Abstract
This study was designed to assess the synergistic effects of nitrogen (N) and phosphorus (P) concentrations on oil content, fatty acid profile, and predicted fuel properties of Dunaliella salina. Axenic D. salina cells were grown in F/2 growth medium of salinity 34 ppt containing 33.6 g.l−1 ultramarine synthetic sea salt. Growth dry weight, cell count, and their relationship were measured, and oils were extracted by soaking following Soxhlet extraction. Growth dry weight was markedly affected by N and P concentrations, with maximum growth dry weights of cultures grown at recommended N and P concentrations (control), half of the recommended N concentration (0.5 N) and (0.5 N/0.5P) being 0.911 g.l−1, 0.755 g.l−1, and 0.615 g.l−1, respectively. Oil content showed the reverse pattern, with cultures grown in the absence of phosphorus (0.0P), full N/P starvation (0.0 N/0.0P), and control resulting in maximum oil contents of 24.86%, 22.85%, and 5.88%, respectively. The majority of fatty acid methyl esters ranged between C14 and C22. Estimated fuel properties of algal cells grown under NP stress conditions were found to meet the American Society for Testing and Materials (ASTM) and European Committee for Standardization (EN) guidelines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing global usage of energy, the depletion of conventional fossil fuels, and the environmental and human health problems related to the widespread combustion of fossil fuels have driven the search for new sources of energy that are renewable, sustainable, and environmentally friendly (Chandra et al. 2019; Yun et al. 2016). In terms of biofuel production, microalga-derived lipids are regarded as a sustainable alternative feedstock (Sabu et al. 2017). Apart from being sustainable, microalgae-derived biofuels are non-toxic and biodegradable. Moreover, they give rise to lower greenhouse gas (GHG) emissions than conventional fuels, establishing them as cleaner alternatives to fossil fuels (El-Sheekh et al. 2019; Chen et al. 2015).
A third generation biofuels derived from algae are considered more sustainable than first- and second-generation biofuels derived from food crops and non-food feedstocks, respectively (Chen et al. 2017). Microalgae are photosynthetic microorganisms with growth rates 20 to 30 times higher than plants and have the ability to grow in non-arable soils using water unsuitable for human consumption and agriculture. Moreover, lipids can be harvested from these microorganisms all year around, resulting in higher lipid productivity than is attained using oleaginous plants (Chen et al. 2017; El-Kassas et al. 2017; Huete-Ortega et al. 2018).
Algal cultures are important sources of many energy products, including oils, alcohols, methane, and hydrogen. For example, Chlorella vulgaris grown under stress conditions have yielded lipid contents of 40–56% (Illman et al. 2000; Abo El-Khair et al. 2015; El-Sayed et al. 2017). Microalgae store excess solar energy captured through photosynthesis as neutral lipids, often as high levels of triacylglycerols (TAGs), which are substrates, along with alcohols and an alkaline/acid catalyst, for the production of biodiesel through transesterification reactions (Chen et al. 2011; Matsakas et al. 2017; Yodsuwan et al. 2017). Despite their potential to produce biodiesel, microalgae have not been utilized commercially because their lipid content is too low, and the cost of biodiesel production is higher than that of fossil fuels (Behzadi and Farid 2009). However, increasing the lipid productivity of microalgae may make their use in the production of biodiesel commercially feasible (Kamalanathan et al. 2015; Burch and Franz 2016; Gao et al. 2018). Reducing these costs and increasing the lipid contents of algal biomasses are therefore necessary for commercial biodiesel production (Converti et al. 2009).
The low productivity of microalgal biomass resulting from low nutrient concentrations has been associated with high lipid content and productivity (Li et al. 2016). However, not all oils produced by algae can be utilized in biodiesel (Guschina and Harwood 2006). For example, biodiesel with a high content of unsaturated fatty acids (USFA) has been shown to emit more nitrogenous oxides and to have lower thermal efficiency than biodiesel with a higher content of saturated fatty acids (SFAs) (Gopinath et al. 2010). Moreover, the fuel properties of biodiesel were found to be influenced by both fatty acids and alcohols contained in fatty acid methyl esters (FAMEs) (Knothe 2008). Therefore, the ability of algal oil to substitute for diesel fuel depends on the fatty acid properties of the former (Da Silva et al. 2009). In addition, certain microalgal species have more appropriate fatty acid profiles, with the unsaponified fraction allowing the produced biodiesel to have high oxidation stability (OS) (Dote et al. 1994; Minowa et al. 1995). Furthermore, FAME properties showed high correlations with fuel parameters, such as cetane number (CN), iodine value (IV), and Kinematic viscosity (KV), indicative of the potential of biofuels to be viable alternatives to conventional fuels for internal combustion engines (Sokoto et al. 2011).
Microalgal lipid metabolism can be manipulated to enhance lipid accumulation, for example by altering the growing conditions and culture parameters of microalgae. Nutrient availability, pH, temperature, and light intensity can enhance lipid accumulation by microalgae (Li et al. 2016). The limitation or deprivation of nutrients, particularly nitrogen and phosphorus, has been reported to enhance the yield of TAGs in several species of microalgae. Lipid accumulation in algal cells is markedly affected by nutrient deficiency (Bharte and Desai 2019; Huete-Ortega et al. 2018). For example, a reduction in sodium nitrate concentration in the medium, from 1.5 g.l−1 to 0.375 g.l−1, increased lipid content three-fold, from 5.90% to 15.31%, in Chlorella vulgaris cells (Converti et al. 2009). Moreover, although nitrogen limitation had a greater influence on lipid accumulation than phosphate limitation, limitation of both nutrients simultaneously synergistically enhanced lipid accumulation (Yodsuwan et al. 2017).
The green alga Dunaliella salina has attracted attention for biofuel production because of its ability to tolerate NaCl concentrations ranging from 0.05 to 5.0 M. D. salina becomes the dominant species in high salinity cultures through limiting contamination with competitors and predators. Under stress conditions, some strains of D. salina produce high amounts of β-carotene and accumulate high concentrations of total lipids, with these strains used commercially in the production of these products (Chen et al. 2015; Bonnefond et al. 2017).
This study analyzed the effects of combined abiotic stress, induced by altering the concentrations of nitrogen and phosphorus, on the yield and quality of lipids produced by D. salina. The aim of this study was to provide a framework for assessing the effects of limiting other nutrients on TAG accumulation by D. salina under stress conditions.
Materials and methods
Microalgal culture and growth medium
D. salina (strain CCAP 19/12) was obtained from the Culture Collection of Algae and Protozoa (CCAP, UK). A liquid D. salina sample was subcultured in F2 growth medium containing 33.6 g.l−1 artificial sea salt (Guillard and Ryther 1962) at 25 ± 1 °C in a 100 ml flask continuously illuminated with LED lights (70 μmol.m−2.s−1).
Experimental design
D. salina cultures were grown in F2 growth medium containing 33.6 g.l−1 artificial sea salt and various nitrogen and phosphorus concentrations to determine their combined effects on lipid accumulation. Experiments were performed in 250 ml flasks with three biological replicates. The normal (control) treatment was defined as the concentrations of nitrogen and phosphorus in F2 medium. The other combinations of N/P concentration examined are listed in Table 1.
The optical density of D. salina cultures at 650 nm was measured daily using a Shimadzu UV-3600 spectrophotometer to determine their growth rates. To measure the biomass productivity of D. salina, samples on the last day of culture were centrifuged at 3000 g for 10 min, and the pellets were transferred to pre-weighed 1.5 m Eppendorf tubes to measure wet weight. The samples were subsequently freeze-dried at − 80 °C, and their dry weight was determined.
Relationship between OD at 650 nm and cell count
Cell count was calibrated relative to optical density at 650 nm as described by Skoog et al. (2007).
Extraction and determination of lipids
The extraction of lipids from D. salina was initiated by overnight soaking in a 3:2 (v/v) mixture of n-hexane: isopropanol in preweighed cellulose extraction thimbles (33 × 94 mm) followed by Soxhlet extraction (El-Sayed et al. 2017). The thimbles were washed with water, dried at 105 °C for 60 min and reweighed, with the differences in weight regarded as the initial lipid content.
FAME profile
Fatty acid methyl esters of D. salina oils were identified and determined by gas chromatography (GC) using a GC Perkin Elmer Auto System XL.
Fuel properties
Fuel properties, including CN, saponification value (SV), IV, degree of unsaturation (DU), long-chain saturated factor (LCSF) and cold filter plugging point (CFPP) were determined as described by Mittelbach and Remschmidt (2004).
Statistical analysis
All experiments were performed in triplicate, and data were recorded as the mean and standard deviation (SD). The effects of the treatments were tested by one-way analysis of variance (ANOVA) using STATISTICA Software (version 7.0) from StatSoft Inc. (2004). Results were considered statistically significant when p < 0.05.
Results and discussion
Growth profile of D. salina
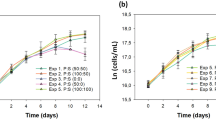
Dry weight, the relationship between optical density at 650 nm and cell count and growth curve of D. salina cultured in media containing different concentrations of nitrogen and phosphorus are shown in Fig. 1. Over the 10-day growth period, optimum growth was observed in cultures supplemented with full nitrogen and phosphorus concentrations (control), resulting in a dry weight of 0.911 ± 0.020 g.l−1. In comparison, previous studies have yielded dry weights of 0.34 g.l−1 (Zhu and Lee 1997) and 2.113 g.l−1 (Emeish 2012). Culture under conditions of nutrient depletion and/or imbalance was found to markedly reduce dry weight. For example, dry weight of D. salina cultured in medium containing (0.0 N) was 0.3 ± 0.023 g.l−1, whereas dry weight after culture in medium lacking both nitrogen and phosphorus (0.0 N/0.0P) was 0.35 ± 0.013 g.l−1. Dry weights were higher, however, when D. salina was cultured in medium containing (0.5 N) (0.775 ± 0.150 g. l−1, p < 0.05).
a Effects of nitrogen and phosphorus concentrations on the growth dry weight (g.l−1) of D. salina. The lower case letters above the bars indicate statistically significant differences from cells grown under control conditions (p < 0.05). b Relationship between cell counts and optical density at 650 nm of D. salina grown under control conditions, and c Growth curves of D. salina at different nitrogen-phosphorus concentrations
Nutrient depletion and imbalance are quite well understood, but their effects vary among algal species, with different growth patterns being due to carotenogenesis factors. For example, carotenogenesis was induced in five algal species of the genus Chlorophyta by 90% nitrogen depletion, but the pigments formed varied among species (El-Sayed 1999). Carotenogenesis, defined as the massive accumulation of carotenes and carotenoids associated with the accumulation of algal oils, is enhanced by nitrogen and phosphorus depletion and by their relationship. Under such conditions, algal metabolism tends to shift to the production of oils and carotene to proceed through their life cycle or to enhance photosynthetic processes by accumulating compounds rich in carbon to form oil, acids, and triglycerides (Gallego-Cartagena et al. 2019; Minyuk et al. 2017; Solovchenko and Neverov 2017). Nitrogen starvation induces growth failure, reducing dry weight and oil production.
Nutrient limitations also greatly affected cell structure and metabolites. Following the decomposition of chlorophyll and proteins, cell division is reduced and spherical cells are produced, resulting in reductions in dry weight (Belotti et al. 2013). Dry weight accumulation was closely associated with the concentrations of nitrogen and phosphorus in the medium (Fig. 1a). Decreased nitrogen content led to blockage or alteration of photosynthetic processes due to chlorophyll decomposition. This effect was markedly enhanced by decreasing phosphorus concentration, which in turn affected energy storage. Dry weight was significantly higher, however, when algal cells were cultured in the presence of (0.5 N/0.5P) than when cultured in the absence of either or both (p < 0.05). A previous study reported that complete omission of nitrogen during the mass cultivation of algae reduced oil and carotenoid contents, with at least 10% nitrogen plus 100% phosphorus required for optimal production (El-Sayed 1999).
Oil content
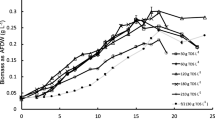
The oil content of D. salina was markedly affected by different nitrogen and/or phosphorus limitations. In contrast with methanol or chloroform, which extract mostly polar compounds, most of the oils extracted from D. salina by n-hexane and isopropanol were neutral or non-polar (Fig. 2).
The neutral lipid content of cells grown under control conditions was found to be 5.88 ± 0.85%. Nitrogen limitation (0.5 N) increased the neutral lipid content to 7.81 ± 1.10%, whereas severe nitrogen limitation (0.0 N) resulted in a neutral lipid content of 10.50 ± 1.30% (p < 0.05). The increase in neutral lipid contents in response to nitrogen limitation is regarded as a defense mechanism of algal cells against protein decomposition and reduced growth dry weight (Chokshi et al. 2017; Khan et al. 2018) (Table 2).
Phosphorus concentration was found to have a greater effect than nitrogen on oil accumulation by D. salina. A 50% reduction in phosphorus increased oil content about 3-fold compared with cultures grown under control conditions (16.45 ± 1.25% vs. 5.88 ± 0.85%), whereas severe phosphorus reduction (0.0P) increased oil content to 24.86 ± 2.30%, more than 4-fold higher than control. Cells grown in (0.5 N/0.5P) medium had an oil content of 12.77 ± 1.10%, whereas cells grown in medium lacking both nitrogen and phosphorus had an oil content of 22.85 ± 1.35% (p < 0.05). Moreover, an inverse correlation (r = − 0.67) was observed between biomass and lipid content (Fig. 3). For example, under control conditions, the oil content was 5.88%, and the biomass was 0.911 g.l−1, whereas at (0.0 N/0.0P) the oil content was 22.85%, and the biomass was 0.35 g.L−1, findings similar to those observed previously (White et al. 2011).
In general, reductions in nitrogen and phosphorus concentrations lead to an increase in lipid content, including an increase in long chain fatty acids and USFA, and a decrease in biomass. Stress induces the production of free radicals, which can injure these cells. Cells tend to protect themselves against free radicals by accumulating di-carbon fragments to form fatty acids and by synthesizing USFA, which act as free radical scavengers. These steps are preceded by chlorophyll decomposition and the accumulation of high concentrations of carotenoids. Carotenoids are oil soluble pigments requiring a pool of intracellular oil. Thus, the combination of nitrogen deficiency and chlorophyll decomposition inversely correlates with high concentrations of carotenoids and oil content (Griffiths and Harrison 2009; Valledor et al. 2014).
Prediction of D. salina fuel properties
Several fuel properties of lipids are related to the ratio of USFAs to SFAs (Table 3). Nutrient limitations do not directly affect fuel properties; rather they affect FAME profiles by increasing both long chain and highly saturated-fatty acids, while having a negative effect on the net gain of dry biomass (Nouri et al. 2019).
The European standard for biodiesel has limited its IV, defined as the DU of a mixture of fatty acids, to 120 g I2/100 g (Mittelbach and Remschmidt 2004). IV was found to correlate highly with the DU (Lin et al. 2006). D. salina grown under control conditions had an IV of 100, but IV was only slightly higher (107.62) when grown at (0.5 N/0.5P). Other treatments resulted in lower IV or had no effect. SV showed an inverse pattern, being higher under nitrogen limiting (197.57) than control (182.77) conditions.
Fuel quality must meet the biodiesel standards for CN, kinematic viscosity (KV), and OS (Mittelbach and Remschmidt 2004), as they are determined by the cloud and pour points (Knothe 2008). CN correlates directly with fatty acid chain length and the number of saturated bonds (Knothe et al. 1998). Polyunsaturated fatty acids, including the esters of linoleic (C18:2) and linolenic (C18:3) acids, have low CN (Knothe et al. 2003).
CFPP has been shown to correlate with saturated FAME (SFAME) content (Ramos et al. 2009), with LCSF being predictive of and directly correlating with CFPP. In contrast, longer carbon chains (C22:0 and C24:0) in biodiesel are associated with poorer low-temperature properties (Wu et al. 2005).
One of the major issues affecting the use of biodiesel containing polyunsaturated FAMEs is OS (Knothe 2006), which is influenced by several factors, mainly the presence of double bonds (Bajpai and Tyagi 2006). OS decreases as the contents of polyunsaturated FAMEs increase (Knothe 2005). The oxidative stability of fuels is therefore affected by the amounts of oleic, linoleic, and linolenic acid esters, with increases in these compounds resulting in fuel deterioration. Small amounts of more highly unsaturated fatty compounds had a disproportionately stronger effect. The most frequent types of SFAMEs observed in D. salina were palmitic acid, C16:0 (12.16–26.03%), and myristic acid, C14:0 (1.29–2.13%). Another study reported that the SFA content of this species was 82% (Mallick et al. 2012), highlighting its high OS and excellent CN. Polyunsaturated fatty acids containing four or more double bonds are frequently observed in microalgal oil. These bonds are susceptible to oxidation, generating higher nitrogen oxide contents and exhibiting lower thermal efficiency than biodiesel containing SFAs (Chisti 2007). Thus, microalgal oils containing polyunsaturated fatty acids are less acceptable for the production of biodiesel (Chisti 2007). The very low oxidative potential of a mixture of C16:1, C18:1, and C14:0 fatty acids in a 5:4:1 ratio suggests that this mixture may be ideal for biodiesel (Schenk et al. 2008). The present work found that this ratio in control cultures of D. salina was nearly 3:30:1, varying in response to changes in nitrogen and phosphorus concentrations, and suggesting that the concentrations of C16:1, C18:1, and C14:0 acids in control cultures require modification. The highly USFA C18:2 and C18:3 have low CNs and high IVs. Also, increasing the contents of polyunsaturated fatty acids reduced OS, but did not change CFPPs, as long carbon chain saturated FAMEs had the worst CFPP values. Thus, appropriate percentages of SFA and USFA are required for these algae to be used as biodiesel feedstock.
Conclusion
Certain nutrients, especially nitrogen, affect the amount of oil produced by growing algae, with the concentration of nitrogen in association with energy storage factors like phosphorus greatly affecting both oil content and fuel priorities. Nitrogen and phosphorus affect the oil content of these organisms by altering their FAME profiles, including both the carbon chain length and the degree of saturation. Biodiesel produced by algae varies widely, depending on the types of FAMEs, particularly the ratio of SFA to USFA. The high sensitivity of algae to ambient nutritional and environmental conditions suggests that fatty acid ratios can be controlled by optimizing nutrient balance, enhancing the feasibility of biodiesel production.
Abbreviations
- FAME:
-
Fatty acid methyl ester
- LCSF:
-
Long-chain saturated factor
- TAG:
-
Triacylglycerol
- DU:
-
Degree of unsaturation
- CN:
-
Cetane number
- USFA:
-
Unsaturated fatty acids
- SV:
-
Saponification value
- CFPP:
-
Cold filter plugging point
- IV:
-
Iodine value
- KV:
-
Kinematic viscosity
- SFA:
-
Saturated fatty acid
- CF:
-
Cold flow
- OS:
-
Oxidation stability
References
Bajpai D, Tyagi VK (2006) Biodiesel: source, production, composition, properties and its benefits. J Oleo Sci 55:487–502
Behzadi S, Farid MM (2009) Production of biodiesel using a continuous gas–liquid reactor. Bioresour Technol 100:683–689
Belotti G, Bravi M, Caprariis BD, Filippis PD, Scarsella M (2013) Effect of nitrogen and phosphorus starvations on Chlorella vulgaris lipids productivity and quality under different trophic regimens for biodiesel production. Am J Plant Sci 4:44–51
Bharte S, Desai K (2019) The enhanced lipid productivity of Chlorella minutissima and Chlorella pyrenoidosa by carbon coupling nitrogen manipulation for biodiesel production. Environ Sci Pollut Res 26:3492–3500
Bonnefond H, Moelants N, Talec A, Mayzaud P, Bernard O, Sciandra A (2017) Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol Biofuels 10:25
Burch AR, Franz AK (2016) Combined nitrogen limitation and hydrogen peroxide treatment enhances neutral lipid accumulation in the marine diatom Phaeodactylumtricornutum. Bioresour Technol 219:559–565
Chandra R, Amit S, Ghosh UK (2019) Effects of various abiotic factors on biomass growth and lipid yield of Chlorella minutissima for sustainable biodiesel production. Environ Sci Pollut Res 26:3848–3861
Chen M, Tang H, Ma H, Holland TC, Ng KY, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655
Chen Y, Tang X, Kapoore RV, Xu C, Vaidyanathan S (2015) Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Convers Manag 106:61–72
Chen B, Wan C, Mehmood MA, Chang JS, Bai F, Zhao X (2017) Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products-a review. Bioresour Technol 244:1198–1206
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmusdimorphus. Biotechnol Biofuels 10:60
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Dote Y, Sawayama S, Inoue S, Minowa T, S-y Y (1994) Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 73:1855–1857
El-Kassas H, El-Sayed A, Mostafa MM, Reda M (2017) Algal growth and nutrients removal as affected by nitrogen source. J Environ Sci 39:51–73
El-Khair BES, Manal GM, Hamed S (2015) Complementary production of biofuels by the green alga Chlorella vulgaris. IJRER 5:963–943
El-Sayed AB (1999) Some physiological studies on green algae. Ph.D. Thesis, Faculty of Agric, Department of Agriculture Botany, Cairo University
El-Sayed AB, Nashwa AH, Fetyan A, El-Fakharany MK (2017) Bioethanol production from Egyptian Enteromorpha sp. Middle East J Appl Sci 7:216–225
El-Sheekh MM, Gheda SF, El-Sayed AB, Abo Shady AM, El-Sheikh ME, Schagerl M (2019) Outdoor cultivation of the green microalga Chlorella vulgaris under stress conditions as a feedstock for biofuel. Environ Sci Pollut Res 26:18520–18532
Emeish S (2012) Production of natural β-carotene from Dunaliella living in the Dead Sea. Jordan J Earth Environ Sci 4(2):23–27
Gallego-Cartagena E, Castillo-Ramirez M, Martinez-Burgos W (2019) Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J Biol Sci 26:1325–1330
Gao B, Liu J, Zhang C, Van de Waal DB (2018) Biological stoichiometry of oleaginous microalgal lipid synthesis: the role of N:P supply ratios and growth rate on microalgal elemental and biochemical composition. Algal Res 32:353–361
Gopinath A, Puhan S, Govindan N (2010) Effect of biodiesel structural configuration on its ignition quality. Int J Energy Environ1:395–306
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonulaconfervacea (cleve) gran. Can J Microbiol 8:229–239
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Huete-Ortega M, Okurowska K, Kapoore RV, Johnson MP, Gilmour DJ, Vaidyanathan S (2018) Effect of ammonium and high light intensity on the accumulation of lipids in Nannochloropsis oceanica (CCAP 849/10) and Phaeodactylumtricornutum (CCAP 1055/1). Biotechnol Biofuels 11:60
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enz Microb Technol 27:631–635
Kamalanathan M, Gleadow R, Beardall J (2015) Impacts of phosphorus availability on lipid production by Chlamydomonas reinhardtii. Algal Res 12:191–196
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36
Knothe G (2005) The history of vegetable oil-based diesel fuels. In: Knothe G (ed) The biodiesel handbook. AOCS Press, Champaign, pp 4–16
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energ Fuel 22:1358–1364
Knothe G, Bagby MO, Ryan TW (1998) Precombustion of fatty acids and esters of biodiesel. A possible explanation for differing cetane numbers. J Am Oil Chem Soc 75:1007–1013
Knothe G, Matheaus A, Ryan T (2003) Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel 82:971–975
Li C, Yu Y, Zhang D, Liu J, Ren N, Feng Y (2016) Combined effects of carbon, phosphorus and nitrogen on lipid accumulation of Chlorella vulgaris in mixotrophic culture. J Chem Technol Biotechnol l91:680–684
Lin C-Y, Lin H-A, Hung L-B (2006) Fuel structure and properties of biodiesel produced by the peroxidation process. Fuel 85:1743–1749
Mallick N, Mandal S, Singh AK, Bishai M, Dash A (2012) Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J Chem Technol Biotechnol 87:137–145
Matsakas L, Gao Q, Jansson S, Rova U, Christakopoulos P (2017) Green conversion of municipal solid wastes into fuels and chemicals. Electron J Biotechnol 26:69–83
Minowa T, Yokoyama S-y, Kishimoto M, Okakura T (1995) Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 74:1735–1738
Minyuk G, Chelebieva E, Chubchikova I, Dantsyuk N, Drobetskaya I, Sakhon E, Chekanov K, Solovchenko A (2017) Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 32:245–259
Mittelbach M, Remschmidt C (2004) Biodiesel: the comprehensive handbook. Martin Mittelbach, Graz
Nouri H, Moghimi H, Nikbakht RM, Ostovar M, Farazandeh MSS, Ghanaatian F, Talebi AF (2019) Enhanced growth and lipid production in oleaginous fungus, Sarocladiumkiliense ADH17: study on fatty acid profiling and prediction of biodiesel properties. Renew Energy 135:10–20
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Sabu S, Singh ISB, Joseph V (2017) Optimisation of critical medium components and culture conditions for enhanced biomass and lipid production in the oleaginous diatom Naviculaphyllepta: a statistical approach. Environ Sci Pollut Res 24:26763–26777
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels:high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20–43
Skoog DA, Holler FJ, Crouch SR (2007) Instrumental analysis. Cengage Learning, New Delhi
Sokoto MA, Hassan G, Dangoggo SM, Ahmad HG, Uba A (2011) Influence of fatty acid methyl esters on fuel properties of biodiesel produced from the seeds oil of Curcubita pepo. Nigerian J Basic Appl Sci 19:81–86
Solovchenko A, Neverov K (2017) Carotenogenic response in photosynthetic organisms: a colorful story. Photosynth Res 133:31–47
Stat Soft, Inc (2004) SATISTICA (Data analysis software system). Version 8.0. https://www.statsoft.com
Valledor L, Furuhashi T, Recuenco-Munoz L, Wienkoop S, Weckwerth W (2014) System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol Biofuels 7:171
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102:1675–1682
Wu M, Wu G, Han L, Wang J (2005) Low-temperature fluidity of bio-diesel fuel prepared from edible vegetable oil. Pet Process Petrochem 36:57–60
Yodsuwan N, Sawayama S, Sirisansaneeyakul S (2017) Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylumtricornutum. Agric Nat Resour 51:190–197
Yun HS, Ji MK, Park YT, Salama E-S, Choi J (2016) Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production. Environ Sci Pollut Res 23:17831–17839
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almutairi, A.W. Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ Sci Pollut Res 27, 32296–32303 (2020). https://doi.org/10.1007/s11356-020-08531-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08531-8