Abstract
Energy crises and climate change attracted less-explored microalgae as renewable resources. Deficiencies of nitrogen and phosphorus are the most effective inducers of lipid accumulation in microalgae but at the cost of biomass productivity. Therefore, nitrogen, phosphorus, and carbon manipulation of the culture medium was adopted for maximizing lipid as well as biomass production in Dunaliella salina. Phosphate deficiency in combination with 1.25 mM KNO3 (1/8 of the basal) resulted in higher lipid content (341.1 mg g−1 dry cell weight, DCW), but lower biomass (13.12 mgL−1d−1 DCW). The addition of 10.00 mM NaHCO3 to such cultures enhanced not only lipid content to 1.17-fold but also biomass productivity to 2.25-fold. The increase in biomass may be correlated with the stress-ameliorating effects of bicarbonate augmentation which helped in maintaining the health of the cells, as reflected by robust photosynthetic performance. The two important enzymes, RuBisCO and ACCase were also monitored for their expressions. RuBisCO possesses large and small subunits (rbcL and rbcS) responsible for incorporation of CO2, and beta carboxyl transferase (accD) of the heteromeric ACCase is associated with the first and committed step of fatty acid biosynthesis. Enhanced biomass and lipid content in D. salina cells after NaHCO3 augmentation may be ascribed to 6.23-fold increase in the expression of accD and > 2.16-fold increase in rbcL and rbcS genes. Thus, the present work recommends a threshold level of nitrogen and bicarbonate in phosphate deficient D. salina cultures for simultaneously maximizing the biomass and lipid content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing utilization of petroleum-derived fossil fuels is leading to rapid climate changes as a consequence of greenhouse gases (GHGs) emissions [1]. The accelerated exploitation of crude oil reserves makes them unsustainable with the ever-increasing prospect of complete exhaustion. Subsequently, biodiesel from microalgal lipids is projected to act as an alternative, renewable and sustainable source of energy [2]. Microalgae are the most efficient photosynthetic systems having a high growth rate, and can be cultivated in non-arable lands to produce 300 times more lipid than oil crop plants with simple salts as growth requirements [3]. A halotolerant microalga, Dunaliella salina can be cultivated in seawater by naturally avoiding bio-fouling, and being wall-less, the resultant oil extraction is sure to be cost-effective [4]. Wastewater can be utilized to cultivate microalgae that can eliminate up to 90% inorganic nutrients of wastewater simultaneously producing high value biomass at low cost [5]. Due to their high CO2 sequestration capacity, microalgae were also cultivated coupled to industrial flu gas emission in a novel photobioreactor [6]. Adoption of this alternative energy is constrained by the non-availability of a suitable, low-cost photobioreactor for mass cultivation and raising the lipid content in microalgae to a substantial level. The lab has already demonstrated the efficacy of a low-cost, photobioreactor (VFP-PBR) of 32 L capacity for mass cultivation of D. salina and its economic harvesting through FeCl3.6H2O and pH-induced flocculation [7]. The bioreactor yielded 17.85 mgL−1d−1 DCW biomass productivity (BMPr). It is anticipated that increasing the intracellular lipid content up to 60–70% of DCW (dry cell weight) at an appreciable biomass output, may overcome the hurdles of commercial viability [3]. Comparatively higher lipid content was produced in different microalgal species through manipulating culture conditions such as salinity [8], light quality and durations [9], pH [10] or altering levels of the phosphorous [11], nitrogen [10, 11], carbon [12] and sulphur [13] etc. These nutrients, being an integral part of the nucleic acids, proteins, chlorophylls, ATP cycle and signalling processes are capable of directing metabolism of the cell rapidly under suboptimal concentrations, streamlining meagrely available resources for the synthesis of energy and carbon reserves [13]. Nitrogen, a major constituent of the amino acids, is an inevitable requirement in higher amounts for the cell division, growth as well as maintenance of the metabolic synergy [14]. Nitrogen deficiency adversely affected protein synthesis as well as photochemistry of the cells leading to higher lipid accumulation in microalgal species like Dunaliella sp., Ankistrodesmus falcatus KJ671624, Phaeodactylum tricornutum, and Scenedesmus sp. [10,11,12]. Likewise, deficiency of the phosphorous rendered deleterious effects on intracellular signalling, energy balance, ATP turnover, respiration and photosynthesis, ultimately compromising normal cell metabolism as well as growth in microalgal species [13, 14]. However, phosphorous deprivation has also been used as a strategy to enhance lipid accumulation in various microalgal species such as Pavlova lutheri, Isochrysis galbana, and Phaeodactylum tricornutum [15]. Thus, nitrogen and phosphorous limitation has proved efficient in augmenting lipid accumulation in some microalgal species, with compromised biomass productivity. Therefore, lipid-inducing factors such as nitrate or phosphate limitation or depletion coupled with stress alleviating supplements such as micronutrients [16] and bicarbonate [12] were optimized for overproduction of lipids vis-à-vis biomass. Simultaneous manipulation of Fe, Na and N levels in the cultures of C. minutissima, yielded higher lipid over that of individual nutrient treatment [17]. Again, C. vulgaris cultures subjected to simultaneous nitrate limitation (0.03 mM) and NaCl stress (0.11 M) resulted in higher lipid productivity (160 mg L−1 DCW) [18]. Microalgae assimilate carbon either in the form of inorganic carbon (HCO3 −) or gaseous CO2 [12]. Saline microalgae such as D. salina, usually grow under higher pH, where lower solubility of CO2 renders gaseous CO2 unavailable to the growing cells. Such situation demands supplementation of the medium with NaHCO3 which is cheaper, easy to handle, and effective in small amounts, thereby, significantly nullifying inorganic carbon unavailability [13]. D. salina also possesses external and internal carbonic anhydrases, enabling it to efficiently utilize the bicarbonate ion and maintain photosynthesis in hypersaline condition [19, 20]. In microalgae, acetyl CoA carboxylase (ACCase) serves as the biocatalyst in the first and rate-limiting step of the lipid biosynthesis, converting acetyl-CoA to malonyl-CoA. Beta carboxyl transferase (β-CT) is known to be the key sub-unit of heteromeric ACCase of chloroplasts, being encoded by the plastidic accD gene [21]. It was reported that lower expression of accD brought down the activity of ACCase and vice versa in tobacco plant [22]. Thus, understanding accD expression pattern in the target organism under varied nutrient regimes may facilitate genetic engineering prospects after maximizing the lipid content through nutrient manipulation strategies. Likewise, RuBisCO, the enzyme involved in the fixation of CO2 via the Calvin-Benson cycle is coded by rbcL (large sub-unit) and rbcS (small sub-unit) and thus, central to the biomass production [23]. Therefore, it was imperative to monitor the expression of rbcL and rbcS genes. There are limited studies with a focus on the inter-relationship of nutrient conditions, photosynthetic performance, and key gene expression patterns in D. salina [15, 24].
Nitrogen limitation strategies to overproduce lipids in microalgae are mainly two types; (a) two-stage cultivation involving culture of microalgal cells in nutrient sufficient medium in the first stage and then transferring to nitrogen deficient medium in second stage [18, 25], (b) one-stage cultivation with the addition of desired nitrogen only and make the cells starve for nitrogen in the due course which may be advantageous over two stage considering the cost and time consumption [26]. Nevertheless, the biomass and lipid accumulation in microalgal system in response to varied nutrient levels is species-specific [27]. Therefore, in the present work, D. salina was investigated for higher lipid production in one stage without compromising the biomass output. The biomass may be used for co-production of commercially valuable compounds such as lipids, glycerol, carotenoids and therapeutic compounds that can offset high cost involved in production [25]. The target strain D. salina was collected from a hypersaline lake, Sambhar, from Rajasthan, India. This strain showed tolerance in range of NaCl from 0.25 to 5.0 M [20]. The strain grew optimally at 0.5 M NaCl in the batch culture. It is a cell factory for many high value compounds, a natural β-carotene source, having high intrinsic lipid production capability and immense environmental stress resilience making it suitable for cultivation under hot climate without requiring fresh water [28]. There are also reports that explored the ways to increase the biomass and lipid production in halophilic D. salina by varying the levels of different nutrients and other factors [29]. Most of these studies lacked the strategy involving the determination of threshold limits of different nutrients for simultaneous improvement of biomass and lipid production and also a better understanding of the physiological status of the cells. A multiphasic approach involving nutrient manipulation, assessment of its impact on photosynthetic performance and expression of key genes involved in photosynthesis and lipid production of D. salina might be useful in developing a suitable way for hyper-accumulation of lipids while maintaining biomass productivity, and thereby paving the way in reducing the cost of biofuel production, as the biomass produced may also be used as a source of valuable products and feedstock for biofuel. Therefore, the objective of the present study was to determine the suitable concentrations of different nutrients (N, P and C) for maximizing lipid yield from D. salina while maintaining optimum biomass productivity in one-stage cultivation. Also, their effects on biomass and lipid production were correlated with the photosynthetic performance of the cells as well as expression of key genes, accD (involved in lipid biosynthesis), rbcL and rbcS (involved in inorganic carbon assimilation and biomass production).
Materials and Methods
Organism and Culture Conditions
Modified Johnson’s Medium (MJM) was used to prepare high-density cultures of D. salina for inoculating the treatments [5]. The basal MJM contained 7.38 mM MgCl2.6H2O, 2.03 mM MgSO4.7H2O, 2.68 mM KCl, 1.36 mM CaCl2.2H2O, 10.00 mM KNO3, 0.5 mM NaHCO3, 0.25 mM KH2PO4, 2.81 µM Na2EDTA, 4.5 µM FeCl3.6H2O, 0.98 µM H3BO3, 0.03 µM (NH4)6Mo7O24.4H2O, 0.024 µM CuSO4.5H2O, 0.021 µM CoCl2.6H2O, 0.03 µM ZnCl2, 0.018 µM MnCl2.4H2O. Media were supplied with 0.5 M NaCl at 80 µmolm−2 s−1 illuminations, light/dark photoperiod of 16/8 h and maintained at 28 ± 2 °C. Sterile media and glass-wares were used under a laminar flow hood (Instech, India) to carry out all operations. All chemicals used were of analytical grade (Merck, India).
Experimental Design
The current experiment involved three steps. In step 1, different concentrations of KNO3 (0.00, 1.25, 2.50, 5.00 and 10.00 mM) and KH2PO4 (0.00, 0.064, 0.128, 0.256 and 0.512 mM) were employed one at a time to assess the impact of nutrient stress on the growth, photosynthetic efficiency, and lipid production in D. salina. During step-1 of the experiment, the concentration of KH2PO4 was kept at 0.256 mM (basal level) with variation of KNO3 levels. Conversely, under different KH2PO4 level, 10.00 mM KNO3 was used. Each experiment continued for 12 days and conducted in triplicates of biological samples (total 27 treatments) and the data recorded in the form of mean ± SD. A combination of nitrate (‘Nlim’ = 1.25 mM KNO3, 1/8 of the basal level and ‘–N’, 0.00 mM KNO3) and phosphate (-P = 0.000 mM KH2PO4 and Plim = 0.064 mM KH2PO4, 1/4 of the basal) that resulted in higher lipid production in step 1 were taken for further study in step 2 and evaluated in triplicates for the above parameters (total 15 experiments). Step 3 involved the addition of 5 (C1) and 20-fold (C2) NaHCO3 over that of basal medium (C1 = 2.5 mM, and C2 = 10.00 mM) to the combined nutrient stress of Step 2 and examined for overall growth, biomass productivity, and lipid yield (total 27 experiments). The photochemistry of such cells along with relative expression of RuBisCO (carbon assimilation) and accD (lipid biosynthesis) genes were also examined. In all the cases, MJM containing 10 mM KNO3, 0.5 mM NaHCO3, and 0.256 mM KH2PO4 (basal level of respective nutrients) was taken as control. The experimental design is briefly described in the following flow chart (Fig. 1).
Estimation of Biomass Productivity
For estimating biomass productivity, the cultures were filtered through 0.45 µm Whatmann glass filter paper (Sartorious, Göttingen, Germany) and the resulting pellet was oven-dried at 80 °C for 12 h. Biomass productivity (BMPr, mgL−1d−1 dry cell weight, DCW) was measured using the following formula [25];
Here, BM1 and BM2corresponded to dry cell weight of cultures at time T1 and T2 respectively measured in an analytical balance (Sigma Aldrich, USA).
Photopigment Estimation
For determining the pigment contents, microalgal culture (2 mL) was centrifuged (Eppendorf 5415R, Hamburg, Germany) at 10,000 rpm, 4 min at room temperature and the pellet was resuspended in an equal volume of 99.9% methanol and incubated at dark (24 h at 4◦C). Pigment concentrations were determined after taking absorbance at 470, 652.4, and 665.2 nm in a UV–VIS spectrophotometer (Thermo Scientific Evolution 160, v8.01, Massachusetts, USA). The following formula was used for pigment content estimation [30].
Lipid Content Estimation
Total lipid was extracted and quantified gravimetrically following the method of Bligh and Dyer [31]. Briefly, the culture of appropriate density was harvested (4000 rpm × 4 min, Remi R-8C, Mumbai, India) and washed twice with double-distilled water. The algal biomass was vacuum freeze-dried (Martin Christ, Alpha 1–2 LDplus, Germany), 100 mg of the dried biomass was taken in a 10 mL falcon tube (Tarson, India) to which 3 mL methanol + chloroform (2:1) was added. Then, the tube was kept at 25 °C for 24 h. Then, it was vortexed and sonicated for 10 min at 20 kHz (VCX 500, Sonics & Materials Inc, USA) with intermittent shaking. To it, 1 mL chloroform was added and mixed vigorously. This was followed by addition of 1.8 mL saturated NaCl solution and vortexing the mixture for 2 min. The mixture was then centrifuged for 15 min (3000 rpm, Remi R-8C, Mumbai, India) and bottom organic phase was transferred to a previously weighed clean vial (V1). The organic bottom phase was evaporated in the thermo block (95 °C) under nitrogen stream. In order to completely remove the solvent, the residue was further dried (104 °C × 30 min). Now, the weight of the vial was again monitored (V2) and the lipid content was calculated as, V2 – V1 and expressed in terms of mg g−1 of dry cell weight.
Neutral Lipid Analysis
To detect the accumulation of neutral lipids, D. salina cells were stained with Nile red (9-diethyl amino 5-hexabenzo α-phenoxazine-5-one, Sigma, USA) soon after washing thrice with fresh media. The quantitative determination of neutral lipids was done by exciting the NR stained cells at 485 and detecting the emission at 612 nm using a fluorescence spectrophotometer (Cary Eclipse, Agilent, USA) following the procedure described by [32]. NR-stained D. salina micrographs with prominent golden yellow emissions (lipid bodies) were imaged using Zeiss LSM 780 laser-scanning confocal microscope–equipped Zen 2010 imaging software (Carl Zeiss, GmbH, Germany) with a common exposure time [33]. The excitation and emission wavelengths were at 525 and 595 nm respectively.
Pulse Amplitude Modulated (PAM) Fluorometry
Photosynthetic performance of the cells under various nutrient treatments was measured with reference to maximum photochemical quantum yield (Fv/Fm) of PS II using PAM Fluorimeter (PAM-2500; Heinz Walz, Effeltrich, Germany). Rapid Light Curve (RLC) generation and data processing were done using PAM-2500 software (v 3.20) [20]. Before the measurement, liquid samples were placed in the dark (20 min) for closing all photosystems. Chlorophyll fluorescence at 0.001 and 3000 µmolm−2 s−1 light intensities were taken for obtaining Minimal (Fo) and maximum fluorescence (Fm) levels. Fv/Fm, representing the maximum quantum output of PS II was calculated using the following formula [34];
\(\frac{Fv}{Fm}=\frac{Fo-Fm}{Fm}\), where, Fv is the differential fluorescence value.
Calculation of relative electron transfer rate (rETR) was done as per the formula described in [34]; rETR = PAR · ETR-Factor · Y(II) · PPS2/PPPS, where ETR-Factor (default value was 0.84) depicts the fraction of incident light absorbed by photo-pigments; Y(II) corresponds to effective quantum yield of PS II; PPS2/PPPS: ratio of photons absorbed by PS II and all photo-pigments (default value was 0.5 assuming uniform electron transfer rates across PS I and II). The highest value for rETR across 0 to 2000 μmol photons m−2 s−1 PAR range was designated as rETRmax.
Calculation of Non-photochemical quenching (NPQ) was done as per the formula described by [34];
Here, Fm' stands for fluorescence maxima during a pulse of non-saturating light.
RNA Extraction, RT-PCR, and Gene Expression Analysis
For total RNA extraction, reverse transcriptase PCR (RT-PCR) and expression profiling of different genes, the methods used were described by [35]. For RNA extraction, the aliquot of cultures (2 mL, OD750 nm = 0.6) was sampled at 0, 6, and 12th day of cultivation. The biomass pellet was collected at 2000 rpm, 5 min (Eppendorf 5415R, Hamburg, Germany), and washed with DEPC water before grounding it with mortar and pestle under liquid nitrogen. Extraction of total RNA was performed using QIAGEN RNAeasy Mini Kit (Qiagen, Germantown, USA) as per the supplemented instruction. Agarose gel (1.2%) electrophoresis and spectro-photometric analysis (NanoDrop One, Thermo Scientific, Massachusetts, USA) were done to ensure the quality of extracted RNA. Subsequently, High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Massachusetts, USA) was used to synthesize the first strand of cDNA from total RNA adopting the manufacture’s protocol. The cDNA obtained from this acted as a template for semi-quantitative RT-PCR assay. Reverse transcription was run in a thermocycler (BioRad T100 Thermal Cycler, Hercules, USA) Primers targeting important genes of lipid synthesis (accD) and carbon fixation (rbcL and rbcS) were designed using the NCBI Blast tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome), synthesized (Agrigenome Labs, Kochi, India), and used for quantitative detection of expression of these genes (Table 1). PCR was performed with a final volume of 25 µL and the reaction mixture contained 25 ng of template cDNA, 200 µM dNTPs, 10 pM each of forward and reverse primers, 25 µM CaCl2, 10X reaction buffer (2.5 µL), and Taq DNA polymerase (0.25 U, Takara, Japan). The reaction condition was 1 cycle of 94 °C (3 min); 25 cycles of denaturation at 94 °C (30 s), annealing at 51/48.8/48.5 °C (30 s) and extension at 72 °C (30 s); final extension for 5 min (72 °C). Sterile DEPC (Diethyl PyroCarbonate) treated Milli-Q® (Millipore, USA) water was used to inactivate RNAse enzyme in water and lab utensils. RNAse-treated Milli-Q water was taken in place of cDNA template to act as a negative control. D. salina actin (D-actin) was taken as reference for the purpose owing to its consistent level of expression across the treatments [36]. The template for designing accD primers was taken from complete CDS of Dunaliella salina acetyl CoA carboxylase beta subunit (GenBank: EF363909.1). The templates for rbcL and rbcS primers were obtained from conserved sequences of respective genes after aligning the mRNA sequences chosen from eight related microalgal species of D. salina. All reactions were performed in triplicates and the products were run in 1.2% agarose gel. Gel Doc 2000 system provided with volumetric analysis tool was used to quantify the signal generated in gel (BioRad, Hercules, USA).
Statistical Analysis
Experiments were operated in triplicates and the results were presented as mean ± SD. One-way Analysis of Variance (ANOVA) was used to validate the statistical significance of the treatments adopting Tukey’s multiple range test at α = 0.05 (95% confidence level) provided in the SPSS statistical package (SPSS Inc Version 20.0, IBM). The value p < 0.05, denotes the statistically significant difference between the obtained values of respective parameters.
Results and Discussion
Effects of Nitrate or Phosphate Limitation
Nitrogen and phosphorous are vital macronutrients for the cell division, growth, and smooth functioning of the cellular machinery, thus affecting biomass and lipid production. Photoautotrophic growth and biomass productivity (BMPr) in D. salina at 0.00 –10.00 mM KNO3, showed concentration-dependent response (Fig. 2A). Such responses have also been reported in other microalgal species [11, 13]. The highest BMPr (25.25 ± 0.86 mgL−1d−1) was recorded at the basal level of nitrate (10.00 mM) and lowest (11.05 ± 0.59 mgL−1d−1) in nitrogen-deficient cultures of D. salina (Table 2). BMPr decreased with the reduction in the nitrate concentration of the culture, indicating that more than 2.50 mM nitrate was required for higher biomass productivity. A minimal requirement of nitrate (1.25 mM, 1/8 of the basal level, called as ‘Nlim’) seemed to be essential for biomass productivity (20.04 ± 0.26 mgL−1d−1). However, there was increase in BMPr with increasing KH2PO4 concentration up to the basal level of the phosphate (0.256 mM), but decreased sharply by 0.34-fold (16.98 ± 2.09 mgL−1d−1), when phosphate level was increased to 0.512 mM (Fig. 2B and Table 2). This decrease in BMPr was very close to –P cultures (0.40-fold).
Photoautotrophic growth behaviour of D. salina under varying concentrations of (A) KNO3 (0.00 –10.00 mM), (B) KH2PO4 (0.00 – 0.512 mM), and (C) concomitant nitrate and phosphate limitation (-N and Nlim referred to 0.00 and 1.25 mM KNO3 while –P and Plim referred to 0.00 and 0.064 KH2PO4 respectively). Each value is represented as mean ± SD of three replicates
Interestingly, when –N and –P cultures were compared, –P cultures yielded 38% higher biomass (15.31 ± 0.38 mgL−1d−1) over that of –N cultures (11.05 ± 0.59 mgL−1d−1). This difference in BMPr was validated with the difference in photosynthetic quantum yield under –P cultures (Fv/Fm = 0.558 ± 0.08) and –N cultures (Fv/Fm = 0.396 ± 0.11) (Table 2). Relatively better growth in –P cultures over –N cultures might be due to the supply of the phosphate from intracellularly stored polyphosphate bodies [37, 38]. The data (Ref. Table 2) indicated that even 1/4 of the basal level of phosphate (0.064 mM, called ‘Plim’) was sufficient to sustain the growth. This data is supported by observations of [24], who have shown a relatively modest decrease in growth under phosphate deprivation over that of nitrate in the marine microalgal species. Phosphorus has been demonstrated to have hormesis effect on the viability of another green alga, Chlorella regularis cells under nitrogen limitations [38]. The excess of phosphate inhibited the growth of C. regularis by formation of excess of polyphosphate bodies and thereafter binding with the proteins, resulting in protonated amide nitrogen [38]. The latter damages the membrane, rendering disorganization of cellular organelles and deformation of the cell wall [38].
Total chlorophyll (TChl) content of D. salina cells reduced marginally (4.1%) in 1/4 of the basal nitrate (2.50 mM) compared to control. However, further decrease in nitrate level (–N or Nlim condition) reduced chlorophyll content, lowering photosynthetic quantum yield, consequently lowering BMPr (Ref. Table 2). This result was consistent with the other reports showing that nitrogen deficiency in D. salina and other microalgae led to drastic reduction in photosynthetic efficiency, thereby reducing the BMPr [13, 24]. The substantial reduction in TChl was coupled with increase in carotenoid content which might have acted as shock absorber due to quenching of reactive oxygen species, ROS [39].
The Pulse Amplitude Modulator technique which measures Chl a fluorescence was used to evaluate the physiological status and the photosynthetic efficiency of microalgal cultures [40]. It was demonstrated that the microalgal cultures under stress can be described in terms of chlorophyll fluorescence when Fv/Fm values measured below 0.4 [41, 42]. Photosynthetic quantum yield recorded was significantly higher (p < 0.05) in the cultures with 1.25 mM KNO3 compared to nitrate-deficient cultures, signifying the importance of nitrogen in maintaining photosynthetic activities even under sub-optimal availability (Table 2). Sustained photosynthetic efficiency (Fv/Fm ≥ 0.558) in –P and Plim cultures may be due to existing intracellular reserve of phosphate. Contrasting to nitrate, a very low amount of phosphate was sufficient for the growth of D. salina cells. The presence of nitrogen in the metabolic hub was of paramount importance as it changes the rate of cell metabolism significantly [14, 27]. Highest LC (298.24 ± 19.9 mg g−1 DCW) was recorded at 1.25 mM nitrate (Nlim), therefore, it may be considered as the critical threshold limit for higher lipid accumulation in D. salina. Similar reports in other microalgae suggested that reduction of nitrate level to half of the ambient (17.65 mM) resulted in higher lipid production (313.12 mg g−1 DCW) in Ankistrodesmus falcatus KJ671624 [11], and diatoms also produced higher lipid under limited nitrogen over deficient ones [12, 43]. Likewise, maximum LC (264.5 ± 14.0 mg g−1) was recorded in –P condition among different phosphate concentrations. Phosphate depletion also induced higher lipid accumulation in D. salina [13] and Chlorella sp. [44]. In another report, phosphate starvation in f/2 media resulted in 290 mg g−1 DCW lipid production in D. salina cells [24]. Thus, assuming that simultaneous application of nitrate and phosphate deficiency could further accelerate the lipid accumulation in D. salina cells, combinations of nitrate and phosphate levels were selected taking 1.25 or 0.00 mM nitrate and 0.00 or 0.064 mM phosphate as these concentrations yielded maximum lipid among all the culture conditions. This sort of strategy with combined nutrient deficiency has been employed in several other microalgal species with considerable success [10, 45].
Effect of Combined Nitrate and Phosphate Limitations
Photoautotrophic growth of D. salina under selected combinations of nitrate and phosphate is shown (Fig. 2C and Table 3). The upper curve demonstrated that the control culture grew better (BMPr = 23.24 ± 2.78 mgL−1d−1) compared to others.
The combined deficiency of N and P (-N-P) had a more deleterious impact on the growth of D. salina than that of the individual nutrient depletion in the culture medium. A similar trend was reported by [46] in a different strain of D. salina under –N-P condition, although only 228.5 mg g−1 DCW lipid could be accumulated. In the present study, a new strategy was adopted through addition of low level of nitrate (Nlim) or phosphate (Plim) to –N–P cultures, which ameliorated the stress considerably, as reflected by an increase in the BMPr (Ref. Table 3). However, BMPr recorded in NlimPlim cultures was significantly higher (p < 0.05) over the other combinations, 16.14% lower than the control. Highest Fv/Fm (0.603 ± 0.18), TChl (55.07 ± 4.85 mg g−1 DCW) were recorded in the control cultures having carotenoids at the lowest level (12.19 ± 1.52 mg g−1 DCW). NlimPlim cultures had slightly lower Fv/Fm (13%), TChl (2% lower), but increased carotenoid content (15%) over that of the control cultures. This indicated that addition of low levels of N and P helped in the sustenance of the algal growth.
Lipid content enhanced significantly (p < 0.05) in all the combinations when compared to the control or respective individual nutrient-limited conditions (Table 3). Highest LC (341.16 ± 27.5 mg g−1 DCW) was recorded in Nlim–P followed by that of NlimPlim cultures (319.22 ± 24.0 mg g−1). Relatively modest BMPr in Nlim-P (13.12 ± 0.57 mgL−1d−1), indicated the scope of BMPr improvement. Phosphate deprivation may have triggered the early onset of lipid accumulation in Nlim–P cultures, and therefore, the cells were able to channelize the resources towards lipid production using minimally available nitrate. In another report of algal species (C. sorokiniana), reducing nitrate to half (5.88 mM) and phosphate to one-fourth (0.057 mM) of the ambient BG-11, resulted in rise in lipid content from 185 to 330 mg g−1 DCW [16]. Simultaneous nitrate limitation and phosphate starvation also yielded 2.55-fold more lipids in A. falcatus KJ671624 cells as compared to the control [10]. Therefore, BMPr was enhanced to a substantial level while producing comparatively higher lipid, employing NaHCO3 augmentation strategy in the subsequent part of the study.
Effects of NaHCO3 Augmentation in Nutrient Limitation Conditions
Determination of Critical Threshold Level of NaHCO3
The possibility of enhancing biomass productivity while keeping higher lipid production in the microalgae can be accompanied through increasing the carbon flux in the organism by supplying more inorganic carbon. The solubility of the atmospheric CO2 significantly decreases in the marine and hyper-saline conditions. Thus, inorganic carbon (IC) plays a limiting factor in these culture systems, affecting photosynthesis and triggering enhanced carbon concentrating mechanisms (CCM) in microalgae [47]. Bicarbonate in the medium acts as a buffer (pKa = 10.3) and helps in supplying nutrition to the microalgal cell in the form of inorganic carbon [48]. Addition of NaHCO3 in D. salina cultures also proved to be effective in enhancing the relative percentage of saturated and mono-unsaturated fatty acids that are suitable for biodiesel production [7]. Therefore, to determine critical concentration of NaHCO3 for enhancing biomass and lipid production under combined nutrient limitations, the D. salina cultures were augmented with varying concentrations of NaHCO3 (0.50 to 20.00 mM) (Fig S1, supplementary information). The increase in BMPr of such D. salina cultures up to addition of 10.00 mM bicarbonate may be attributed to the enhanced availability of the carbon skeleton, higher TChl content leading to robust photosynthetic quantum yield (Fv/Fm > 0.6) with lowered carotenoid content, indicating the role of bicarbonate in alleviation of the nutrient (N or P) stress (Table S1, supplementary information). The addition of bicarbonate beyond 10.00 mM in the present experiment was non-conducive for algal growth as it might have drastically changed the pH of the media, causing salt formation. Thus, the highest BMPr was obtained at 10.00 mM NaHCO3 supplemented D. salina culture, although lipid content in these cultures (0.50 to 10.00 mM) did not vary significantly (Ref. Table S1). Therefore, it was aimed to evaluate 2.50 and 10.00 mM NaHCO3 augmented D. salina cells growing in individual pr combined nutrient (N and P) deficiency or limited condition for enhancing their BMPr as well as lipid yield.
NaHCO3 Addition to Individual Nutrient-limited Cultures
D. salina grows at high pH, therefore, supplementation with higher level of NaHCO3 in culture medium may overcome the lower availability of the dissolved inorganic carbon in media under high pH, resulting in better biomass as well as lipid productivity in this and other microalgal species [12, 13, 49,50,51]. The photoautotrophic growth of D. salina in the media, having individual nutrient deficiency (-N or –P) or limitation (Nlim or Plim), upon augmentation with 2.50 or 10.00 mM NaHCO3, showed the highest BMPr (57.03% more than control) in Nlim + C2 (1.25 mM nitrate + 10.00 mM NaHCO3) cultures (Fig. S2 and Table S2, supplementary information). Similar findings were reported in the diatom RGd-1 and the green alga Scenedesmus sp., where cultures amended with high NaHCO3 resulted in faster growth [48, 52]. In terms of lipid accumulation, nitrate starved cultures resulted in increased cellular lipid content upon the addition of NaHCO3 (Fig S3, supplementary information). It was also demonstrated in another green alga Scenedesmus sp. that increasing NaHCO3 level under nutrient starvation conditions led to de novo synthesis of lipids, and the carbon flux is channelized towards lipid anabolism [52]. Again, the role of HCO3 − was highlighted in augmenting the PS II electron transport efficiency as well as assembly of the Mn4CaO5 inorganic core of the oxygen evolving complex, thus validating the consensus that IC in the form bicarbonate can not only serve to replenish depleting CO2 in the medium under high pH, but also ameliorate cellular stress caused by possible factor [53]. However, Chavoshi and Shariati have reported that D. bardawil accumulated around 400 mg g−1 DCW of lipid upon treatment with 100 mM acetate (organic carbon), keeping the KNO3 concentration at 5.00 mM, suggesting that the mixotrophic growth may be useful for higher lipid production [54]. This indicates that the carbon flux increases either in the form of inorganic or organic carbon supplied to microalgal cells, inducing higher lipid production. There is also a report of increased lipid content (up to 540 mg g−1 DCW) in D. salina using De Walne’s media augmented with 100 mM NaHCO3, [13]. In contrast, in the current study, NaHCO3 levels beyond 20 mM formed precipitation (in MJM media), thus, limiting its application at a very high concentration. Therefore, the quantitative application of inorganic carbon seemed to be species-specific, and it largely depends on the medium composition.
NaHCO3 Addition to Combined Nutrient-limited Cultures
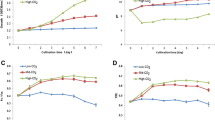
Effect of NaHCO3 addition on the photoautotrophic growth, biomass, and lipid production of D. salina cells under simultaneous nitrate and phosphate limitations is shown in Figs. 3A-E. The data revealed that the addition of bicarbonate to combined nutrient stress cultures whether limited or deficient for N and P, increased the BMPr. This again emphasized that carbon supplementation played important role in biomass production. The biomass productivity of D. salina cells was much dependent on the presence of nitrogen over phosphorus, although the latter was also necessary for the sustenance of the alga. Phosphate deficiency in combination with 1.25 mM KNO3 (Nlim-P) resulted in lower biomass (13.12 mgL−1d−1 DCW). The addition of 10.00 mM NaHCO3 to such cultures significantly enhanced the BMPr to 1.27 and 2.25-fold over the control and Nlim-P cultures respectively. Thus, augmented NaHCO3 might have enabled the D. salina cells to overcome low availability of CO2 in high pH media. As far as lipid content was concerned, a substantial increase was recorded in Nlim-P cultures supplemented with 10.00 mM NaHCO3 reaching 400.1 ± 25.1 mg g−1 DCW which was 2.02 and 1.17-fold over that of the control and Nlim-P cultures without 10.00 mM NaHCO3 respectively. This increment in BMPr compared to Nlim –P cultures might be affiliated to the stress ameliorating effect of bicarbonate which helped in maintaining the photosynthetic health of the cells as reflected by robust photosynthetic performance. The enhancement in lipid content may be correlated to the stress triggered out of phosphate deficiency at the beginning of the culture. Addition of higher bicarbonate only provided more carbon skeleton and energy (ATP) through robust photosynthesis for still higher lipid production [12, 13]. The lipid content in bicarbonate-augmented nitrate and phosphate-deficient cultures was in consonance with the reports for other microalgae such as Scenedesmus sp. [52], P. lutheri [55], and Chlorella sp. [56]. Bicarbonate-amended media (10.00 mM) under nitrate deficient conditions, showed relatively lower improvement in the BMPr owing to lowered chlorophyll content (< 48.66 ± 4.18 mg g−1 DCW) and photosynthetic inefficiency (Fv/Fm < 0.395 ± 0.17). Ameliorating effects of bicarbonate under nutrient stress has also been reported that showed increase in antioxidant activities of another microalga, Pachycladella codatii, thus playing a role in neutralizing ROS [57]. Gordillo and co-workers had also established the decreased carbon fixing ability in S. platensis under nitrate deprivation, despite the fact that inorganic carbon was available in the plenty [58]. It was inferred from the data presented in Fig. 3 that Nlim-P + C2 or NlimPlim + C2 cultures were appropriate for the biomass vis-à-vis lipid production.
Effect of NaHCO3 augmentation on photoautotrophic growth behaviour of D. salina cells under (A) both nitrate and phosphate deprivation (-N –P), (B) nitrate deprived and phosphate limitation (-N Plim), (C) nitrate limited and phosphate deprivation (Nlim –P), and (D) both nitrate and phosphate limited condition (NlimPlim) and Fig. 3E showed the effect of NaHCO3 augmentation on BMPr and LC in D. salina cultures after 12 d under concomitant KNO3 and KH2PO4 limitation. C1 ad C2 referred to addition of 2.50 and 10.00 mM NaHCO3 to the respective medium. Values are represented as mean ± SD with triplicate readings. Different letters suggest to significant difference (Tukey’s test; p < 0.05) in BMPr or lipid content at different nutrient conditions
Analysis of Effects of Individual/Concomitant Nutrient Limitations and NaHCO3 Addition at Different Time Points
It was clear from the preceding results that among the nitrate, phosphate, and bicarbonate treatments, nitrate was the most important nutrient for biomass as well as lipid production. It also helped in maintaining the cells healthy and even photosynthetic performance of the algal cultures. To understand the temporal role of each factor, and change in relative carbon flux towards lipid biosynthesis, a comparative analysis was done at three-time points i.e., 0, 6, and 12th d of the selected cultures. The experimental data including gene expression profiling was done at 0th d after 2 h of incubation period to allow stabilization of the cultures. Cultures at day 6 and 12 represented the logarithmic and stationary phases of the experiments respectively.
Effects on Biomass Productivity and Neutral Lipid Accumulation
Temporal variations in the BMPr, neutral lipid accumulation, and photosynthetic performance of D. salina cultures grown at different nutrient regimes are shown in Figs. 4 and 5. It was already seen that 10 mM NaHCO3 (C2) addition in all the cultures proved better for both biomass and lipid productions, and therefore, only C2-augmented cultures were selected for comparative analysis keeping aside C1-augmented cultures. The significant decrease in BMPr in Nlim-P + C2 and NlimPlim cultures after day 6 might be due to consumption of limited nitrate content (1.25 mM) that could again be the reason why neutral lipid content increased sharply in these cultures after the 6th d. Consequently, the production of neutral lipid was enhanced significantly both at 6th and 12th d under nutrient deficiency conditions. Higher values of NRF (nile red fluorescence) in –N and Nlim–P cultures at 6th d over that of Nlim (p < 0.05) suggested the immediate induction of lipid biosynthesis owing to the absence of nitrate and phosphate in these cultures respectively. Neutral lipid level reached an equal level in the Nlim and NlimPlim cultures at the 12th d which can be ascribed to exhaustion of limited nitrate available in the media. From this data, it can also be inferred that limited phosphate may have been sufficient for growth and did not cause significant stress. The increase in the neutral lipid content was qualitatively verified with confocal fluorescence micrographs of Nile Red stained D. salina cells at the 12th d showing prominent golden yellow fluorescence in –N, Nlim, Nlim–P and Nlim –P + C2 culture cells (Fig. S4, supplementary information).
Variation of BMPr and LC in different nutrient limitations along with NaHCO3 augmentation at three different time points; (A) biomass productivity and (B) neutral lipid accumulation in terms of Nile Red fluorescence. The mean value ± SD of triplicate readings are represented as vertical bars. Different letters suggest that means are significantly different (Tukey’s test; p < 0.05) with upper case letters stand for significant differences in folds of biomass productivity or Nile Red fluorescence at various time points within the same nutrient treatment while lower case letters signify significant differences in parameters between different nutrient conditions at same time points
Photosynthetic performance of D. salina cells exposed to deficiency or limitation of nitrate, phosphate individually or in combination or augmented with 10.00 mM NaHCO3 (C2); (A) Maximum photosynthetic quantum yield (Fv/Fm), (B) relative electron transport rate (rETR), and (C) non-photochemical quenching (NPQ). Values represented are mean ± SD of triplicate readings
Photosynthetic Status of D. salina Cells under Different Nutrient Conditions
The maximum quantum yield of cells at the 6th d was most adversely affected in –N (0.42 ± 0.02) and –N + C2 (0.55 ± 0.03) which reduced to 0.17 ± 0.01 and 0.19 ± 0.027 respectively at the 12th d, suggesting malfunctioning of photosystems due to nitrate non-availability (Fig. 5). Gao and co-workers had also recorded a sharp decline in Fv/Fm (17%) and rETR (36%) upon nitrate deprivation in D. salina [24]. Among the nitrate limited conditions without extra bicarbonate, Fv/Fm value was lowest in the Nlim–P followed by that of Nlim and NlimPlim. Thus, the absence of phosphate under Nlim condition had greater detrimental effect on photosystems over phosphate availability. However, Nlim and Nlim–P showed similar quantum yield at the 12th d signifying the exhaustion of nitrate at the end of the culture period in both the sets. Similarly, the performance of photosystems was completely compromised of –N and –N + C2 cells at the 12th d, inferring that extra bicarbonate can only have a limited ameliorating effect under nitrate-deprived condition, hence, improved quantum efficiency in all the Nlim cultures. Interestingly, rETR values in NlimPlim condition (28.97) were superior to control (25.96) and NlimPlim + C2 (28.76) owing to the cell’s desperate measure to sustain under such nutrient limitation with the availability of nitrate and phosphate at a critical level to maintain cellular photochemistry. The surplus light energy is dissipated in the form of heat (NPQ), keeping the entropy of photosystems under check. Data revealed the rise in NPQ with an increase in culture duration as well as deficiency of either of the nutrients. However, the addition of extra bicarbonate rendered stress amelioration effects.
Semi-quantitative Gene Expression Profiling of Selected Enzymes
Figures 6A-C represented the differential expression of accD, rbcL and rbcS genes at 0, 6, and 12th d in the selected cultures of D. salina. ACCase, the enzyme responsible for catalyzing the first and rate-limiting step of lipid biosynthesis, has been the point of interest for the genetic engineering perspective [59]. Thus, optimization of lipid over-production strategies must be accompanied by intricate evaluation of accD (the chloroplastidic subunit of ACCase) expression in such conditions. Nitrate-deprived cultures of D. salina triggered 1.84-fold surge in the expression of accD at the 6th d (p < 0.05), but only 1.62-fold at the 12th d. Post hoc analysis revealed that the change in accD level in –N cultures was significant between 0 and 6th d (p < 0.05) and non-significant between 6 and 12th d, indicating the triggering of accD gene expression in the earlier stage under –N condition. Conversely, under Nlim condition, maximum expression (2.23-fold) was monitored at 12th d against 1.14-fold at the 6th d (p < 0.05 between 6 and 12th d) indicating triggering of lipid synthesis at a relatively later stage in Nlim cultures with greater vigour. Nlim cultures afforded the acceleration of lipid production beyond the 6th d (since nitrogen remained available for a longer period). These data were in tune with the temporal variations in the NRF levels of the respective cultures. The data are supported by the findings of [60] who had shown significant up-regulation of accD expression in nitrate-limited C. sorokiniana cells. Similarly, Singh and co-workers found 4.4-fold increase in accD expression in C. sorokiniana cells cultured under limited nitrate and phosphate simultaneously [16]. It was also clear from the data that nitrogen deficient media and even its augmentation with that of C2 (10 mM HCO3 −) didn’t favour the expression of rbcL and rbcS genes as was evident from post hoc analysis (p = 0.243 and 0.993 for rbcL and rbcS at the 12th d respectively compared to control). This reduction in BMPr may be explained in the light of reports of [61] that inferred that nitrogen deprivation conditions inflicted reduced activities of RuBisCO, tryptophan synthase (β-subunit) and 16S ribosomal subunit. This led to inefficient photosynthesis, low amino-acid turnover, and a subsequent reduction in BMPr. Nutrient starvation in another green alga C. pyrenoidosa also negatively affected RuBisCO function as rbcL expression was down-regulated by 3–5 fold [62].
Differential expressions of (A) accD (first and rate limiting gene of lipid biosynthesis), (B) rbcL and (C) rbcS (involved in inorganic carbon fixation) genes in relation to varying culture regimes at three different time points. Vertical bars represent mean value ± SD of triplicate readings. Different letters suggest means are significantly different (Tukey’s test; p < 0.05). Upper case letters correspond to significant differences in folds of gene expression at different time points within the same nutrient treatment while lower case letters signify significant differences in relative gene expression between different nutrient conditions at the same time points
Interestingly, C2 addition to Nlim culture significantly raised the larger and smaller subunits of the RuBisCO on the 12th d by 2.53 and 1.78-fold respectively (p < 0.05) indicating that a critical threshold limit of nitrogen was necessary for over-expression of RuBisCO subunits for the assimilation of abundant inorganic carbon. Such cultures with a critical threshold limit of nitrogen and deficient in phosphate (Nlim–P) were recorded with 3.23-fold increase in accD gene transcripts compared to the control (p < 0.05). The addition of C2 resulted in the highest level of accD expression (6.23-fold, p < 0.05) which might be due to the concerted action of the cells to channelize all the resources towards lipid production as the phosphate deficiency triggered it from the beginning. A study on another algal species, C. sorokiniana has shown 9.6 and 2.8-fold enhancement in expression of accD and rbcL under combined stress involving metal and nutrients [16]. Expression of rbcL in Nlim–P remained higher and equivalent to the Nlim cultures, indicating that the phosphate deprivation had little impact on carbon capture in the D. salina cells. Therefore, it can be inferred that carbon augmentation to Nlim-P cultures may be adjusted accordingly for accD over-expression. As far as our knowledge goes, this is the first report in D. salina deciphering the critical threshold limit of carbon and nitrogen level in culture media coupled with the expression profiling of key genes involved in biomass and lipid accumulation to achieve higher levels simultaneously.
Conclusion
Overproduction of the lipid in nutrient(s) deficient growth media suffers from lower biomass productivity in D. salina. Among all the tested combinations of selected nutrients, limited nitrogen supplementation (1.25 mM), phosphate starvation along with augmentation of bicarbonate (10 mM), led lipid accumulation (~ 40% DCW) in D. salina with significant increase in biomass (27%) also, compared to the control. Such cultures were recorded with up-regulation of rbcL and rbcS as well as accD genes, indicating enhanced carbon flux in the cells. These cells showed photosynthetic quantum yield (> 0.5) reflecting healthy status. Results outlined the basis of selecting applicable nutrient stress condition for mass cultivation of D. salina. The over-expressed genes may act as target(s) for hyper production of lipids using genetic engineering approaches. Thus, the halophilic D. salina, a treasure of high value compounds including lipids must be studied in a well-defined manner for application prospective in industrial sector.
References
Field JL, Richard TL, Smithwick EA, Cai H, Laser MS, LeBauer DS, Long SP, Paustian K, Qin Z, Sheehan JJ, Smith P (2020) Robust paths to net greenhouse gas mitigation and negative emissions via advanced biofuels. Proc Natl Acad Sci 117(36):21968–21977. https://doi.org/10.1073/pnas.1920877117
Urtubia HO, Betanzo LB, Vásquez M (2016) Microalgae and cyanobacteria as green molecular factories: tools and perspectives. In: Thajuddin N, Dhanasekaran D (ed) Algae—Organisms for Imminent Biotechnology June 2016, Intech Open, London, pp-1–27
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131. https://doi.org/10.1016/j.tibtech.2007.12.002
Pirwitz K, Rihko-Struckmann L, Sundmacher K (2015) Comparison of flocculation methods for harvesting Dunaliella. Bioresour Technol 196:145–152. https://doi.org/10.1016/j.biortech.2015.07.032
Cardoso LG, Duarte JH, Costa JAV, de Jesus Assis D, Lemos PVF, Druzian JI, ... Chinalia FA (2021) Spirulina sp. as a bioremediation agent for aquaculture wastewater: production of high added value compounds and estimation of theoretical biodiesel. Bioenergy Res 14(1): 254–264. https://doi.org/10.1007/s12155-020-10153-4
Wilson MH, Shea A, Groppo J, Crofcheck C, Quiroz D, Quinn JC, Crocker M (2021) Algae-based beneficial re-use of carbon emissions using a novel photobioreactor: a techno-economic and life cycle analysis. Bioenergy Res 14(1):292–302. https://doi.org/10.1007/s12155-020-10178-9
Khadim SR, Singh P, Singh AK, Tiwari A, Mohanta A, Asthana RK (2018) Mass cultivation of Dunaliella salina in a flat plate photobioreactor and its effective harvesting. Bioresour Technol 270:20–29. https://doi.org/10.1016/j.biortech.2018.08.071
Ahmed RA, He M, Aftab RA, Zheng S, Nagi M, Bakri R, Wang C (2017) Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-07540-x
Bonnefond H, Moelants N, Talec A, Bernard O, Sciandra A (2016) Concomitant effects of light and temperature diel variations on the growth rate and lipid production of Dunaliella salina. Algal Res 14:72–78. https://doi.org/10.1016/j.algal.2015.12.018
Byrd SM, Burkholder JM (2017) Environmental stressors and lipid production in Dunaliella spp. II. Nutrients, pH, and light under optimal or low salinity. J Exp Mar Biol Ecol 487:33–44. https://doi.org/10.1016/j.jembe.2016.11.006
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29. https://doi.org/10.1016/j.bej.2014.10.019
Gardner RD, Cooksey KE, Mus F, Macur R, Moll K, Eustance E, Carlson RP, Gerlach R, Fields MW, Peyton BM (2012) Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp. and the diatom Phaeodactylum tricornutum. J Appl Phycol 24(5):1311–1320. https://doi.org/10.1007/s10811-011-9782-0
Srinivasan R, Mageswari A, Subramanian P, Suganthi C, Chaitanyakumar A, Aswini V, Gothandam KM (2018) Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-25417-5
Behera B, Unpaprom Y, Ramara R, Maniam GP, Govindan N, Paramasivan B (2021) Integrated biomolecular and bioprocess engineering strategies for enhancing the lipid yield from microalgae. Renew Sust Ener Rev 148:111270. https://doi.org/10.1016/j.rser.2021.111270
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energy J 5(5):1532–1553. https://doi.org/10.3390/en5051532
Singh P, Kumari S, Guldhe A, Singh G, Bux F (2017) ACCase and rbcL gene expression as a function of nutrient and metal stress for enhancing lipid productivity in Chlorella sorokiniana. Energy Convers Manag 148:809–819. https://doi.org/10.1016/j.enconman.2017.06.054
Cao J, Yuan H, Li B, Yang J (2014) Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour Technol 152:177–184. https://doi.org/10.1016/j.biortech.2013.10.084
Mirizadeh S, Nosrati M, Shojaosadati SA (2020) Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stage cultivation. Bioenergy Res 13(2):507–517. https://doi.org/10.1007/s12155-019-10077-8
Goyal A, Shiraiwa Y, Husic HD, Tolbert NE (1992) External and internal carbonic anhydrases in Dunaliella species. Mar Biol 113(3):349–355. https://doi.org/10.1007/BF00349158
Singh P, Khadim R, Singh AK, Singh U, Maurya P, Tiwari A, Asthana RK (2019) Biochemical and physiological characterization of a halotolerant Dunaliella salina isolated from hypersaline Sambhar Lake. India J Phycol 55(1):60–73. https://doi.org/10.1111/jpy.12777
Sasaki Y, Nagano Y (2004) Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci Biotechnol Biochem 68(6):1175–1184. https://doi.org/10.1271/bbb.68.1175
Madoka Y, Tomizawa KI, Mizoi J, Nishida I, Nagano Y, Sasaki Y (2002) Chloroplast transformation with modified accD operon increases acetyl-CoA carboxylase and causes extension of leaf longevity and increase in seed yield in tobacco. Plant Cell Physiol 43(12):1518–1525. https://doi.org/10.1093/pcp/pcf172
Andersson I, Backlund A (2008) Structure and function of Rubisco Plant Physiol. Biochem 46(3):275–291. https://doi.org/10.1016/j.plaphy.2008.01.001
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491. https://doi.org/10.1016/j.biortech.2013.08.066
Bongiovani N, Popovich CA, Martínez AM, Constenla D, Leonardi PI (2020) Biorefinery approach from Nannochloropsis oceanica CCALA 978: neutral lipid and carotenoid co-production under nitrate or phosphate deprivation. Bioenergy Res 13(2):518–529. https://doi.org/10.1007/s12155-019-10045-2
Yen HW, Hu IC, Chen CY, Ho SH, Lee DJ, Chang JS (2013) Microalgae-based biorefinery–from biofuels to natural products. Bioresour Technol 135:166–174. https://doi.org/10.1016/j.biortech.2012.10.099
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546. https://doi.org/10.3389/fmicb.2016.00546
Borovkov AB, Gudvilovich IN, Avsiyan AL (2020) Scale-up of Dunaliella salina cultivation: from strain selection to open ponds. J Appl Phycol 32(3):1545–1558. https://doi.org/10.1007/s10811-020-02104-5
Chen Y, Tang X, Kapoore RV, Xu C, Vaidyanathan S (2015) Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Convers Manag 106:61–72. https://doi.org/10.1016/j.enconman.2015.09.025
Wellburn AR, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls A and B of leaf extracts in different solvents. In: Sybesma C (ed) Advances in photosynthesis research. Advances in Agricultural Biotechnology, vol 2. Springer, Dordrecht, pp 9–12. https://doi.org/10.1007/978-94-017-6368-4_3
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 9(3):e91957. https://doi.org/10.1371/journal.pone.0091957
Hathwaik LT, Redelman D, Samburova V, Zielinska B, Shintani DK, Harper JF, Cushman JC (2015) Transgressive, reiterative selection by continuous buoyant density gradient centrifugation of Dunaliella salina results in enhanced lipid and starch content. Algal Res 9:194–203. https://doi.org/10.1016/j.algal.2015.03.009
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998. https://doi.org/10.1093/jxb/ert208
Tiwari A, Singh P, Riyazat Khadim S, Singh AK, Singh U, Singh P, Asthana RK (2019) Role of Ca2+ as protectant under heat stress by regulation of photosynthesis and membrane saturation in Anabaena PCC 7120. Protoplasma 256(3):681–691. https://doi.org/10.1007/s00709-018-1328-8
Hong L, Liu JL, Midoun SZ, Miller PC (2017) Transcriptome sequencing and annotation of the halophytic microalga Dunaliella salina. J Zhejiang Univ Sci B 18(10):833–844. https://doi.org/10.1631/jzus.B1700088.10.1631/jzus.B1700088
Bental M, Pick U, Avron M, Degani H (1991) Polyphosphate metabolism in the alga Dunaliella salina studied by 31P-NMR. Biochim Biophys Acta Mol Cell Res 1092(1):21–28. https://doi.org/10.1016/0167-4889(91)90173-U
Fu L, Li Q, Yan G, Zhou D, Crittenden JC (2019) Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol Biofuels 12(1):1–9. https://doi.org/10.1186/s13068-019-1458-z
Uarrota VG, Stefen DLV, Leolato LS, Gindri DM, Nerling D (2018) Revisiting carotenoids and their role in plant stress responses: from biosynthesis to plant signalling mechanisms during stress. In: Gupta D, Palma J, Corpas F (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham. https://doi.org/10.1007/978-3-319-75088-0_10
Cui M, Liu Y, Zhang J (2020) The variation of growth rate, photosynthetic activity, and biodiesel productivity in Synechocystis sp. PCC 6803 under antibiotic exposure. Bioenergy Res 13(3):955–962. https://doi.org/10.1007/s12155-020-10114-x
Brindley Alías C, García-Malea López MC, Acién Fernández FG, Ferníndez Sevilla JM, García Sánchez JL, Molina Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87(6):723–733. https://doi.org/10.1002/bit.20179
Bemejo-Padilla E, Kinsou H, Filali R, Perez-Bibbins B, Taidi B (2021) Rapid indicators for monitoring the health of Chlamydomonas nivalis biomass during preservation. J Appl Phycol 33(5):2723–2732. https://doi.org/10.1007/s10811-021-02517-w
Burrows EH, Bennette NB, Carrieri D, Dixon JL, Brinker A, Frada M, Baldassano SN, Falkowski PG, Dismukes GC (2012) Dynamics of lipid biosynthesis and redistribution in the marine diatom Phaeodactylum tricornutum under nitrate deprivation. Bioenergy Res 5(4):876–885. https://doi.org/10.1007/s12155-012-9201-7
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25(1):311–318. https://doi.org/10.1007/s10811-012-9865-6
Burch AR, Franz AK (2016) Combined nitrogen limitation and hydrogen peroxide treatment enhances neutral lipid accumulation in the marine diatom Phaeodactylum tricornutum. Bioresour Technol 219:559–565. https://doi.org/10.1016/j.biortech.2016.08.010
Almutairi AW (2020) Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ Sci Pollut Res 27(26):32296–32303. https://doi.org/10.1007/s11356-020-08531-8
Burns BD, Beardall J (1987) Utilization of inorganic carbon by marine microalgae. J Exp Mar Biol Ecol 107(1):75–86. https://doi.org/10.1016/0022-0981(87)90125-0
Moll KM, Gardner RD, Eustance EO, Gerlach R, Peyton BM (2014) Combining multiple nutrient stresses and bicarbonate addition to promote lipid accumulation in the diatom RGd-1. Algal Res 5:7–15. https://doi.org/10.1016/j.algal.2014.04.002
Srinivasan R, Kumar VA, Kumar D, Ramesh N, Babu S, Gothandam KM (2015) Effect of dissolved inorganic carbon on β-carotene and fatty acid production in Dunaliella sp.. Appl Biochem Biotechnol 175(6):2895–906. https://doi.org/10.1007/s12010-014-1461-6
White DA, Pagarette A, Rooks P, Ali ST (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25(1):153–165. https://doi.org/10.1007/s10811-012-9849-6
Qi M, Yao C, Sun B, Cao X, Fei Q, Liang B, Ran W, Xiang Q, Zhang Y, Lan X (2019) Application of an in situ CO2–bicarbonate system under nitrogen depletion to improve photosynthetic biomass and starch production and regulate amylose accumulation in a marine green microalga Tetraselmis subcordiformis. Biotechnol Biofuels 12(1):1–21. https://doi.org/10.1186/s13068-019-1523-7
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Shevela D, Eaton-Rye JJ, Shen JR (2012) Photosystem II and the unique role of bicarbonate: a historical perspective. Biochim Biophys Acta (BBA)-Bioenergetics 1817(8):1134–1151. https://doi.org/10.1016/j.bbabio.2012.04.003
Chavoshi ZZ, Shariati M (2019) Lipid production in Dunaliella bardawil under autotrophic, heterotrophic and mixotrophic conditions. Braz J Oceanogr 67. https://doi.org/10.1590/S1679-87592019024906709
Guihéneuf F, Stengel DB (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11(11):4246–4266. https://doi.org/10.3390/md11114246
Mondal M, Ghosh A, Oinam G, Tiwari ON, Gayen K, Halder GN (2017) Biochemical responses to bicarbonate supplementation on biomass and lipid productivity of Chlorella sp. BTA9031 isolated from coalmine area. Environ Prog Sustain Energy 36(5):1498–1506. https://doi.org/10.1002/ep.12594
Fawzy MA, Abdel-Wahab DA, Hifney AF (2017) Physiological and biochemical responses of the green alga Pachycladella chodatii (SAG 2087) to sodicity stress. Egypt J Basic Appl Sci 4(1):30–36. https://doi.org/10.1016/j.ejbas.2016.11.001
Gordillo FJ, Jiménez C, Figueroa FL, Niell FX (1998) Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J Appl Phycol 10(5):461–469. https://doi.org/10.1023/A:1008090402847
Talebi AF, Tohidfar M, Bagheri A, Lyon SR, Salehi-Ashtiani K, Tabatabaei M (2014) Manipulation of carbon flux into fatty acid biosynthesis pathway in Dunaliella salina using AccD and ME genes to enhance lipid content and to improve produced biodiesel quality. Biofuel Res J 1(3):91–97. https://doi.org/10.18331/BRJ2015.1.3.6
Li YX, Zhao FJ, Yu DD (2015) Effect of nitrogen limitation on cell growth, lipid accumulation and gene expression in Chlorella sorokiniana. Braz Arch Biol Technol 58(3):462–467. https://doi.org/10.1590/S1516-8913201500391
Msanne J, Xu D, Konda AR, Casas-Mollano JA, Awada T, Cahoon EB, Cerutti H (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydomonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59. https://doi.org/10.1016/j.phytochem.2011.12.007
Fan J, Cui Y, Wan M, Wang W, Li Y (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7(1):1–4. https://doi.org/10.1186/1754-6834-7-17
Acknowledgements
We gratefully acknowledge the Head and Coordinators of CAS in Botany, DST-FIST, ISLS, BHU for providing research facilities.
Funding
The financial supports are also acknowledged by RKA (IOE Scheme No 6031), SRK (09/013/(0574)/2015-EMR-I), AM (09/013(0733)/2017-EMR-I) to CSIR, New Delhi; PS (DSKPDF; No. F.4–2/2006(BSR)/BL/19–20/162) to UGC, PM (19/06/2016(i)EU-V).
Author information
Authors and Affiliations
Contributions
SRK: Design of the experiments, execution, data collection and ms writing; AM: Methodology characterization and experimentation; PS: Isolation of D. salina from natural sample, supply of related gene data for primer designing after transcriptomic analysis, Software, data interpretation; PM: Data analysis/interpretation and support in mass cultivation during experimentation; AKS1: Coordination in doing fluorescence microscopy and discussion, software; AKS2: Visualization, Discussion and designing help in validation; RKA: Sample collection from hypersaline Sambhar lake Rajsthan, India. Conceptualization and the direction in designing, writing of ms and compilation. All authors approve the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Riyazat Khadim, S., Mohanta, A., Singh, P. et al. A Study on Dunaliella salina Under Selected Nutrient Manipulation with Reference to the Biomass, Lipid Content Along with Expression of ACCase and RuBisCO Genes. Bioenerg. Res. 16, 622–637 (2023). https://doi.org/10.1007/s12155-022-10460-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10460-y