicellulose can be formed from mixture of different monomer units (hetero-polymer). For example, the hemicellulose of hardwood trees comprises a xylan backbone with a minor amount of glucomannan and arabinoxylan. On the other hand, the hemicellulose of softwoods comprises a galacto-glucomannan backbone with a small amount of xylan. The lateral side chains in hemicelluloses are linked to the backbone polymer chain through β-1,4-glucan and β-1,3-glucan bonds [15].
Lignin is a hetero-polymer comprising of three phenyl propane units: (i) coniferyl alcohol (guaiacyl alcohol) (ii) coumaryl alcohol (p-hydroxyphenyl alcohol) (iii) sinapyl alcohol (syringyl alcohol) (Fig. 2c) [16]. The composition of the monomer units differs as per the plant species, maturity, etc. For example, lignin from softwoods comprises only coniferyl monomer; hardwood lignin comprises both coniferyl and sinapyl units, while herbaceous plants have all three monomer units [16].
The percentage of the three major components, i.e., lignin, cellulose, and hemicellulose, can vary depending upon the maturity and type of plant. Therefore, the percentage composition of some representatives of hardwood, softwood, and crop residues is provided in Table 1.
Anaerobic Digestion: Process and Parameters
AD is the process operated by a consortium of microorganisms that degrades the organic substrate in anaerobic conditions and produces methane as the final product. Since the AD process provides a good amount of biomass without major adverse environmental impact when properly managed, it may be considered one of the most competent methods of transforming waste into energy [25]. The four stages of AD process are as follows: (1) enzymatic hydrolysis, (2) acidogenesis, (3) acetogenesis, and (4) methanogenesis [26, 27]. In the first step of hydrolysis, complex organic compounds present in lignocellulosic biomass are degraded to form simple organic compounds by hydrolytic enzymes produced by the microorganisms. Here, the polysaccharides are transformed into monosaccharides, while proteins and lipids are respectively converted into amino acids and fatty acids [26, 28]. The second step is acidogenesis, where simple organic compounds are utilized by fermentative bacteria and converted into volatile organic acids (i.e., acetic acid, propionic acid, butyric acid), alcohols, ketones, aldehydes, CO2, H2, etc. [26, 29]. Both obligate and facultative anaerobes are active during acidogenesis. The third step is acetogenesis, where volatile acids and alcohols are used to produce acetate via two routes, namely hydrogenation and dehydrogenation reaction [28]. During acetogenic hydrogenation, CO2 and H2 are combined to form acetate. While in the regular hydrogenation process, volatile fatty acids are oxidized anaerobically to produce acetate [28]. The fourth step is methanogenesis. In this stage, methanogens utilize acetate, CO2, and H2 and convert them to methane and CO2. The acetoclastic methanogens use acetate as substrate and convert it into methane and CO2 [30]. On the other hand, the hydrogenotrophic methanogens use hydrogen and CO2 to produce methane [30]. The major families of bacteria and archaea involved in the different stages of AD are depicted in Fig. 3.
The key factors that affect the activity of microorganisms involved in these four phases are pH in digester, chemical oxygen demand, alkalinity, concentration of ammonia, volatile fatty acids, and micronutrients [31]. These factors further depend on and are regulated by process parameters, including temperature, total solids concentration, substrate particle size, mixing, C/N ratio, inoculum to feed ratio, hydraulic retention time, and organic loading rate [31].
Recalcitrance of Lignocellulosic Biomass
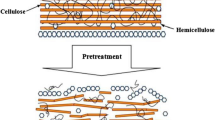
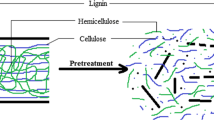
The hydrolyzability of lignocellulosic biomass depends on the percentage composition of the constituents and recalcitrance [6, 31]. While some plants cell walls are less lignified and consist relatively ‘easy to digest’ (less recalcitrant), other plant cell walls are highly lignified, resulting in ‘too tough to digest’ (highly recalcitrant). During the AD hydrolysis step, less recalcitrant plant cell walls are hydrolyzed first to produce methane leaving behind recalcitrant plant cell walls that are inaccessible by enzymes due to high lignin content [6, 8]. To make the recalcitrant plant cell wall more readily degradable and accessible by hydrolytic enzymes, the biomass needs to undergo pretreatment either by physical, chemical, or biological methods. Other physical characteristics of cellulose (viz. distribution of amorphous and crystalline portions, pore size, surface area, etc.) also influence the rate of hydrolysis (Fig. 4) [8, 26]. Amorphous cellulose, increased pore size, and surface area are accompanied by high hydrolysis efficiency when compared to crystalline cellulose that are highly recalciatrant. Hence, lignocellulosic biomass with a high crystallinity index is often less readily degradable [32].
Pretreatment decreases the resistance of biomass for enzymatic degradation by increasing its surface area and pore size and improving the accessibility of cellulose and hemicellulose to enzymes. Hence, higher methane yields are reported using the pretreated biomass [1, 21, 32].
Pretreatment
Pretreatment helps to cleave the lignin carbohydrate complex (LCC) and improve biomass digestibility, which is essential for producing higher biogas generation during the AD process [13]. Different types of pretreatments viz. physical, chemical, physio thermal, thermochemical, and biological have been reported in the literature. Overall, pretreatment facilitates enzymatic hydrolysis by de-lignifying the biomass, reducing the crystallinity, increasing the porosity, and enabling the enzymes to access carbohydrates more efficiently (Fig. 5) [13].
Physical Pretreatment
Physical or mechanical pretreatments viz. grinding, milling, and freezing have been designed to reduce the particle size and degree of polymerization and loosen the compact arrangement of cellulose fibers in lignocelluloses. Particle size reduction may be achieved through the milling or grinding techniques carried out by employing ball, knife, hammer, two-roll, extruders, colloid, and attrition, as shown in Fig. 6 [31]. The appropriate physical pretreatment method depends upon the percentage of moisture present in the substrate. Dry biomass (moisture content < 15%) can be effectively comminuted by two-roll, hammer, attrition, and knife mills, while wet disk milling, ball milling, and colloid mills are appropriate for comminuting wet biomass (moisture content > 15%) [31]. On the other hand, vibrio and ball milling may be employed for dry and wet biomass. Grinding also decreases the particle size, thereby improving the digestibility of straw pertaining to the increased surface area. However, none of the physical or mechanical pretreatment methods removes lignin; hence, a significant increase in methane production is needed [31, 33]. Therefore, the physical pretreatment methods are generally coupled with other pretreatment methods.
Kim et al. [34] examined the impact of planetary and attrition milling process on crystallinity and enzyme saccharification of RS. The reduction in percentage crystallinity was observed for RS after planetary milling and attrition milling from 48 to 11% and to 33%, respectively, which improved the enzymatic saccharification due to the increased accessibility of enzymes. The extrusion method also involves shearing, mixing, and heating biomass [35]. The extrusion treatment reduces particle size and crystallinity along with a larger surface area due to shearing forces [36]. Chen et al. [35] reported a 72% rise in methane yield after subjecting the RS to extrusion pretreatment.
In microwave treatment, biomass is exposed to microwaves for a certain time—the heat energy results in increased solubilization of organic material in biomass. The radiation intensity, exposure time, and substrate composition are the controlling factors during microwave treatment [37]. Numerous studies are previously undertaken to inspect the impact of different physical pretreatment on agricultural residues (Table 2). Jackowiak et al. [38] optimized the microwave pretreatment for WS at 150 and 180 °C to increase solubilization and enhance biodegradability during AD. Due to improved soluble chemical oxygen demand, the treated biomass led to amplified methane production (28%) when compared to untreated WS. Sapci [37] investigated microwave pretreatment at higher oven temperatures (200 and 300 °C) compared to Jackowiak et al. [38] to determine its effect on biomass digestibility during AD under mesophilic conditions. However, no significant impact on methane yield was detected at raised temperatures (200 to 300 °C). A possible reason for such low methane yield may be accredited to generating heat-induced inhibitors viz. phenolic compounds and furfural at higher temperatures.
Steam explosion involves exposing the lignocellulose to high-pressure steam for a short duration, followed by abrupt de-pressuring that results in shearing of lignocellulosic biomass, resulting in higher methane production [39,40,41]. On the other hand, freeze treatment disrupts the hydration layer during the freeze–thaw cycle and crystal formation during freezing, leading to the structural breakdown in lignocellulosic materials. Chang et al. [42] evaluated the outcome of freeze pretreatment on digestibility of RS, which was reported to increase from 48 to 84%. In addition, the reducing sugar yield attained after enzymatic digestion of RS exposed to freeze treatment was also reported to be higher (417 g kg−1) when compared to its untreated counterpart (227 g kg−1).
Though the physical pretreatment has the efficiency to decrease the particle size and crystallinity of biomass and growing surface area in a short time span, it is not feasible due to high energy consumption. In some cases, physical pretreatment generates heat-induced inhibitors, which are not desirable for downstream processing.
Chemical Pretreatment
In chemical pretreatment, chemicals (acids, alkali, ionic liquids, organic solvents, ozone etc.) are employed to disrupt the interactions between macromolecules of lignocellulosic materials. H2SO4 is the most widely explored biomass pretreatment process as it is inexpensive, easily available, and effective in pretreating the lignocellulosic biomass under mild treatment conditions of temperature, pressure, concentration, and treatment duration [46, 47]. Alkali treatment (mostly NaOH) also causes the breaking of the ester and glycosidic bonds, resulting in variations in lignocelluloses' structure [18]. Organosolv pretreatment uses organic solvents viz. methanol, tetra hydro furan, acetone, ethanol, and N-methyl morpholine N-oxide (NMMO). Ammonia fiber explosion (AFEX), CO2 explosion, and wet oxidation are other common examples of physicochemical pretreatments. AFEX encompasses treating the substrate using ammonia in liquid or gaseous form with (called extractive ammonia pretreatment or EA) or without extraction step to remove recalcitrant lignin molecule [48,49,50,51]. Both ammonolysis and hydrolysis occurring during pretreatment result in cleavage of ester cross-linkages between the cell wall components, producing respective organic amides (acetamide, feruloylamide, etc., p-coumorylamide) or organic acids (acetic acid, ferulic acid, p-coumaric acid), respectively. Solubilized lignin degradation produced during ammonia pretreatment gets relocated to the surface when ammonia is vented from the reactor. A 3- to fivefold upsurge in sugar conversion was reported after AFEX or Extractive ammonia (EA) pretreatment [52]. The lab-scale studies that were undertaken to inspect the impact of chemical pretreatment on agricultural residues have been discussed in Table 3.
McIntosh et al. [53] studied the outcomes of varying parameters during alkaline pretreatment, including pretreatment time, temperature, and alkali concentration on WS. Diluted sodium hydroxide solutions of 0.75, 1.0, and 2.0% concentrations were used for pretreatment at 60 °C and 121 °C. A 6.3-fold increase in enzymatic saccharification was attained after pretreatment with 2% NaOH (30 min, 121 °C), while treatment with 2% NaOH (90 min, 60 °C) resulted into 4.9-fold increase. The enhanced saccharification can be attributed to the disruption of ester bonds which covalently connect the lignin and xylan. Taherdanak et al. [54] investigated alkaline pretreatment of wheat plants including grains and straw using 8% NaOH solution (w/v) at varying temperatures ranging from 0 to 100 °C. The peak cumulative methane yield was attained from pretreatment at 75 °C for 60 min, 54% more than the methane yield attained from untreated biomass. The rise in methane yield may be due to effective lignin and hemicelluloses removal along with a reduction in crystallinity.
Zhao et al. [55] treated RS with a mixture of propionic and acetic acid and investigated processing parameters viz. concentration of acids, residence time, and solid–liquid ratio. Performing batch AD of untreated and treated biomass gave ~ 36% rise in methane yield for pretreated RS due to 35% of lignin removal. Kim et al. [56] explored a two-stage method for pretreating the RS by employing aqueous ammonia trailed by sulfuric acid (4% w/w) pretreatment. The comparison of single and two-stage pretreatment revealed higher glucose release for the later one, providing maximum digestibility of 96.9%.
Reilly et al. [57] studied the impact of calcium hydroxide (7.4% w/w, 48 h) pretreatment on WS in terms of biochemical methane potential. They reported a threefold rise in methane potential was achieved after 5 days of AD. The enhanced methane potential was attributed to the measured increase of soluble chemical oxygen demand after pretreatment due to hemicellulose degradation. Increased soluble chemical oxygen demand reduced hydrolytic activity in the initial stage of AD. Liu et al. [58] explored the impact of potassium hydroxide on the chemical structure, composition, and enzymatic degradation of WS. When WS was treated with KOH solutions ranging between 2 to 50% for 24 h and a positive relation was observed between lignin degradation and KOH concentration. The highest lignin removal of 54.7% was attained at 50% KOH pretreatment due to disruption of ester and glycosidic bonds, which led to a 77.1% rise in methane yield.
Solé-Bundó et al. [59] studied anaerobic co-digestion of microalgae biomass and WS in the presence and absence of alkali catalyzed thermochemical pretreatment. Alkaline pretreatment with 10% CaO (75 °C, 24 h) resulted in a 15% rise in methane production compared to the untreated substrate, which was accredited to the upsurge of carbohydrates and protein solubilization after pretreatment.
Xiong et al. [60] investigated pretreatment of RS with Fenton reagent [hydrogen peroxide (H2O2) with ferrous iron as a catalyst], standalone ultrasound, and ultrasound-assisted Fenton pretreatment. The results revealed that the quantity of reducing sugars released after ultrasound-assisted Fenton pretreatment was 4 and 1.5 times higher when compared to untreated straw and Fenton reagent pretreated straw without ultrasound. The analytical studies revealed that the ultrasound pretreatment disrupted the crystalline structure of cellulose and increased the pore volume and surface area by the cavitation effect. On the other hand, Fenton reagent preferentially degraded hemicellulose and lignin by generating hydroxide free radicals.
A recent study explored the application of urea for corn straw pretreatment [61]. The study also justified the use of urea as a pretreatment chamical for its capability to simultaneously adjusting the C/N ratio of the substrate. The study reported 7% lignin reduction which led to 23.9% higher methane yield compared to the untreated corn straw.
As discussed before, the cost factor related to reagents and inhibitory molecule formation are the key drawbacks associated with the chemical pretreatment process. These drawbacks were the major drivers for researchers to explore other greener methods, such as biological pretreatment methods [11]. Also, most of the non-volatile chemical (acid, alkali, or ionic liquid) used in pretreatment are expensive to recover and are left behind in the biomass, which has to be neutralized using acid or based before AD. This results in the formation of high salt concentration, which will impact anaerobic microbes, the biogas yield, and downstream processing of wastewater treatment after AD process. In that respect, using volatile chemical during pretreatment, as in the case of AFEX, could be advantageous since 97% of ammonia could be removed, leaving behind pretreated biomass with residual chemicals. Also, removing recalcitrant lignin, as in the case of the EA process, could positively impact the AD process.
Biological Pretreatment
While physical pretreatment methods require a significant amount of energy, the chemical methods produce toxic by-products that could inhibit the hydrolysis process during AD [10]. These shortcomings have motivated many researchers to explore the biological methods of pretreatment, which are environment-friendly and economically feasible [10]. Biological pretreatment employs the ligninolytic enzyme system produced by fungi (white and brown-rot fungi) and bacteria, which degrade the lignin in biomass and simultaneously hydrolyze cellulose and hemicellulose [11, 69]. Since brown rot fungi largely degrade the cellulose and hemicellulose fractions of lignocellulosic biomass, there are no preferred organisms for biological pretreatment [11].
The ligninolytic enzymes are omnipresent in different organism’s viz. bacteria, fungi, and insects [70]. In bacteria, three groups consisting of actinomycetes, α-proteobacteria, and γ-proteobacteria are known to possess the ability to degrade lignin. Streptomyces viridosporus, Thermobifida fusca, and actinomycetes species exhibited lignin peroxidase activities while Azospirillum lipoferum, Bacillus subtilis, Marinomonas mediterranea, Thermus thermophilus, and Streptomyces cyaneus exhibited the laccase activity [71]. In insects, ligninolytic enzymes are primarily involved in the sclerotization and pigmentation of cuticles and the oxidation of toxic compounds [72].
Lignin breakdown is exhibited by a different class of fungi: ascomycetes, basidiomycetes, and Deuteromycetes. White rot fungi principally belong to basidiomycetes and partly to ascomycetes. They secrete extracellular enzymes comprising of hydrolytic and ligninolytic enzymes (Fig. 7) for degrading lignocelluloses [73]. Hydrolytic enzymes are categorized as exo-, endo-cellulase, and β-glucosidases. The ligninolytic enzyme system comprises laccase, manganese peroxidase (MnP), lignin peroxidase (LiP), and versatile peroxidase (VP) [74, 75]. Table 4 summarizes the target substrates for these enzymes in lignocellulosic biomass and their mode of action to carry out the biomass degradation. However, white-rot fungi do not secrete all the enzymes mentioned above. They possess either one of the three or the combination of two of the enzymes. Ruttimann-Johnson et al. [76] could isolate only LiP and MnP from Phanerochaete chrysoporium with no laccase activity. Rajakumar et al. [77] detected the production of MnP and laccase in Ceriporiopsis subvermispora, but no LiP.
Dashtban et al. [16] explained the enzymatic process of lignin degradation by ligninolytic enzymes. Laccase, MnP, LiP, and VP, which cannot pierce the cell wall, oxidize small intermediates that catalyze electron extraction for lignin aromatic rings. The reaction generates free radicals that cleave the cell wall, thus facilitating the entry of fungal hyphae in the lignin matrix (Fig. 8).
Laccases (E.C. 1.10.3.2) belong to the class multicopper oxidase, having 4 Cu molecules in its active site to employ oxygen as an oxidizer and degrade the polyphenol structure of lignin. Out of 4 Cu atoms, one is paramagnetic Type-I Cu, the oxidation site for the reducing substrate [72]. The other three copper atoms (one atom of Type-II and two atoms of Type-III Cu) create a trinuclear cluster where oxygen reduction takes place to form two molecules of water. Laccase is competent to degrade both phenolic and non-phenolic units in lignin. During the oxidation of phenolic structures, phenoxy radicals are generated, resulting in ketones production via alkyl aryl cleavage or α-β cleavage. Laccase may also oxidize the non-phenolic structures of lignin with the assistance of mediators such as hydroxyl benzotriazole [78]. Laccases exist in bacteria, plants, fungi, lichens, etc. While bacterial laccases are mostly intracellular, fungal laccase is extracellular with different glycosylation degrees. In bacteria, laccases participate in various mechanisms, including morphogenesis, oxidation of toxic compounds, and pigmentation [79]. In fungi, the major role of laccase is related to host–pathogen interaction and lignin degradation.
Enzyme LiP (E.C. 1.11.1.14) is a monomeric heme protein that oxidizes the nonphenolic structures in lignin and its analogous compounds. The structure of LiP consists of a central Fe+3 atom having coordination bonds with four heme tetrapyrroles and a histidine residue. LiP degrades the non-phenolic component of lignin through an oxidization reaction mediated by hydrogen peroxide form phenoxy radicals which are decomposed subsequently through α-β cleavage. LiP is transformed to LiP-I having Fe + 4 and a free radical on a tetrapyrrole ring in hydrogen peroxide. LiP-I oxidizes the donor substrate (lignin) into a radical cation and converts it into LiP-II having Fe+4 only (no free radical on tetrapyrrole ring) [78].
Enzyme MnP (E.C.1.11.1.13) catalyzes the chemical reaction that degrades both phenolic and non-phenolic units of lignin in the presence of Mn+2. MnP is more widely spread in fungi than LiP and reported to be found in many species such as P. chrysosporium, Trametes sp., P. ostreatus, and other families of Coriolaceae, Polyporaceae, etc. The structure of MnP consists of one molecule of heme (iron protoporphyrin), three sugar residues, 357 amino acids, and two calcium ions [78]. The presence of hydrogen peroxide initiates the catalytic cycle, oxidizing Mn2+ to Mn3+, which converts the phenol rings to phenoxy radicals disintegrating lignin [80].
Since biological pretreatment using fungi is an economical and environment-friendly process to augment the enzymatic degradation of lignocellulosic residues, this area of research has attracted many researchers in recent years. Numerous studies have been undertaken to examine the impact of fungal pretreatment on biomass (Table 5). Ghosh and Bhattacharyya [81] reported a 32% rise in methane production by implementing fungal treatment of RS using Polyporus ostreformis. Coriolus versicolor was investigated by Phutela et al. [82] for pretreating RS. The pretreated RS resulted in lignin removal (19.1%) with enhanced biogas production (26.2%). Recently, some researchers have employed co-culturing of fungal isolates instead of cultivating pure colonies of isolates. Rastogi et al. [83] investigated pretreatment of RS with fungal monoculture such as Pyrenophora phaeocomes and reported high laccase, xylanase, and mannanase production after four days of solid-state fermentation. P. phaeocomes, when grown on RS for 40 days, resulted in 63% degradation of lignin and 51% removal of hemicellulose. The enzymatic hydrolysis of this pretreated straw showed 50% saccharification efficiency, which resulted in 470 mg g−1 released sugars.
Simultaneous pretreatment and enzyme saccharification were studied in rice husk by Potumarthi et al. [84]. The pretreatment was performed by growing P. chrysosporium on sterilized rice husk with 60 to 70% moisture. The highest concentration of reducing sugar was witnessed on the eighteenth day of pretreatment. Similarly, the treatment of WS using P. chrysosporium was explored by Singh et al. [85]. The study reported 30% lignin loss within three weeks of pretreatment.
Wan and Li [86] examined the effect of fungal treatment on CS, switchgrass, and wood. The study reported a two to three-fold rise in released reducing sugar compared to the control samples. Song et al. [87] reported 43.8% lignin degradation after pretreating CS feedstock with the fungal consortium for 42 days. In addition, the pretreated CS was reported to have a seven-fold increase in saccharification efficiency compared to untreated CS. Taha et al. [88] reported a sevenfold rise in enzyme saccharification by co-culturing fungal consortia. The study also revealed that the activity of enzymes from fungal isolates was twofold higher than the enzyme cocktail obtained from a bacterial consortium.
Anaerobic rumen fungi have also been suggested for simultaneous pretreatment and bioaugmentation during AD. These fungi are stated to enhanced the methane yield in two ways: (i) releasing holocellulose or reducing sugars from lignocelluloses and (ii) by catabolizing acetate and formate during AD to assist the methanogens in higher methane production [89]. However, there is a scarcity of studies regarding the anaerobic fungi for biological pretreatment [89].
Other than fungi, the lignolytic bacteria have also been employed for degradation of lignocellulosic biomass. In a recent study, Comamonas testosteroni, Agrobacterium sp., Lysinibacillus sphaericus, and Paenibacillus sp. were investigated to degrade oil palm empty fruit bunches for enhancing the methane yield [69]. The study reported highest lignin degradation of 25.8% by Lysinibacillus sphaericus. Though the observed lignin degradation is lesser in comparison to the degradation achieved in earlier reports of fungi pretreatment. However, the 25.8% ligning degradation by using lignolytic bacteria was achieved in 7 days which is much shorter duration than the 30 to 40 days of fungal pretreatment.
Combinatorial Pretreatment Strategies
There are also reports of employing combinatorial strategies for biological treatment alongside physical and thermochemical treatments (Table 6). Since every pretreatment method has some drawbacks, two or more pretreatment may be coupled to enhance the substrate degradability and overall methane yield through their synergistic effects. Yu et al. [97] carried out two-step pretreatment involving physical (ultrasound) and chemical pretreatment (2% H2O2), respectively, followed by biological pretreatment with P. ostreatus. Combined treatment of rice hulls assisted in decreasing the pretreatment time to 18 days compared to the sole treatment by P. ostreatus for 60 days. Ma et al. [98] had implemented acid pretreatment with 0.25% H2SO4 followed by biological treatment with Echinodontium taxodii. The coupled pretreatment was more efficient than acid pretreatment alone, leading to a 1.3 to 2.1-fold rise in the yield of reducing sugars. Zhang et al. [99] explored the coupling of steam explosion and fungal treatment of RS. In this study, RS pretreated with a steam explosion was further exposed to P. chrysosporium resulting in 50% lignin degradation after ten days of treatment. This was significantly faster than the lignin removal obtained with fungal treatment alone.
Mustafa et al. [107] examined the coupling of milling and fungal pretreatment in terms of its impact on the methane production from AD of RS. Milled RS of < 2 mm size was treated with P. ostreatus for varying 10, 20, and 30 days durations. A surge in methane yield up to 165% was attained after pretreating straw with P. ostreatus for 30 days compared to untreated straw. Guan et al. [108] inspected the coupling of chemical and biological treatment for RS, which was pretreated with a mixture of CaO and liquid fraction of digestant (LFD) for 5 days. Results revealed 21% higher removal of lignocellulosic components after CaO-LFD treatment with 57% higher methane yield. Si et al. [109] also investigated the combination of chemical and bacterial treatment for RS. In this study, RS treated with acid was exposed to ligninolytic bacterium Pandoraea sp. B-6 for degrading the remaining lignin. Alternatively, Acinetobacter sp. B-2 was utilized for treating the alkali-treated RS. The research outcomes revealed a 41% and 32% rise in sugar release for acid/ Pandoraea and alkali/ Acinetobacter combinations, respectively. Balan et al. [111] employed P. ostreatus for pretreating RS trailed by AFEX treatment which led to a rise in glucan and xylan conversions compared to RS pretreated with AFEX only [111]. After harvesting mushrooms, the spent substrate is discarded in the mushroom industry, which is an excellent biologically pretreated substrate for producing biogas. Using such industrial waste for producing biomass is more advantageous when compared to performing biological pretreatment steps exclusively for the anaerobic digestion process.
Martínez-Patiño et al. [112] studied fungal pretreatment of olive tree biomass with I. lacteus for 28 days. The fungal-treated biomass was further exposed to acid pretreatment with 2% H2SO4 (w/v) (90 min, 130 °C). The fungal treatment coupeled with acid pretreatment resulted in a 34% increase in enzymatic degradation of biomass.
Genetic Engineering of Fungi for Improving Bio-delignification
The efficiency of bio-delignification and improved degradability of lignocellulosic substrate relies upon the activity of lignocellulolytic enzymes. The engineering of fungal strains producing a high quantity of lignocellulolytic enzymes can prove to be a low-cost method for improving bio-delignification [113]. In this regard, genetic engineering techniques may be used to modify the genetic makeup of fungi for more efficient production of enzymes degrading the complex organization of lignocellulosic constituents. The genes responsible for the production and secretion of enzymes may be targeted along with the genes involved in the cellular processes such as transcription, folding, and secretion of enzymes (Fig. 9). Furthermore, genetic modifications for improving the substrate utilization pathway can also enhance the capability of fungi to grow in minimal and inexpensive mediums [114].
The first strategy for genetic manipulation is to improve the transcription efficiency of enzyme coding genes. The transcriptional regulation of enzymes involves numerous activators and suppressors and their combinations [115]. Overexpressing the activators or inactivating the suppressors may result in enhanced levels of enzyme production. This strategy has been effectively implemented for augmenting the production of cellulase in Trichoderma reesei, Myceliophthora thermophila, and Penicillium oxalicum. xyr1 and clr2 are the most targeted activators, while cre1, res1, and ace1 are the targeted cellulase repressor. Wang et al. [116] achieved a 1.35-fold rise in cellulase production by Trichoderma reesei after overexpression of xyr1 and the downregulation of ace1. Similarly, Yao et al. [117] investigated the overexpression of clr2 along with downregulation of cre-A in P. oxalicum and observed a 27-fold upsurge in cellulase production. Liu et al. [118] observed a ninefold rise in cellulase production in M. thermophila by downregulating cre1, res1, along with gh1 and alp1.
Manipulation of genes involved in post-translational modification, protein folding, and secretory pathways of lignocellulosic enzymes may also lead to altered levels of enzyme production in fungi. Recently, Gao et al. [119] targeted HAC-1 responsible for regulating protein folding in T. reesei. Overexpression of hac-1 led to a noteworthy rise in cellulase production T. reesei. Moreover, manipulation of chromatin remodeling factors such as lae1 having a major role in gene expression may also be explored for altering the production of lignocellulolytic enzymes. For example, the deletion of laeA gene in P. oxalicum caused reduced production of cellulase and xylanases, which suggested the possibility of reversed outcomes in case of overexpression of these factors (Li et al.) [120].
In addition to manipulating the transcriptional regulators, chimeric regulators can also be designed and screened for improved lignocellulolytic enzymes. The chimeric regulators with engineered functional domains can be designed by incorporating new effector and DNA binding domains. Zhang et al. [121] engineered a more effective T. reesei by employing an artificial activator comprising ace1 DNA binding domain and vp16 effector domain. The engineered strain showcased improved production of cellulase and xylanase. Novel genes for producing chimeric laccases with improved efficiency were also prepared by employing DNA recombination methods. Nakagawa et al. [122] recombined cDNA of two dissimilar laccases from Lentinula edodes (< 60% identity). The engineered chimeric laccase showcased expression levels similar to the wild type laccase and intermediate temperature and pH profiles lying between both wild type laccases. Bleve et al. [123] recombined ery4 and ery3 isoforms of laccases from P. eryngii, resulting in chimeric laccase with improved activity and thermal stability. Though there are several advantages of producing higher biogas using genetically modified organisms, there are practical difficulties in using such organisms on a large scale due to regulatory approvals.
Conclusion and Future Perspectives
Biological pretreatment aids in de-polymerizing lignocellulosic biomass, which sequentially increases the enzymatic saccharification. Despite having numerous advantages over physical and chemical methods, biological pretreatments are relatively slow and still need to address various challenges. Biological pretreatment has been suggested as a cost-effective technique compared to other pretreatments. However, longer residence time requires more floor space to carry out the biological treatment. Also, sterile conditions are required to grow microorganisms that lead to high operational costs. Using mushroom-spent substrate could overcome this problem since the revenue generated using mushroom cultivation will offset substrate conditioning and sterilization costs. In addition, the biogas generated could be locally used, which further reduces the transportation cost. Other advantages of biological treatment include genetically engineered microorganisms, paving the way for developing new fungal and bacterial strains for selective lignin degradation for improved biogas conversion. Exploring the the naturally occurring anaerobic fungi or genetically engineer is also a new direction of research to employ fungal pretreatment simultaneously with bioaugmentation of AD. However, there are environmental regulatory challenges while using genetically modified organisms in the AD process. Therefore, more research is needed to optimize combinatorial strategies of biological treatment with other pretreatment methods that will result in a better outcome. Due to the much faster growth rate compared to fungi, lignolytic bacteria should be explored more intensively as they have the capability to address the major challenges of fungal pretreatment such as longer pretreatment time and low tolerance to environmental factors.
Abbreviations
- AD:
-
Anaerobic digestion
- WS:
-
Wheat straw
- RS:
-
Rice straw
- CS:
-
Corn stover
- Mt:
-
Million tons
- NMMO:
-
N-Methyl Morpholine N-oxide
- AFEX:
-
Ammonia fiber explosion
- EA:
-
Extractive ammonia pretreatment
- LiP:
-
Lignin peroxidase
- MnP:
-
Manganese peroxidase
- VP:
-
Versatile peroxidase
References
Vadenbo C, Tonini D, Astrup TF (2017) Environmental multiobjective optimization of the use of biomass resources for energy. Environ Sci Technol 51(6):3575–3583
Renewable Capacity Statistics (2018) International Renewable Energy Agency (IRENA), 2018 (https://www.irena.org/publications/2018/mar/renewable-capacity-statistics-2018)
Balan V (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. 1-32 ISRN biotechnology
Callegari A, Bolognesi S, Cecconet D, Capodaglio AG (2019) Production technologies, current role, and future prospects of biofuels feedstocks: a state-of-the-art review. Crit Rev Environ Sci Technol 2019:1–53
Fivga A, Speranza LG, Branco CM, Ouadi M, Hornung A (2019) A review on the current state of the art for the production of advanced liquid biofuels. AIMS Energy 7(1):46–76
Yadav M, Singh A, Balan V, Pareek N, Vivekanand V (2019) Biological treatment of lignocellulosic biomass by Chaetomium globosporum: process derivation and improved biogas production. Int J Biol Macromol 128:176–183
Meyer MA, Leckert FS (2018) A systematic review of the conceptual differences of environmental assessment and ecosystem service studies of biofuel and bioenergy production. Biomass Bioenergy 114:8–17
Yadav M, Paritosh K, Pareek N, Vivekanand V (2019) Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J Clean Prod 213:75–88
Satchwell AJ, Scown CD, Smith SJ, Amirebrahimi J, Jin L, Kirchstetter TW, Brown NJ, Preble CV (2018) Accelerating the deployment of anaerobic digestion to meet zero waste goals. Environ Sci Technol 52(23):13663–13669
Carrere H, Antonopoulou G, Affes R, Passos F, Battimeli A, Lyberatos G, Ferrer I (2016) Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol 199:386–397
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass – an overview. Bioresour Technol 199:76–82
Devi S, Gupta C, Jat SL, Parmar MS (2017) Crop residue recycling for economic and environmental sustainability: the case of India. Open Agric 2:486–494
Zheng Y, Shi J, Tu M, Cheng YS (2017) Chapter one - principles and development of lignocellulosic biomass pretreatment for biofuels. Adv Bioenergy 2:1–68
Gupta R, Lee YY (2009) Mechanism of cellulase reaction on pure cellulosic substrates. Biotechnol Bioeng 102(6):1570–1581
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800
Dashtban M, Schraft H, Syed TA, Qin W (2010) Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mole Biol 1(1):36–50
Sathitsuksanoh N, Zhua Z, Zhang YHP (2012) Cellulose solvent and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour Technol 117:228–233
Park YC, Kim JS (2012) Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 47(1):31–35
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
Sills DL, Gossett JM (2012) Using FTIR spectroscopy to model alkaline pretreatment and enzymatic saccharification of six lignocellulosic biomasses. Biotechnol Bioeng 109(4):894–903
Shirkavand E, Baroutian S, Gapes D, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment – a review. Renew Sustain Energy Rev 54:217–234
Persson T, Ren JL, Joelsson E, Jönsson AS (2009) Fractionation of wheat and barley straw to access high-molecular-mass hemicelluloses prior to ethanol production. Bioresour Technol 100(17):3906–3913
Gnansounou E, Dauriat A (2010) Techno-economic analysis of lignocellulosic ethanol: a review. Bioresour Technol 101(13):4980–4991
Saha BC, Yoshida T, Cotta MA, Sonomoto K (2013) Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind Crops Prod 44:367–372
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 90:877–891
Kumar S, Paritosh K, Pareek N, Chawade A, Vivekanand V (2018) De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: an overview. Renew Sustain Energy Rev 90:160–170
Peng L, Appels L, Su H (2018) Combining microwave irradiation with sodium citrate addition improves the pre-treatment on anaerobic digestion of excess sewage sludge. J Environ Manag 213:271–278
Oh ST, Kang SJ, Azizi A (2018) Electrochemical communication in anaerobic digestion. Chem Eng J 353:878–889
Nizami AS (2012) Anaerobic digestion: processes, products, and applications. In: Caruana DJ, Olsen AE (eds) Potential of activated sludge utilization. Nova Science, Commack
Paritosh K, Kushwaha SK, Yadav M, Pareek N, Chawade A, Vivekanand V (2017) Food waste to energy: an overview of sustainable approaches for food waste management and nutrient recycling. BioMed Res Int 1-20 Article ID 2370927
Croce S, Wei Q, D’Imporzano G, Dong R, Adan F (2016) Anaerobic digestion of straw and corn stover: the effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol Adv 34(8):1289–1304
Park S, Venditti RA, Abrecht DG, Jameel H, Pawlak JJ, Lee JM (2007) Surface and pore structure modification of cellulose fibers through cellulase treatment. J Appl Polym Sci 103(6):3833–3839
Zhang R, Zhang Z (1999) Biogasification of rice straw with an anaerobic-phased solids digester system. Bioresour Technol 68:235–245
Kim HJ, Lee S, Kim J, Mitchell RJ (2013) Lee JH (2013) Environmentally friendly pretreatment of plant biomass by planetary and attrition milling. Bioresour Technol 144:50–56
Chen X, Zhang YL, Gu Y, Liu Z, Shen Z, Chu H, Zhou X (2014) Enhancing methane production from rice straw by extrusion pretreatment. Appl Energy 122:34–41
Duque A, Manzanares P, Ballesteros M (2017) Extrusion as a pretreatment for lignocellulosic biomass: fundamentals and applications. Renew energy 114B:1427–1441
Sapci Z (2013) The effect of microwave pretreatment on biogas production from agricultural straws. Bioresour Technol 128:487–494
Jackowiak D, Bassard D, Pauss A, Ribeiro T (2011) Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour Technol 102:6750–6756
Vivekanand V, Olsen EF, Eijsink VGH, Horn JS (2013) Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour Technol 127:343–349
Vivekanand V, Olsen EF, Eijsink VGH, Horn SJ (2014) Methane potential and enzymatic saccharification of steam exploded bagasse. BioResources 9:1311–1324
Ferreira LC, Nilsen PJ, Fdz-Polanco F, Pérez-Elvira SI (2014) Biomethane potential of wheat straw: influence of particle size, water impregnation and thermal hydrolysis. Chem Eng J 242:254–259
Chang KL, Thitikorn-amorn J, Hsieh JF, Ou BM, Chen SH, Ratanakhanokchai K, Huang PJ, Chen ST (2011) Enhanced enzymatic conversion with freeze pretreatment of rice straw. Biomass Bioenergy 35(1):90–95
Hjorth M, Gränitz K, Adamsen APS, Møller HB (2011) Extrusion as a pretreatment to increase biogas production. Bioresour Technol 102:4989–4994
Ferreira LC, Donoso-Bravo A, Nilsen PJ, Fdz-Polanco F, Pérez-Elvira SI (2013) Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour Technol 143:251–257
Eskicioglu E, Monlau F, Barakat A, Ferrer I, Kaparaju P, Trably E, Carrere H (2017) Assessment of hydrothermal pretreatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res 120:32–42
Qi W, Liu G, He C, Liu S, Lu S, Yue J, Wang Q, Wang Z, Yuan Z, Hu J (2019) An efficient magnetic carbon-based solid acid treatment for corncob saccharification with high selectivity for xylose and enhanced enzymatic digestibility. Green Chem 21(6):1292–1304
Gu Y, Guo J, Nawaz A, ul Haq I, Zhou X, Xu Y (2021) Comprehensive investigation of multiples factors in sulfuric acid pretreatment on the enzymatic hydrolysis of waste straw cellulose. Bioresour Technol 340:125740
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Industrial Eng Chem Res 48:3713–3729
da Costa SL, Jin M, Chundawat SP, Bokade V, Tang X, Azarpira A, Lu F, Avci U, Humpula J, Uppugundla N, Gunawan C (2016) Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ Sci 9(4):1215–1223
da Costa SL, Foston M, Bokade V, Azarpira A, Lu F, Ragauskas AJ, Ralph J, Dale B, Balan V (2016) Isolation and characterization of new lignin streams derived from extractive-ammonia (EA) pretreatment. Green Chem 18(15):4205–4215
Stoklosa RJ, Orjuela AP, da Costa SL, Uppugundla N, Williams DL, Dale BE, Hodge DB, Balan V (2017) Techno-economic comparison of centralized versus decentralized biorefineries for two alkaline pretreatment processes. Bioresour Technol 226:9–17
Jin M, da Costa SL, Schwartz C, He Y, Sarks C, Gunawan C, Balan V, Dale BE (2016) Toward lower cost cellulosic biofuel production using ammonia based pretreatment technologies. Green Chem 18(4):957–966
McIntosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 35:3094–3103
Taherdanak M, Zilouei H (2014) Improving biogas production from wheat plant using alkaline pretreatment. Fuel 115:714–719
Zhao R, Zhang Z, Zhang R, Li M, Lei Z, Utsumi M, Sugiura N (2010) Methane production from rice straw pretreated by a mixture of acetic–propionic acid. Bioresour Technol 101:990–994
Kim JW, Kim KS, Lee JS, Park SM, Cho HY, Park JC, Kim JS (2011) Two-stage pretreatment of rice straw using aqueous ammonia and dilute acid. Bioresour Technol 102:8992–8999
Reilly M, Dinsdale R, Guwy A (2015) Enhanced biomethane potential from wheat straw by low temperature alkaline calcium hydroxide pre-treatment. Bioresour Technol 189:258–265
Liu X, Zicari SM, Liu G, Li Y, Zhang R (2015) Pretreatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour Technol 185:150–157
Solé-Bundó M, Eskicioglu C, Garfí M, Carrère H, Ferrer I (2017) Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pretreatment. Bioresour Technol 237:89–98
Xiong ZY, Qina YH, Ma JY, Yang L, Wu ZK, Wang TL, Wang WG, Wang CW (2017) Pretreatment of rice straw by ultrasound-assisted Fenton process. Bioresour Technol 227:408–411
Xie Z, Zou H, Zheng Y, Fu SF (2022) Improving anaerobic digestion of corn straw by using solid-state urea pretreatment. Chemosphere 293:133559
Teghammar A, Karimi K, Sárvári Horváth I, Taherzadeh MJ (2011) Enhanced biogas production from rice straw, triticale straw and softwood spruce by NMMO pretreatment. Biomass Bioenergy 36:116–120
Chandra R, Takeuchi H, Hasegawa T (2012) Hydrothermal pretreatment of rice straw biomass: a potential and promising method for enhanced methane production. Appl Energy 94:129–140
Song Z, Yang G, Guo Y, Zhang T (2012) Comparison of two chemical pretreatments of rice straw for biogas production by anaerobic digestion. BioResources 7:3223–3236
Heiske S (2013) Improving anaerobic digestion of wheat straw by plasma-assisted pretreatment. J At Mol Phys 2013:1–8 Article ID 791353
Song Z, Yang G, Liu X, Yan Z, Yuan Y, Liao Y (2014) Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 9(4):e93801
Harun S, Geok SK (2016) Effect of sodium hydroxide pretreatment on rice straw composition. Indian J Sci Technol 9(21):1–9
Chang KL, Chena XM, Wang XQ, Han YJ, Potprommanee L, Liu JY, Liao YL, Ning XA, Sun SY, Huang Q (2017) Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour Technol 227:388–392
Nurika I, Shabrina EN, Azizah N, Suhartini S, Bugg TD, Barker GC (2022) Application of ligninolytic bacteria to the enhancement of lignocellulose breakdown and methane production from oil palm empty fruit bunches (OPEFB). Bioresour Technol Reports 17:100951
Placido J, Capareda S (2015) Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour Bioprocess 2:23
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33:25–40
Baker PW, Charlton A, Hale MDC (2015) Increased delignification by white rot fungi after pressure refining Miscanthus. Bioresour Technol 189:81–86
Rouches E, Gimbert IH, Steyer JP, Carrere H (2016) Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review. Renew Sustain Energy Rev 59:179–198
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valor 10(2):235–251
Ruttimann-Johnson C, Salas L, Vicuna R, Kirk TK (1993) Extracellular enzyme production and synthetic lignin mineralization by Ceriporiopsis subvermispora. Appl Environ Microbiol 59:1792–1797
Rajakumar S, Gaskell J, Cullen D, Lobos S, Karahanian E, Vicuna R (1996) Lip-like genes in Phanerochaete sordida, and Ceriporiopsis subvermispora, white rot fungi with no detectable lignin peroxidase activity. Appl Environ Microbiol 62:2660–2663
Millati R, Syamsiah S, Niklasson C, Cahyanto MN, Ludquist K, Taherzadeh MJ (2011) Biological pretreatment of lignocelluloses with white-rot fungi and its applications: a review. BioResources 6(4):5224–5259
Singh G, Bhalla A, Kaur P, Capalash N, Sharma P (2011) Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol 10:309–326
Frigon JC, Mehta P, Guiot SR (2012) Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 36:1–11
Ghosh A, Bhattacharyya BC (1999) Biomethanation of white rotted and brown rotted rice straw. Bioprocess Eng 20:297
Phutela UG, Sahni N, Sooch SD (2011) Fungal degradation of paddy straw for enhancing biogas production. Indian J Sci Technol 4:660–665
Rastogi S, Soni R, Kaur J, Soni SK (2016) Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour Technol 222:458–469
Potumarthi R, Baadhe RR, Nayak P, Jetty A (2013) Simultaneous pretreatment and saccharification of rice husk by Phanerochete chrysosporium for improved production of reducing sugars. Bioresour Technol 128:113–117
Singh D, Zeng J, Laskar DD, Deobald L, Hiscox WC, Chen S (2011) Investigation of wheat straw biodegradation by Phanerochaete chrysosporium. Biomass Bioenergy 35:1030–1040
Wan C, Li Y (2011) Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feed stocks. Bioresour Technol 102:7507–7512
Song L, Yu H, Ma F (2013) Zhang X (2013) Biological pretreatment under non-sterile conditions for enzymatic hydrolysis of corn stover. BioResources 8:3802–3816
Taha M, Shahsavari E, Al-Hothaly K, Mouradov A, Smith AT, Ball AS, Adetutu EM (2015) Enhanced biological straw saccharification through co-culturing of lignocellulose degrading microorganisms. Appl Biochem Biotechnol 175:3709–3728
Panahi HK, Dehhaghi M, Guillemin GJ, Gupta VK, Lam SS, Aghbashlo M, Tabatabaei M (2022) A comprehensive review on anaerobic fungi applications in biofuels production. Sci Total Environ 829:154521
Dongyan Y, Jin LX, Jian GZ, Wu WY (2003) Improving biogas production of corn stalk through chemical and biological pretreatment: a preliminary comparison study. Trans Chinese Soc Agric Eng 16:209–213
Zeng J, Singh D, Chen S (2011) Biological pretreatment of wheat straw by Phanerochaete chrysosporium supplemented within organic salts. Bioresour Technol 102:3206–3214
Du W, Yu H, Song L, Zhang J, Weng C, Ma F, Zhang X (2011) The promising effects of by-products from Irpex lacteus on subsequent enzymatic hydrolysis of bio-pretreated corn stalks. Biotechnol Biofuels 4:37
Wan C, Li Y (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30(6):1447–1457
Cianchetta S, Maggio BD, Burzi PL, Galletti S (2014) Evaluation of selected white-rot fungal isolates for improving the sugar yield from wheat straw. Appl Biochem Biotechnol 173:609–623
Saha BC, Qureshi N, Kennedy GJ, Cotta MA (2016) Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int Biodeter Biodegr 109:29–35
Karimi M, Esfandiar R, Biria D (2017) Simultaneous delignification and saccharification of rice straw as a lignocellulosic biomass by immobilized Trichoderma viride sp. to enhance enzymatic sugar production. Renew Energy 104:88–95
Yu J, Zhang J, He J, Liu Z, Yu Z (2009) Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour Technol 100:903–908
Ma F, Yang N, Xu C, Yu H, Wu J, Zhang X (2010) Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour Technol 101:9600–9604
Zhang S, Jiang M, Zhou Z, Zhao M, Li Y (2012) Selective removal of lignin in steam-exploded rice straw by Phanerochaete chrysosporium. Int Biodeter Biodegr 75:89–95
Ishola MM, Isroi TMJ (2014) Effect of fungal and phosphoric acid pre-treatment on ethanol production from oil palm empty fruit bunches (OPEFB). Bioresour Technol 165:9–12
Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N (2015) Influence of alkaline catalyst addition on compressed liquid hot water pretreatment of rice straw. Chem Eng J 278:85–91
Dai Y, Si M, Chen Y, Zhang N, Zhou M, Liao Q, Shi D, Liu Y (2015) Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour Technol 198:725–731
Zhang Y, Chen X, Gu Y, Zhou X (2015) A physicochemical method for increasing methane production from rice straw: extrusion combined with alkali pretreatment. Appl Energy 160:39–48
Shi F, Xiang H, Li Y (2015) Combined pretreatment using ozonolysis and ball milling to improve enzymatic saccharification of corn straw. Bioresour Technol 179:444–451
Yin Y, Wang J (2016) Enhancement of enzymatic hydrolysis of wheat straw by gamma irradiation–alkaline pretreatment. Radiat Phys Chem 123:63–67
Kaur K, Phutela UG (2016) Enhancement of paddy straw digestibility and biogas production by sodium hydroxide-microwave pretreatment. Renew Energy 92:178–184
Mustafa AM, Poulsen TG, Xia Y, Sheng K (2017) Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour Technol 224:174–182
Guan R, Li X, Wachemo AC, Yuan H, Liu Y, Zou D, Zuo X, Gu J (2018) Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci Total Environ 637–638:9–17
Si M, Liu D, Liu M, Yan X, Gao C, Chai L, Shi Y (2019) Complementary effect of combined bacterial-chemical pretreatment to promote enzymatic digestibility of lignocellulose biomass. Bioresour Technol 272:275–280
Tsegaye B, Balomajumder C, Roy P (2019) Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Conver Manag 186:82–92
Balan V, da Costa SL, Chundawat SP, Vismeh R, Jones AD, Dale BE (2008) Mushroom spent straw: a potential substrate for an ethanol-based biorefinery. J Ind Microbiol Biotechnol 35(5):293–301
Martínez-Patiño JS, Lu-Chau TA, Gullón B, Ruiz E, Romero I, Castro E, Lema JM (2018) Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind Crops Prod 121:10–17
Liu G, Qu Y (2018) Engineering of filamentous fungi for efficient conversion of lignocellulose: tools, recent advances and prospects. Biotechnol Adv 37(4):519–529
Ellilä S, Fonseca L, Uchima C, Cota J, Goldman GH, Saloheimo M, Sacon V, Siika-Aho M (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10:30
Benocci T, Aguilar-Pontes MV, Zhou M, Seiboth B, de Vries RP (2017) Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol Biofuels 10:152
Wang S, Liu G, Wang J, Yu J, Huang B, Xing M (2013) Enhancing cellulase production in Trichoderma reesei RUT C30 through combined manipulation of activating and repressing genes. J Ind Microbiol Biotechnol 40(6):633–641
Yao G, Li Z, Gao L, Wu R, Kan Q, Liu G, Qu Y (2015) Redesigning the regulatory pathway to enhance cellulase production in Penicillium oxalicum. Biotechnol Biofuels 8:71
Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C (2017) Development of a genomeediting CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10(1):1–14
Gao F, Hao Z, Sun X, Qin L, Zhao T, Liu W, Luo H, Yao B, Su X (2018) A versatile system for fast screening and isolation of Trichoderma reesei cellulase hyperproducers based on DsRed and fluorescence-assisted cell sorting. Biotechnol Biofuels 11:261
Li Y, Zheng X, Zhang X, Bao L, Zhu Y, Qu Y, Zhao J, Qin Y (2016) The different roles of Penicillium oxalicum LaeA in the production of extracellular cellulase and β-xylosidase. Front Microbiol 7:2091
Zhang J, Wu C, Wang W, Wang W, Wei D (2018) Construction of enhanced transcriptional activators for improving cellulase production in Trichoderma reesei RUT C30. Bioresour Bioprocess 5(1):40
Nakagawa Y, Sakamoto Y, Kikuchi S, Sato T, Yano A (2010) A chimeric laccase with hybrid properties of the parental Lentinula edodes laccases. Microbiol Res 165:392–401
Bleve G, Lezzi C, Spagnolo S, Rampino P, Perrotta C, Mita G, Grieco F (2014) Construction of a laccase chimerical gene: recombinant protein characterization and gene expression via yeast surface display. Appl Biochem Biotechnol 172:2916–2931
Funding
This study was funded by Department of Biotechnology, Ministry of Science and Technology, Government of India (Ramalingaswami Re-Entry fellowship No. BT/RLF/Reentry/04/2013). Dr. Balan thank University of Houston and State of Texas for startup funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, M., Balan, V., Varjani, S. et al. Multidisciplinary Pretreatment Approaches to Improve the Bio-methane Production from Lignocellulosic Biomass. Bioenerg. Res. 16, 228–247 (2023). https://doi.org/10.1007/s12155-022-10489-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10489-z