Abstract
In this work, we proposed a short-term adaptation strategy to improve xylitol production on sugarcane bagasse hemicellulosic hydrolysate through the maximization of Candida guilliermondii FTI 20037 tolerance to inhibitors. Hemicellulosic hydrolysate obtained by diluted acid hydrolysis (1.0% (wv−1) H2SO4, 1:10 solid/liquid ratio, 121 °C, 10 min) was concentrated up to fivefold to obtain hydrolysates with different concentration factors. Yeast was cultivated in each hydrolysate for 24-h and consecutively transferred to the subsequent more concentrated hydrolysate to obtain different adaptation degrees. Adapted cells were used as inoculum in fermentations with the same hydrolysate in which they were adapted. The performance of adapted and non-adapted yeast was compared to validate the adaptation strategy employed. The beneficial effects of adaptation were more pronounced in the hydrolysates with higher inhibitor concentration (twofold concentrated and non-treated, H2N; and fivefold concentrated and treated, H5). It improved xylose assimilation and xylitol production as well as xylitol yield and xylitol volumetric productivity in both hydrolysates. A 62.5% increase in productivity (0.24 to 0.39 gL−1 h−1) and a 15.7% increase in yield (0.51 to 0.59 gg−1) were observed for H5 hydrolysate, while for H2N hydrolysate these increases were 54.5 and 29.6%, respectively. Yeast adaptation also improved arabinose consumption and reduced glycerol production. The reduction in glycerol production indicates a greater tolerance of adapted cells to the inhibitors present in hydrolysates. Short-term adaptation proved to be an efficient strategy to improve yeast tolerance as well as its fermentative performance on sugarcane bagasse hemicellulosic hydrolysate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioeconomy has emerged as an alternative economic system to the use of non-renewable and non-sustainable resources in order to reduce greenhouse gas emissions in the last decades [1]. Biorefining is the main element in the framework of bioeconomy as the broad spectrum of biomass resources offers great opportunities for a wide-ranging product portfolio such as biofuels, biochemicals, bioenergy/biopower, and other biomaterials to satisfy the different needs of society [2, 3]. Sugarcane is one of the most competitive carbon sources applied as raw material in the biorefinery context, given its high efficiency in low-cost carbon generation and its contribution to mitigate the effects of fossil fuel using [4]. Its application in this context also enables expanding the sugarcane agro-industry product portfolio [1], which comprises xylitol, a polyol classified as one of the top 12 high value-added chemicals capable of supporting the technical and economic viability of biorefineries [5]. Xylitol has consolidated applications in the food, dental, pharmaceutical, and cosmetics industries due to its interesting properties. In addition, it can also be used in the medical field and as a chemical platform to produce new molecules and materials [6].
Xylitol is commercially produced through the catalytic hydrogenation of xylose deriving from xylan-rich materials [7]. However, given the disadvantages associated with this production route—such as huge energy demand, extensive xylose purification steps, the requirement of specialized and expensive equipment, and complicated catalyst deactivation and recycling—the biotechnological production of xylitol appears to be an attractive alternative due to its better cost benefit, less energy demand, and more environmentally friendly nature [7, 8]. Plant cell wall deconstruction and hemicellulosic sugars’ solubilization is one of the key stages in xylitol production through the biotechnological route [9]. Diluted acid hydrolysis is often used for such a purpose due its low cost, easy operation, short reaction times, and effectiveness since it enables recovering 70–95% of monomeric sugars [6]. However, diluted acid hydrolysis also releases/forms toxic compounds capable of inhibiting microorganisms, as well as reducing biomass growth and fermentative performance [10,11,12,13].
Furfural, 5-hydroxymethylfurfural (5-HMF), acetic acid, phenolic compounds, and inorganic ions are the most common inhibitors often found in lignocellulosic hydrolysates [10, 13, 14]. Furfural and 5-HMF are generated through the thermochemical degradation of pentoses and hexoses, respectively. Their toxicity is associated with the inhibition of metabolism enzymes, plasma membrane integrity disturbance, DNA damage, and RNA and protein synthesis inhibition [15, 16]. The toxicity of acetic acid, which results from hemicellulose deacetylation [17], is associated with its non-dissociated form penetration into the cell and with cytoplasm acidification; this process leads to protein inactivation, metabolic processes’ inhibition, and oxidative stress induction [18]. However, low acetic acid concentrations can favor xylose metabolism by Candida guilliermondii [19]. Phenolic compounds, which are considered the main inhibitors found in hydrolysates [16], derive from the partial degradation of lignin and extractives [17]. Their toxicity is mainly associated with plasma membrane damage, which results in loss of integrity and function as a selective barrier [20]. Inorganic ions generated mainly from biomass and hydrolysis reactor corrosion [13, 17] inhibit enzymes and metabolic pathways and form non-specific cytotoxic compounds [21].
Hydrolysate detoxification is often used as a strategy to mitigate inhibitors’ toxicity and, consequently, to improve microbial performance. Adsorption on activated charcoal treatments and overliming are often used for this purpose [8, 9]; however, these methods show some disadvantages, such as loss of sugars and waste generation, which are difficult to recycle or properly dispose of. Furthermore, these strategies do not eliminate inhibitors; therefore, even in detoxified hydrolysates, microorganisms are still exposed to toxic compounds, growing in a harmful environment [12, 22]. In this sense, cell adaptation techniques have been employed together with or replacing detoxification methods to develop more robust and tolerant strains and to overcome hydrolysates’ toxicity [20, 23]. Short-term adaptation strategies have been described as efficient adaptive techniques to increase microbial tolerance to inhibitors found in hydrolysates. They are carried out through microorganism pre-exposure to non-lethal inhibitor concentrations [20, 24]. Unlike the evolutionary adaptation that leads to genetic alterations, short-term adaptation mainly affects metabolism [24], and it induces the generation of a more resistant cell phenotype [25]. When compared to parental strains, short-term adaptation can improve both microbial tolerance to inhibitors and fermentative performance, even at high lignocellulosic inhibitor concentrations [20, 26].
Although several studies have reported the application of short-term adaptation strategies to improve ethanol production by different yeasts [24,25,26,27,28,29], few studies have applied it in xylitol production processes. Therefore, considering hemicellulosic hydrolysates toxicity, the beneficial effect of short-term adaptation on xylitol production by C. guilliermondii FTI 20037 was evaluated. The main objective of the present study was to obtain cells with improved tolerance to inhibitors present in sugarcane bagasse hemicellulosic hydrolysates (SBHH), confirming the potential of this strategy in obtaining robust yeasts, mainly for application in the biotechnological xylitol production at industrial scale.
The adaptive strategy performed consisted of growing C. guilliermondii in SBHH with increasing concentration factors and, consequently, increasing inhibitor concentrations. Initially, cells were cultivated at raw hydrolysate. After that, they were transferred to twofold concentrated hydrolysate and subsequently to three, four, and fivefold concentrated hydrolysates. Thus, yeast cells with different adaptation degrees were obtained. To validate the short-term adaptation strategy proposed, fermentations were performed at the same concentrated hydrolysate that yeast was adapted.
Materials and Methods
SBHH Preparation
Sugarcane bagasse, which was kindly donated by Usina Guarani, Olímpia, SP, Brazil, was subjected to diluted acid hydrolysis in a 250-L stainless steel reactor under the following conditions: 1.0% (wv−1) H2SO4, 1:10 solid/liquid ratio at 121 °C for 10 min [30]. The hydrolysate was filtered and concentrated two, three, four, and fivefold under vacuum at 70 °C [31]. Next, hydrolysates were treated (detoxified) based on pH adjustment to 7.0 and 2.5 by using CaO and H3PO4, respectively. This procedure was followed by new filtration and adsorption treatment with 1.0% (wv−1) activated charcoal at 60 °C, 100 rpm, for 30 min [32]. Subsequently, pH was adjusted to 5.5 by using 6 mol L−1 NaOH and treated hydrolysates were autoclaved at 115 °C, for 15 min. A twofold concentrated hydrolysate portion was used as a control to evaluate the effect of fermentation on the non-detoxified hydrolysate. This hydrolysate only had its pH adjusted to 5.5. The composition of each hydrolysate is described in Table 1.
Inoculum Preparation and Short-term Adaptation
Candida guilliermondii FTI 20037 was preserved at 4 °C on malt extract agar (Difco, BD, France) to be used for inoculum preparation. A loop of cells was aseptically transferred to 125-mL Erlenmeyer flasks filled with 50 mL of semi-defined medium (xylose 30.0 gL−1, (NH4)2SO4 2.0 gL−1, CaCl2·H2O 0.1 gL−1, and rice bran extract 20.0 gL−1). The flasks were incubated in an orbital shaking incubator (New Brunswick Scientific Co. Inc.) at 30 °C, 200 rpm for 24 h. Cells were recovered by centrifugation (800 × g for 15 min), washed, and resuspended in sterile distilled water to be used as inoculum in the non-concentrated and treated hydrolysate at 0.4 gDCW.L−1 initial concentration [33]. After 24 h growth (30 °C, 200 rpm), cells were recovered from the non-concentrated and treated hydrolysate by centrifugation as described above, washed with sterile distilled water, and transferred to the twofold concentrated hydrolysate at 0.4 gDCW.L−1 initial concentration. Growth was also conducted for 24 h. After that, cells were recovered by centrifugation and transferred to the more concentrated hydrolysate. This process was carried out consecutively until fivefold concentrated hydrolysate to adapt yeast to the same concentration factor of hydrolysate used in fermentation, thus obtaining different adaptation degrees. All hydrolysates were also supplemented with ammonium sulfate, calcium chloride, and rice bran extract at the same concentrations used in the semi-defined medium. Growth in each concentrated hydrolysate was performed for 24 h.
Fermentation Conditions
Batch fermentations were carried out on untreated and twofold concentrated hydrolysate (H2N) and two (H2), three (H3), four (H4), and fivefold (H5) concentrated and treated hydrolysates by using cells adapted and non-adapted to the same hydrolysate concentration factor. Fermentations were performed in a 2.4-L bench-scale KLF 2000 bioreactor (BioEngineering Co., Switzerland) filled with 1.6 L of hydrolysate at initial pH 5.5 and 30 °C during incubation time corresponding to the consumption of at least 80% of xylose. A kLa value of 20 h−1 was applied in all fermentations. It was determined through the gassing-out method. To this, the cell-free culture medium was aerated with nitrogen gas. The increase of dissolved oxygen over time was monitored and used to calculate the kLa value [34]. Samples were collected every 12 h to monitor cell growth, substrate consumption, and product and by-product formation. All fermentations were performed in triplicate.
Analytical Methods
Xylose, glucose, arabinose, ethanol, glycerol, and acetic acid concentrations were determined by High-Performance Liquid Chromatography (HPLC) (Waters, Milford, LA) equipped with refractive index detector and Aminex HPX-87H column (Bio-Rad, CA, USA) operating at 45 °C; 0.01 mol L−1 H2SO4 was used as eluent at a flow rate of 0.6 mL min−1. Furfural and 5-hydroxymethylfurfural were also quantified by HPLC (Shimadzu-LCl110AD) equipped with a UV detector (SPD-10A UV–Vis, Waters, Milford, MA, USA) and RP-18 column (Hewlett-Packard, CA, USA) operating at 25 °C; acetonitrile/water (1:8) added with 1% acetic acid (wv−1) was used as eluent at a flow rate of 0.8 mL min−1 [31]. Total phenolic compounds were spectrophotometrically determined at 280 nm after samples’ pH was adjusted to 12. Cell concentration was monitored by measuring absorbance at 600 nm, which was correlated to dry weight based on a previously established calibration curve. All analyzes were performed in triplicate.
Xylitol yield (YP/S) was determined as the ratio between produced xylitol and consumed xylose (gg−1). Xylitol volumetric productivity (QP) was defined as the ratio between produced xylitol and fermentation time (gL−1 h−1), whereas xylose/arabinose consumption rates (QXS and QAS, respectively) were calculated as the ratio between consumed xylose/arabinose and fermentation time (gL−1 h−1).
Results and Discussion
Effect of Short-term Adaptation on Xylose Bioconversion into Xylitol
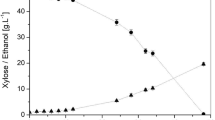
Candida guilliermondii FTI 20037 was pre-exposed to hemicellulosic hydrolysates with different chemical compositions to evaluate the effect of short-term adaptation on xylose-to-xylitol bioconversion. Since the inhibitors usually found in hydrolysates are known to reduce microbial growth as well as fermentative performance, short-term adaptation may be a strategy capable to develop a tolerant and robust phenotype, improving xylitol production. Acetic acid and phenolic compounds were the main inhibitors present in the hydrolysates used in this work (Table 1). Furfural and 5-HMF were also generated during diluted acid hydrolysis (0.08 and 0.01 gL−1, respectively—data not shown); however, the detoxification step reduced them to non-detectable concentrations. Fermentations carried out with non-adapted cells had the purpose to analyze yeast behavior against different hydrolysate concentration factors. According to the results shown in Fig. 1, xylose assimilation took place since the first fermentation hours. Similar consumption profiles were observed in all evaluated adaptation conditions. However, it is interesting to point out the favored xylose consumption in H2N (twofold concentrated and non-treated) and H5 (fivefold concentrated and treated) hydrolysates (Fig. 1a and e, respectively), which recorded the highest total phenolic compounds content; these compounds are well-known for inhibiting the fermentative activity of the investigated yeast [13]. Xylose consumption favoring by cell adaptation in H2N hydrolysate fermentation was supported by QXS increase by 22.5% (0.40 to 0.49 gL−1 h−1). Similarly, an increase by 10.4% (0.48 to 0.53 gL−1 h−1) in this parameter was also observed for the H5 hydrolysate (Fig. 1e). Full xylose consumption was observed after 96-h cultivation in H5 hydrolysate fermentation. On the other hand, negligent xylose consumption (70.1%) was observed when non-adapted cells were used as inoculum (Fig. 1e). Concerning the H2N hydrolysate, adapted cells promoted 96.9% xylose consumption after 60-h fermentation, which corresponded to increase by 15.6% in xylose consumption in comparison to that promoted by non-adapted cells (Fig. 1a). These results have indicated that the short-term adaptation technique may be capable of helping microorganisms to overcome toxicity caused by high toxic compound concentrations found in hemicellulosic hydrolysates (Table 1).
Xylose assimilation (circles) and xylitol production (triangles) by non-adapted (white) and adapted (black) C. guilliermondii over sugarcane bagasse hemicellulosic hydrolysate fermentations. a H2N [twofold concentrated and non-treated hydrolysate], b H2 [two], c H3 [three], d H4 [four], and e H5 [fivefold concentrated and treated hydrolysates]
Tomás-Pejó and Olsson [27] have described increased xylose consumption when short-term adaptation was performed to adapt a recombinant Saccharomyces cerevisiae strain to wheat straw hydrolysate; their findings corroborate results in the present study. The aforementioned authors observed xylose consumption ranging from 40 to 98% after 120-h fermentation of wheat straw hydrolysate comprising 4.25 gL−1 acetic acid, 0.65 gL−1 5-HMF, 3.85 gL−1 furfural, and 0.025 gL−1 vanillin, whereas non-adapted yeast did not consume this pentose. van Dijk et al. [24] have also reported increased xylose consumption by a recombinant Saccharomyces cerevisiae strain during wheat straw hydrolysate fermentations comprising approximately 3.76 gL−1 acetic acid, 0.48 gL−1 5-HMF, and 2.4 gL−1 furfural. Adapted cells consumed 46% of initial xylose after 48-h fermentation, whereas the xylose consumption by non-adapted cells only reached 22%. Zhang et al. [29] observed the highest xylose consumption by recombinant S. cerevisiae cells adapted to sweet sorghum hydrolysate. More than 80% of initial xylose had been consumed by the adapted strain after 24-h fermentation, whereas non-adapted cells only consumed 45% of it. Another study has found an increase in total xylose consumption (from 26.3 to 62.7%) by Escherichia coli adapted to increasing kenaf hydrolysate concentration factors [35].
The positive effect of short-term adaptation at the beginning of fermentation was also found in this study. Increased xylose assimilation was observed in the first 12-h fermentation, mainly in hydrolysates presenting higher inhibitor concentrations (H2N and H5). Greater tolerance to inhibitors promoted by cell adaptation to hydrolysates results in faster and more complete xylose consumption [25]. In response to previous exposure to inhibitors, microorganisms reprogram their metabolic pathways and regulatory machineries. This process enables the development of phenotypes tolerant to harmful environments [36]. Thus, adapted strains might induce stress response faster than the non-adapted ones, as well as allow a faster cell development [20].
The important role played by the detoxification stage was also evidenced in the current study, since xylose consumption in H2N hydrolysate (twofold concentrated and not treated) by non-adapted cells at 60-h fermentation was 16.1% lower than that observed in H2 hydrolysate (twofold concentrated and treated). Decreased xylose assimilation observed for adapted cells was only 2.17%, and this outcome has evidenced the benefit of adaptation strategies against the toxic effect of hydrolysates.
Xylitol production profile has followed similar xylose consumption behavior in all evaluated adaptation conditions. The production of this polyol by cells adapted to hydrolysates presenting higher toxic compound concentrations (H2N and H5) has clearly increased (Fig. 1a and e). Results have evidenced a significant increase by approximately 58.7% (from 23.5 to 37.3 gL−1) in xylitol production by cells adapted to H5 hydrolysate after 96-h fermentation, which corresponded to YP/S increase by 15.7% (0.51 to 0.59 gg−1) and QP increase by 62.5% (0.24 to 0.39 gL−1 h−1) (Fig. 2e). As we can see from some current studies on xylitol production by non-adapted yeasts (Table 2), short-term adaptation led to an improvement in both xylitol yield and productivity that are comparable or even better than the achieved by other authors. With regard to H2N hydrolysate, the increase in xylitol production due to cell adaptation was approximately 60.9% (from 6.4 to 10.3 gL−1) (Fig. 1a) after 60-h fermentation, which led to increasing in both yield (29.6%) and volumetric productivity (54.5%) (Fig. 2a). Although xylitol production from H4 hydrolysate (Fig. 1d) by adapted C. guilliermondii was not significantly different from non-adapted (Student’s t-test. P < 0.05), adapted cells exhibited a significant increase in xylitol yield (Fig. 2d). This is due to the lower initial xylose concentration in the medium fermented by adapted cells. No significant difference in xylitol production was observed in H2 and H3 hydrolysates fermentation; this outcome makes it clear that cell adaptation is not necessary when these hydrolysates are fermented. It likely happens due to their inhibitor concentrations, which are lower than those of H2N and H5 hydrolysates (Table 1). According to Sene et al. [37], the greater the cell adaptation degree, the greater their ability to metabolize toxic compounds. Xylitol production from H2N hydrolysate by non-adapted cells was 123.4% lower than that observed in H2 hydrolysate after 60 h (Fig. 1a and b). On the other hand, xylitol production by adapted cells has decreased by approximately 17.5%. There was also a delay in xylitol production onset when hydrolysate concentration factor increased (Fig. 1), likely due to increased inhibitors contents (Table 1). However, the performance of adapted C. guilliermondii in H5 hydrolysate fermentation has improved (Fig. 1e), and it coincided with the highest final xylitol concentration, with the highest volumetric productivity, and with maximum xylose consumption in 96-h fermentation. Regarding biomass growth, as the hydrolysate concentration factor increased, C. guilliermondii improved biomass generation due to the increased availability of carbon sources in the medium. However, no significant differences were observed between adapted and non-adapted conditions for all the five hydrolysates evaluated (Student’s t-test. P < 0.05, data not shown). Similar behavior was also reported by Sene et al. [38] who adapted C. guilliermondii to increasing sugarcane bagasse hemicellulosic hydrolysate concentration factors.
Xylitol yield and xylitol volumetric productivity over sugarcane bagasse hemicellulosic hydrolysate fermentation time by non-adapted and adapted C. guilliermondii. a H2N [twofold concentrated and non-treated hydrolysate], b H2 [two], c H3 [three], d H4 [four], and e H5 [fivefold concentrated and treated hydrolysates]. *Xylitol yield and xylitol volumetric productivity values significantly differed from each other (Student’s t-test. P < 0.05). **Xylitol yield values significantly differed from each other (Student’s t-test. P < 0.05). ***Xylitol volumetric productivity values significantly differed from each other (Student’s t-test. P < 0.05)
Cell adaptation has also improved xylitol production by a recombinant E. coli strain adapted to increasing concentrations of kenaf hemicellulosic hydrolysate and, consequently, of toxic compounds [35]. These authors observed a twofold increase in xylitol production, as well as 10.7% and 100% improvement in xylitol yield and xylitol volumetric productivity, respectively. Sene et al. [38] observed an increase by 34% in xylitol production by C. guilliermondii adapted to fourfold concentrated sugarcane bagasse hemicellulosic hydrolysate in comparison to non-concentrated hydrolysate. Wang et al. [41] observed that the number of propagation steps during Candida tropicalis cell adaptation to corn cob hemicellulosic hydrolysate was important to gradually improve inhibitors’ tolerance, increase xylitol yield, and decrease residual xylose concentration. Similar behavior was reported by Kim [36] during C. tropicalis adaptation to empty palm fruit bunch fiber hydrolysate. Tomás-Pejó and Olsson [27] reported an increase of 33.3% in ethanol yield when a recombinant S. cerevisiae strain was adapted to wheat straw hydrolysate (23% vv−1) in comparison to adaptation to lesser concentrated hydrolysate (12% vv−1). Silva et al. [42] observed an increase by 22% and 49% in ethanol yield and ethanol volumetric productivity, respectively, by Scheffersomyces stipitis adapted to sugarcane bagasse hemicellulosic hydrolysate. Nouri, Azin, and Mousavi [28] also reported an improvement in both ethanol yield and productivity by Barnettozyma californica adapted to sugarcane bagasse hydrolysate. An increase of 50% in lactic acid volumetric productivity was also observed for Bacillus coagulans adapted to wheat straw hemicellulosic hydrolysate [43].
Based on xylose consumption and xylitol production results, it was possible seeing a correlation between inhibitor concentrations in hydrolysates and the need for cell adaptation. Adapted C. guilliermondii performed better in H2N and H5 hydrolysates than non-adapted cells; these hydrolysates comprised higher inhibitor concentrations than H2, H3, and H4 hydrolysates (Table 1). Thus, it is reasonable to assume that is necessary to adapt C. guilliermondii cells to increase xylitol production from hydrolysates, mainly the ones presenting higher inhibitor concentrations. According to Sene et al. [37], the higher the hydrolysate concentration factor, and consequently the higher the inhibitor levels, the greater the need for cell adaptation. Nouri et al. [28] observed the importance of adapt yeast in face of the hydrolysate concentration factor. According to the aforementioned authors, Barnettozyma californica adapted to concentrated sugarcane bagasse hydrolysate presented a higher increase in ethanol yield and productivity, as well as in growth rate, than that of the yeast adapted to the non-concentrated hydrolysate. The need for adaptation in face of increased toxicity was also observed for S. stipitis grown in semi-defined media comprising glucose and xylose, as well as increasing acetic acid concentrations [44].
Short-term adaptation has favored both glucose and arabinose consumption (data not shown). However, glucose was fully consumed in the first 12-h fermentation, regardless of the hydrolysate concentration factor, whereas arabinose was slowly and partially consumed. Tomás-Pejó and Olsson [27] have also observed glucose consumption favoring by a recombinant S. cerevisiae strain adapted to wheat straw hydrolysate. The yeast consumed all glucose in less than 24 h, whereas non-adapted cells have partly consumed this hexose. Sene et al. [37, 38] have also observed slow arabinose assimilation by C. guilliermondii adapted to different sugarcane bagasse hemicellulosic hydrolysate concentration factors. This behavior may be justified by the catabolite repression of arabinose assimilation by xylose. According to Fonseca et al. [45], Pichia guilliermondii, the teleomorph form of Candida guilliermondii, and Candida arabinofermentans have two arabinose transportation systems, namely proton symport and facilitated diffusion. The first one is a high arabinose affinity transporter; however, it is strongly inhibited by xylose in the case of C. arabinofermentans. As for P. guilliermondii, the presence of xylose leads to reduced arabinose transportation since the proton symport system has similar affinities for both pentoses. Although xylose does not inhibit facilitated diffusion transport, it has a low affinity for arabinose. According to another study, arabinose assimilation by S. cerevisiae expressing Kluyveromyces marxianus and P. guilliermondii arabinose transporters genes was strongly inhibited by xylose (75% and 100%, respectively) [46]. Arabinose absorption inhibition by xylose was also verified for recombinants S. cerevisiae strains expressing Penicillium chrysogenum [47] and Arabidopsis thaliana [48] arabinose transporters genes. Arabinose consumption by some yeasts appears to be associated with reduced xylose levels in the medium since this pentose can inhibit arabinose assimilation. Xylose consumption throughout fermentation could mitigate arabinose assimilation repression and favor its use as a carbon source. QAS in the current study has significantly increased due to yeast’s short-term adaptation to H2N and H5 hydrolysates (250% and 117%, respectively – Student’s t-test. P < 0.05). According to the results, xylose assimilation improvement may be the reason why arabinose assimilation was favored.
Effect of Short-term Adaptation on Acetic Acid Consumption
In addition to consuming sugars in hydrolysates, C. guilliermondii was capable of fully consume acetic acid. The consumption of this aliphatic acid was followed by an increase in medium pH (Fig. 3). Similar behavior was reported for this yeast in semi-defined media [19], as well as in sugarcane bagasse [49], barley straw [14], and rapeseed straw [11] hemicellulosic hydrolysates. Full acetic acid consumption took place regardless of cell adaptation and hydrolysate concentration factor. In addition, yeast adaptation did not improve or reduce acetic acid assimilation. There were no changes in the acetic acid assimilation profile due to cell adaptation; its depletion took place concomitantly with that of xylose (Fig. 1) for all hydrolysates evaluated in the current study.
Acetic acid consumption (circles) and pH variation (triangles) over sugarcane bagasse hemicellulosic hydrolysate fermentations by non-adapted (white) and adapted (black) C. guilliermondii. a H2N [twofold concentrated and non-treated hydrolysate], b H2 [two], c H3 [three], d H4 [four], and e H5 [fivefold concentrated and treated hydrolysates]
Effect of Short-term Adaptation on Ethanol and Glycerol Production
The formation of by-products, such as ethanol and glycerol, was observed in all fermentations, regardless of hydrolysate concentration factor and yeast adaptation (Fig. 4). Ethanol formation took place since the first fermentation hours. Its maximum production took place between 12- and 24-h fermentation. The highest ethanol concentration (2.14 gL−1) was produced in H5 hydrolysate at 12-h fermentation. The aforementioned higher ethanol production is likely associated with increased glucose concentration in hydrolysates. Sene et al. [38] have also reported increased ethanol production by C. guilliermondii adapted to increasing sugarcane bagasse hydrolysate concentrations. According to the aforementioned authors, an increase by 40% in ethanol production was observed for cells adapted to fourfold concentrated hydrolysate in comparison to the non-concentrated hydrolysate. In addition, ethanol production coincides with the glucose consumption period (first 12-h fermentation—data not shown). Ethanol consumption over fermentation time was also observed. Likewise, glycerol production was also observed in the first hours for all evaluated fermentations, mainly in hydrolysates presenting higher toxic compound concentrations. Maximum glycerol production (2.52 gL−1) was observed for non-adapted cells in H2N hydrolysate after 60-h fermentation (Fig. 4a). Non-adapted cells tended to increase glycerol generation as hydrolysate concentration factor and, consequently, inhibitor concentrations increased. Increased glycerol production due to increased inhibitor concentrations was also reported by Zhang et al. [29]. It is worth highlighting the lower production of this by-product by adapted cells than by non-adapted cells, mainly in fermentations of hydrolysates with higher inhibitor concentrations. A decrease by approximately 300% in glycerol production was observed for cells adapted to H2N hydrolysate after 60-h fermentation (Fig. 4a), whereas a decrease by 102% in it was observed for H5 hydrolysate after 96-h fermentation (Fig. 4e). Despite increased hydrolysates’ toxicity, glycerol production by adapted cells remained low. Unlike ethanol, glycerol was not consumed by yeast; consequently, it accumulated in the medium throughout the entire fermentation.
Glycerol (circles) and ethanol (triangles) production by non-adapted (white) and adapted (black) C. guilliermondii over sugarcane bagasse hemicellulosic hydrolysate fermentations. a H2N [twofold concentrated and non-treated hydrolysate], b H2 [two], c H3 [three], d H4 [four], and e H5 [fivefold concentrated and treated hydrolysates]
Glycerol is a compatible solute typically produced by yeasts under stress conditions. Lower glycerol formation by adapted cells has indicated that short-term adaptation made C. guilliermondii more tolerant to the inhibitors present in hydrolysates, as well as decreased their toxic effects on cell metabolism and favored its fermentative performance. In addition to cell osmoregulation, glycerol generation plays a significant role in NAD+ regeneration and cell redox balance maintenance [18]. Zhang et al. [29] reported higher glycerol production by the recombinant S. cerevisiae strain that was not adapted to sweet sorghum hydrolysate than by the adapted yeast. The highest amounts of this by-product were produced by the yeast in hydrolysates of greater toxicity; this behavior corroborated the association between increased cellular stress and glycerol production. Narayanan et al. [50] and Sànchez I Nogué et al. [51] have also observed decreased glycerol production by S. cerevisiae adapted cultivated in semi-defined medium and adapted to inhibitors often found in hydrolysates.
Conclusions
The short-term adaptation strategy of C. guilliermondii proposed in this study improved xylose consumption and xylitol production on H2N and H5 sugarcane bagasse hemicellulosic hydrolysates, which contained the higher inhibitor concentrations among all hydrolysates. This led to a significant increase in both xylitol yield and xylitol volumetric productivity. Yeast adaptation also increased arabinose consumption rate and, consequently, arabinose assimilation. Acetic acid consumption and biomass growth were not altered by adaptation. On the other hand, glycerol production by adapted cells was reduced indicating a greater tolerance to the inhibitors present in the sugarcane bagasse hydrolysates. Considering the results obtained in the present study, short-term adaptation proved to be a strategy capable to improve fermentative performance through the increase of yeast tolerance to the inhibitors. It is a simple, promising, and inexpensive technique that could be applied to overcome hydrolysates toxicity, enabling the commercial use of non-recombinant strains in the production of high value-added products from lignocellulosic hydrolysates.
Data Availability
Datasets generated and/or analyzed during the current study are available in the Engineering School of Lorena – University of São Paulo (EEL-USP) repository, [http://sistemas.eel.usp.br/bibliotecas/antigas/2004/BIT04003OCR.pdf].
Code Availability
Not applicable.
References
Hernández-Pérez AF, Chaves-Villamil AC, Arruda PV et al (2020) Sugarcane syrup improves xylitol bioproduction from sugarcane bagasse and straw hemicellulosic hydrolysate. Waste Biomass Valorization 11:4215–4224. https://doi.org/10.1007/s12649-019-00742-6
Ng DKS, Ng KS, Ng RTL (2017) Integrated biorefineries. Encycl Sustain Technol 4:299–314. https://doi.org/10.1016/B978-0-12-409548-9.10138-1
Hingsamer M, Jungmeier G (2019) Biorefineries. In: Lago C, Caldés N, Lechón Y (eds) The role of bioenergy in the emerging bioeconomy: resources, technologies, sustainability and policy, 1st edn. Academic Press, Cambridge, pp 179–222
Lago AC, Bonomi A, Cavalett O et al (2012) Sugarcane as a carbon source: the Brazilian case. Biomass Bioenergy 46:5–12. https://doi.org/10.1016/j.biombioe.2012.09.007
Werpy T, Petersen G (2004) Top value added chemicals from biomass. Volume I: Results of screening for potential candidates from sugars and synthesis gas. https://www.osti.gov/biblio/15008859-top-value-added-chemicals-from-biomass-volume-results-screening-potential-candidates-from-sugars-synthesis-gas. Accessed 13 Feb 2021
Arcaño YD, Valmaña García OD, Mandelli D et al (2020) Xylitol: a review on the progress and challenges of its production by chemical route. Catal Today 344:2–14. https://doi.org/10.1016/j.cattod.2018.07.060
Dasgupta D, Bandhu S, Adhikari DK, Ghosh D (2017) Challenges and prospects of xylitol production with whole cell bio-catalysis: a review. Microbiol Res 197:9–21. https://doi.org/10.1016/j.micres.2016.12.012
Hernández-Pérez AF, Arruda PV, Sene L et al (2019) Xylitol bioproduction: state-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit Rev Biotechnol 39:924–943. https://doi.org/10.1080/07388551.2019.1640658
Puligundla P, Oh SE, Mok C (2016) Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon Lett 17:1–10. https://doi.org/10.5714/CL.2016.17.1.001
Chandel AK, Silva SS, Singh OV (2013) Detoxification of lignocellulose hydrolysates: biochemical and metabolic engineering toward white biotechnology. Bioenergy Res 6:388–401. https://doi.org/10.1007/s12155-012-9241-z
López-Linares JC, Romero I, Cara C et al (2018) Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour Technol 247:736–743. https://doi.org/10.1016/j.biortech.2017.09.139
Santos JC, Marton JM, Felipe MGA (2014) Continuous system of combined columns of ion exchange resins and activated charcoal as a new approach for the removal of toxics from sugar cane bagasse hemicellulosic hydrolysate. Ind Eng Chem Res 53:16494–16501. https://doi.org/10.1021/ie502712j
Silva-Fernandes T, Santos JC, Hasmann F et al (2017) Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour Technol 243:384–392. https://doi.org/10.1016/j.biortech.2017.06.064
Moraes EJC, Silva DDV, Dussán KJ et al (2018) Xylitol-sweetener production from barley straw: optimization of acid hydrolysis condition with the energy consumption simulation. Waste Biomass Valorization 11:1837–1849. https://doi.org/10.1007/s12649-018-0501-9
Quéméneur M, Hamelin J, Barakat A et al (2012) Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int J Hydrogen Energy 37:3150–3159. https://doi.org/10.1016/j.ijhydene.2011.11.033
Rao LV, Goli JK, Gentela J, Koti S (2016) Bioconversion of lignocellulosic biomass to xylitol: an overview. Bioresour Technol 213:299–310. https://doi.org/10.1016/j.biortech.2016.04.092
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:1–10. https://doi.org/10.1186/1754-6834-6-16
Guo Z, Olsson L (2014) Physiological response of Saccharomyces cerevisiae to weak acids present in lignocellulosic hydrolysate. FEMS Yeast Res 14:1234–1248. https://doi.org/10.1111/1567-1364.12221
Felipe MGA, Vieira DC, Vitolo M et al (1995) Effect of acetic acid on xylose fermentation to xylitol by Candida guilliermondii. J Basic Microbiol 35:171–177. https://doi.org/10.1002/jobm.3620350309
Gu H, Zhu Y, Peng Y et al (2019) Physiological mechanism of improved tolerance of Saccharomyces cerevisiae to lignin-derived phenolic acids in lignocellulosic ethanol fermentation by short-term adaptation. Biotechnol Biofuels 12:1–14. https://doi.org/10.1186/s13068-019-1610-9
Mudhoo A, Kumar S (2013) Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int J Environ Sci Technol 10:1383–1398. https://doi.org/10.1007/s13762-012-0167-y
El Gamal M, Mousa HA, El-Naas MH et al (2018) Bio-regeneration of activated carbon: a comprehensive review. Sep Purif Technol 197:345–359. https://doi.org/10.1016/j.seppur.2018.01.015
Guo Z, Khoomrung S, Nielsen J, Olsson L (2018) Changes in lipid metabolism convey acid tolerance in Saccharomyces cerevisiae. Biotechnol Biofuels 11:1–15. https://doi.org/10.1186/s13068-018-1295-5
van Dijk M, Erdei B, Galbe M et al (2019) Strain-dependent variance in short-term adaptation effects of two xylose-fermenting strains of Saccharomyces cerevisiae. Bioresour Technol 292:121922. https://doi.org/10.1016/j.biortech.2019.121922
Nielsen F, Tomás-Pejó E, Olsson L, Wallberg O (2015) Short-term adaptation during propagation improves the performance of xylose-fermenting Saccharomyces cerevisiae in simultaneous saccharification and co-fermentation. Biotechnol Biofuels 8:1–15. https://doi.org/10.1186/s13068-015-0399-4
Biazi LE, Santos SC, Kaupert Neto AA et al (2021) Adaptation strategy to increase the tolerance of Scheffersomyces stipitis NRRL Y-7124 to inhibitors of sugarcane bagasse hemicellulosic hydrolysate through comparative studies of proteomics and fermentation. Bioenergy Res 23:9–21. https://doi.org/10.1007/s12155-021-10267-3
Tomás-Pejó E, Olsson L (2015) Influence of the propagation strategy for obtaining robust Saccharomyces cerevisiae cells that efficiently co-ferment xylose and glucose in lignocellulosic hydrolysates. Microb Biotechnol 8:999–1005. https://doi.org/10.1111/1751-7915.12280
Nouri H, Azin M, Mousavi SL (2018) Enhanced ethanol production from sugarcane bagasse hydrolysate with high content of inhibitors by an adapted Barnettozyma californica. Environ Prog Sustain Energy 37:1169–1175. https://doi.org/10.1002/ep.12769
Zhang K, Wells P, Liang Y et al (2019) Effect of diluted hydrolysate as yeast propagation medium on ethanol production. Bioresour Technol 271:1–8. https://doi.org/10.1016/j.biortech.2018.09.080
Pessoa A Jr, Mancilha IM, Sato S (1997) Acid hydrolysis of hemicellulose from sugarcane bagasse. Brazilian J Chem Eng 14:309–312. https://doi.org/10.1590/S0104-66321997000300014
Rodrigues RCLB, Felipe MGA, Silva JBA et al (2001) The influence of pH, temperature and hydrolyzate concentration on the removal of volatile and non volatile compounds from sugarcane bagasse hemicellulosic hydrolyzate treated with activated charcoal before or after vacuum evaporation. Brazilian J Chem Eng 18:299–311. https://doi.org/10.1590/S0104-66322001000300009
Marton JM, Felipe MGA, Silva JBA, Pessoa A (2006) Evaluation of the activated charcoals and adsorption conditions used in the treatment of sugarcane bagasse hydrolysate for xylitol production. Brazilian J Chem Eng 23:9–21. https://doi.org/10.1590/S0104-66322006000100002
Felipe MGA, Vitolo M, Mancilha IM, Silva SS (1997) Environmental parameters affecting xylitol production from sugar cane bagasse hemicellulosic hydrolyzate by Candida guilliermondii. J Ind Microbiol Biotechnol 18:251–254. https://doi.org/10.1038/sj.jim.2900374
Pirt SJ (1975) Principles of microbe and cell cultivation. Blackwell Scientific Publications, London
Shah SSM, Luthfi AAI, Jahim JM et al (2020) An improvement in fermentability of acid hydrolysed hemicellulose from kenaf stem for xylitol production. Int J Food Eng 16:1–10. https://doi.org/10.1515/ijfe-2019-0230
Kim S (2019) Xylitol production from byproducts generated during sequential acid-/alkali-pretreatment of empty palm fruit bunch fiber by an adapted Candida tropicalis. Front Energy Res 7:1–9. https://doi.org/10.3389/fenrg.2019.00072
Sene L, Felipe MGA, Vitolo M et al (1998) Adaptation and reutilization of Candida guilliermondii cells for xylitol production in bagasse hydrolysate. J Basic Microbiol 38:61–69. https://doi.org/10.1002/(SICI)1521-4028(199803)38:1%3c61::AID-JOBM61%3e3.0.CO;2-2
Sene L, Converti A, Zilli M et al (2001) Metabolic study of the adaptation of the yeast Candida guilliermondii to sugarcane bagasse hydrolysate. Appl Microbiol Biotechnol 57:738–743. https://doi.org/10.1007/s002530100816
Misra S, Raghuwanshi S, Saxena RK (2013) Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydr Polym 92:1596–1601. https://doi.org/10.1016/j.carbpol.2012.11.033
Albuquerque TL, Gomes SDL, Marques JE et al (2015) Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal Today 255:33–40. https://doi.org/10.1016/j.cattod.2014.10.054
Wang L, Yang M, Fan X et al (2011) An environmentally friendly and efficient method for xylitol bioconversion with high-temperature-steaming corncob hydrolysate by adapted Candida tropicalis. Process Biochem 46:1619–1626. https://doi.org/10.1016/j.procbio.2011.05.004
Silva DDV, Arruda PV, Dussán KJ, Felipe MGA (2014) Adaptation of Scheffersomyces stipitis cells as a strategy to the improvement of ethanol production from sugarcane bagasse hemicellulosic hydrolysate. Chem Eng Trans 38:427–432. https://doi.org/10.3303/CET1438072
Aulitto M, Fusco S, Nickel DB et al (2019) Seed culture pre-adaptation of Bacillus coagulans MA-13 improves lactic acid production in simultaneous saccharification and fermentation. Biotechnol Biofuels 12:1–11. https://doi.org/10.1186/s13068-019-1382-2
Soares LB, Bonan CIDG, Biazi LE et al (2020) Investigation of hemicellulosic hydrolysate inhibitor resistance and fermentation strategies to overcome inhibition in non-saccharomyces species. Biomass Bioenergy 137:105549. https://doi.org/10.1016/j.biombioe.2020.105549
Fonseca C, Romão R, Sousa HR et al (2007) L -Arabinose transport and catabolism in yeast. FEBS J 274:3589–3600. https://doi.org/10.1111/j.1742-4658.2007.05892.x
Knoshaug EP, Vidgren V, Magalhães F et al (2015) Novel transporters from Kluyveromyces marxianus and Pichia guilliermondii expressed in Saccharomyces cerevisiae enable growth on L-arabinose and D-xylose. Yeast 32:615–628. https://doi.org/10.1002/yea
Bracher JM, Verhoeven MD, Wisselink HW et al (2018) The Penicillium chrysogenum transporter Pc AraT enables high-affinity, glucose-insensitive l -arabinose transport in Saccharomyces cerevisiae. Biotechnol Biofuels 11:1–16. https://doi.org/10.1186/S13068-018-1047-6
Rottmann T, Klebl F, Schneider S et al (2018) Sugar transporter STP7 specificity for l-arabinose and d-xylose contrasts with the typical hexose transporters STP8 and STP12. Plant Physiol 176:2330–2350. https://doi.org/10.1104/PP.17.01493
Arruda PV, Rodrigues RCLB, Silva DDV, Felipe MGA (2011) Evaluation of hexose and pentose in pre-cultivation of Candida guilliermondii on the key enzymes for xylitol production in sugarcane hemicellulosic hydrolysate. Biodegradation 22:815–822. https://doi.org/10.1007/s10532-010-9397-1
Narayanan V, SàncheziNogué V, van Niel EWJ, Gorwa-Grauslund MF (2016) Adaptation to low pH and lignocellulosic inhibitors resulting in ethanolic fermentation and growth of Saccharomyces cerevisiae. AMB Express 6:59–71. https://doi.org/10.1186/s13568-016-0234-8
Sànchez I, Nogué V, Narayanan V, Gorwa-Grauslund MF (2013) Short-term adaptation improves the fermentation performance of Saccharomyces cerevisiae in the presence of acetic acid at low pH. Appl Microbiol Biotechnol 97:7517–7525. https://doi.org/10.1007/s00253-013-5093-5
Funding
The current study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Maria das Graças de Almeida Felipe. The first draft of the manuscript was written by Italo de Andrade Bianchini and all authors commented on previous versions of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article is not associated with any study with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Informed Consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bianchini, I.d., Sene, L., da Cunha, M.A.A. et al. Short-term Adaptation Strategy Improved Xylitol Production by Candida guilliermondii on Sugarcane Bagasse Hemicellulosic Hydrolysate. Bioenerg. Res. 15, 1182–1194 (2022). https://doi.org/10.1007/s12155-021-10324-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10324-x