Abstract
This study compared the growth, proximate composition, and photosynthesis of Chlorella vulgaris cultures between a compound parabolic concentrator (CPC) coupled to a glass tube and a plain tube without the concentrator. The system with a concentrator increased the irradiance level on the culture by 351% and the average light intensity on cells by 462% compared to the plain tube system. During the exponential growth phase, the growth rate of C. vulgaris was higher in the tube system (1.14 div by day−1) versus the system with a concentrator (0.98 div by day−1), but the cell size was higher in the system with the concentrator (11.23 μm) compared with the tube system (6.28 μm). From day 2 to 4 of culture, biomass production (as organic dry weight: 3.43 to 9.85 pg cell−1) and proximate composition (proteins: 12.13 to 31.36 pg cell−1, lipids: 10.44 to 17.55 pg cell−1, and carbohydrates: 13.04 to 38.38 pg cell−1) were higher in the system with concentrator versus tube system. The biomass productivity (0.07 to 0.37 g L−1) and lipid productivity (1.23 to 5.94 g L−1 day−1) were significantly higher in the tube system in the same period. From a practical perspective, these results emphasize the importance of controlling irradiance in C. vulgaris cultures and suggest that a CPC can be used for microalgae cultures to increase the irradiance and induce stress by light. The CPC system can be used to shift the biochemical composition of microalgae cells toward the production of fine chemicals (e.g., pigments and lipids).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are photosynthetic organisms that can grow under aquatic environments and can convert CO2 into chemicals for food, feed, and high-value products. Microalgae cells can be used for feedstock for applications in cosmetics, pharmacy, and nutrition. Microalgal cultures are traditionally used to produce a variety of bioactive substances, such as carotenoids, polysaccharides, proteins, lipids, and antioxidants. The new application of microalgal cultures is for biofuels [1]. The Chlorophyceae Chlorella vulgaris is widely used as a nutritional supplement, cosmetics, pharmacy, and bioenergy feedstock due to its rapid growth rate, biochemical composition, and high tolerance to various culture conditions [2, 3].
Microalgae are organisms with rapid growth that can be used as biofuel precursors, based on their versatile metabolisms [4, 5]. The supply of uncontaminated, sustainable, and affordable energy is one of the main goals for the next several decades. Because 30% of the energy that is produced is used in the transportation industry, there is an urgent need to develop sustainable energy sources for this area. There are various types of biofuels that are developed from plant sources, such as crops of sugar cane, soybean, palm oil, and rapeseed. The use of crops for the production of biofuels competes directly with food production in terms of land and water resources [6]. Microalgae cultures are being considered as a new source of biofuels due to their high lipid production, tolerance to variations in temperature, environmentally friendly production, and simple life cycle, and the potential for the use of wastewaters as a source of nutrients [4,5,6]. The large-scale production of biofuels from microalgae is a new endeavor, with several limitations impeding the development of industrial biofuel production, such as growth and lipid content, carbon dioxide absorption, light penetration, use of artificial light, and seasonality using solar light [4,5,6].
Light is the driving force in photosynthesis and can vary in intensity, photoperiod, and spectral composition—such changes influence photosynthesis, metabolic pathways, and, consequently, growth and proximate composition [7,8,9]. The availability of light in a microalgae culture changes throughout over time due to the inverse relationship between light irradiance and cell concentration. Thus, it is important to provide sufficient light to favor the growth of various microalgae species. However, under controlled growing conditions, light is increased by expanding the number of lamps that are used. As a result, the economic cost of using lamps to illuminate microalgae cultures and providing an adequate supply of light is one of the factors that should be optimized.
Appropriate light management technologies such as spectral filtration, plasmonic waveguides, spectral shifting, wireless light emitters, and insulated glazing have been tested to improve light conversion efficiency and temperature control of algal photobioreactors. Increasing light efficiency and distribution in microalgal cultures can enhance photosynthesis and biomass production in open or closed systems [10, 11].
One option for increasing the irradiance of microalgae cultures is the use of compound parabolic concentrator (CPC), which has been used to concentrate solar energy, increasing the temperature of the fluid that is irradiated. However, the resulting temperatures generally range from 60 to 100 °C, depending on the characteristics of the system. CPC systems have been proposed for the treatment of wastewater and water distillation [12,13,14,15]. But, the use of these devices to increase the irradiance of microalgae cultures has not been developed due to these limitations.
The objective of this work was to measure the effect of light distribution by a CPC in cultures of Chlorella vulgaris and its influence on growth, biomass production, proximate composition, and photosynthesis. This study is the first attempt to use solar concentrator technology in culturing microalgae and characterizing the quality of the biomass that is produced.
Materials and Methods
Microalgae Strain and Culture Conditions
The Chlorophyceae Chlorella vulgaris strain Beyerinck (Beijerinck) 1890 was obtained from the Institute of Applied Microbiology (IAM), Tokyo University, Bunkyo-Ku, Tokyo, Japan. Nonaxenic and monospecific batch cultures of C. vulgaris were maintained in duplicate in 250 ml Erlenmeyer flask with 100 ml of “f” medium [16] with tap water. The cultures were maintained with daily manual stirring at 22 ± 3 °C and continuous light.
Control System: Plain Tube
Nonaxenic and monospecific batch cultures of C. vulgaris were maintained in duplicate in 1-L Pyrex® borosilicate tubes positioned vertically (length 45 cm, internal diameter 4.6 cm, and tube thickness 0.2 cm); 1000 ml of “f” medium [16] in tap water was added to each. The cultures were maintained with the addition of air bubbles and no supplementation of a carbon source. The temperature of the culture was maintained at 22 ± 3 °C and pH between 8.1 and 10.8.
Experimental System: Compound Parabolic Concentrator
The experimental system consisted of a CPC vertically aligned of 1.15 m2 of reflector area (Aref), 47° half aperture angle (\(\theta_\text{C}\)), 1.37 concentration radio (c), 90° tilt (\(\beta\)), and a Pyrex® tube (length 45 cm, internal diameter 4.6 cm, and thickness 0.2 cm) that was placed on the focal line of the concentrator (Fig. 1a). The 1-L Pyrex® tubes were maintained in duplicate nonaxenic and monospecific batch cultures of C. vulgaris under similar culture conditions that the described to the control system (plain tube). The CPC comprised 2 symmetrical sections, forming an involute curve (K-L), and a parabolic segment (L-M) that reflected all the incident rays between 0-\({\theta }_{\mathrm{m}\mathrm{a}\mathrm{x}}\) toward the tube (Fig. 1b).
Compound parabolic concentrator (CPC) with a tubular receiver and its various components: (1) reflective surface of the CPC, (2) glass tube, and (3) CPC stands. K-L, limits of the involute curve; L-M, limits of the parabola segment; r, radius of the glass tube; \(\varphi\), rim angle; \({\theta }_{\mathrm{m}\mathrm{a}\mathrm{x}}\), acceptance half-angle of compound parabolic concentrator; X–Y, coordinates in the Cartesian plane. (a) Isometric form of the glass tube coupled with a CPC. (b) Cross-section of CPC
The equations for the involute section are [17]
For the parabola curve are [17]
where X and Y are the coordinates in the Cartesian plane.
Light Measurement and Simulation
For both systems (control and experimental), the light was provided in 11:13-h dark:light photoperiod. The light source was a Sunlight Supply Inc® Sun System lamp with a Sylvania® metal halide light bulb. Light spectra of the lamp were measured with an International Light ILT900W spectroradiometer between 350 and 1000 nm.
Based on the Monte Carlo Ray Tracing Method, Trace Pro software was used to estimate the light distribution on the perimeter of the tubes containing the C. vulgaris culture in both systems. Incident photon flux density (PFD0) was measured inside at five different positions of the tubes in both systems, with a 4 π Bioespherical Instrument radiometer model QSL-100 (400–700 nm wavelength).
From the measured PFD0, the average light intensity (Iav) on cells of C. vulgaris inside the tubes was calculated following the model described by Evers [18], assuming that the attenuation of the incident light intensity caused by the cell concentration (C) obeys Lambert–Beer’s law:
where C is the biomass concentration, r is the radius of the vessel for both systems, \({I}_{0}\) is the incident light intensity, S is the distance of a given point inside the tube wall (0 < S ≤ r), the angle of the light path with the axis of symmetry of the tube is represented by \(\phi\) (0 <\(\phi\) ≤ 2π, although for symmetry can be used 0 <\(\phi\) ≤ π), and \({K}_{a}=0.45\, {m}^{2}{g}^{-1}\) is the absorption coefficient of daylight source for C. vulgaris taken from the work presented by Paladino et al. [19].
Growth Rate and Cell Size
Samples of the cultures that were maintained in the system were collected daily at different hours to measure their growth rates. Cell concentration was measured by direct count using a hemocytometer and log2-transformed to determine the growth rate (μ) and generation time (G) per Fogg and Thake [20].
Cell size was measured on days 0 and 4 for 10 randomly selected cells per treatment. Cell diameter was measured with a compound microscope at 40x using an Evolution MP digital camera with Image Pro-Discovery (version 5.1).
Biomass Production and Proximate Composition
Samples were collected twice daily for 5 days (9 a.m and 5 p.m.). To measure total dry weight (TDW), duplicate 14-ml samples were passed through washed and preweighed 47-mm VWR® GF/C glass fiber filters (1.0-μm pore), rinsed with 10 ml ammonium formate to remove salt residues, and dried at 60 °C to a constant weight. To determine ash content (ASH), samples were incinerated at 450 °C for 12 h. Organic dry weight (ODW) was calculated as the difference between the total dry weight and ash content per Sorokin [21].
To measure the proximate composition for each culture, triplicate 10-ml samples were washed with 7 ml ammonium formate, passed through preweighed 24-mm VWR® GF/C glass fiber filters (1.0-μm pore), and stored at − 20 °C until analysis. Protein content was extracted with 0.1 N NaOH at 100 °C for 60 min and determined per Lowry et al. [22]. Carbohydrates were extracted with sulfuric acid and analyzed by the phenol–sulfuric acid method [23]. Lipid content was extracted using chloroform–methanol–water per Blight and Dyer [24] and measured per Pande et al. [25].
Biomass productivity by day (BP) for each microalgae strain was calculated as the ratio of growth rate to total dry weight per day. Lipid productivity (LP) was calculated as the ratio of biomass productivity and lipid content.
Photosynthetic Analysis and Pigment Content
Samples were collected twice daily for 5 days. Photosynthetic activity was analyzed using rapid light curves on a pulse amplitude modulation (PAM) fluorometer (Walz, Junior PAM). The relative electron transport rate (ETR) and maximum quantum efficiency of photosystem II (Fv/Fm) values were obtained for each treatment [26]. The following photosynthetic parameters were obtained from each photosynthetic curve: maximum relative electron transport rate (ETRmax), photosynthetic efficiency (α), and irradiance of saturation (Ik), per Schreiber et al. [27].
Chlorophyll fluorescence was based on measurements of fluorescence parameters of the sample in response to saturating light in the dark or light-adapted. The maximum photochemical quantum efficiency of photosystem II (\({\mathrm{F}}_{\mathrm{v}}/{\mathrm{F}}_{\mathrm{m}}\)) was determined per Eq. 7:
where \({F}_{m}\) is the maximal chlorophyll fluorescence yield when the reaction centers of PS II are closed due to a strong light pulse, and \({F}_{0}\) is the basic fluorescence yield that was recorded with low light. Fv/Fm values were obtained by calculating the absorbance with the Solver program per Eilers and Peeters [26].
The pigment samples from each culture (10-ml were passed through 25-mm VWR® GF/C glass fiber filters and stored at − 20 °C until analysis. Chlorophyll a, b, and c1 + c2 and carotenoid were extracted with acetone and quantified per Parsons et al. [28].
Statistical Analysis
All data were tested for homoscedasticity and normality. Differences in growth rate, TDW, ODW, ASH, biochemical composition (proteins, carbohydrates, and lipids), pigments (chlorophyll a, b, and c1 + c2 and carotenoids), and photosynthetic parameters (α, ETRmax, Ik, and Fv/Fm) were analyzed for each variable in a triplicate set by Student’s t-test with OriginPro® 8.5. When significant differences were detected, Tukey a posteriori test was used. The significance level for all analyses was set to p < 0.05.
Results and Discussion
Light Analysis
The incident radiation with the use of the CPC (Fig. 2a) makes it possible to illuminate the circumferential region of the culture between 180 and 360°, unlike the plain tube in which the same circumferential section remains unilluminated. According to the analysis of incident light distribution on the perimeter of the tube containing the C. vulgaris culture, it was expected an increment of 483% in the system with CPC compared to the plain tube. This increase of irradiance is due to the contribution of the reflective surfaces that direct the incident radiation to the bottom surface of the tube between 180 and 360 degrees (Fig. 2a). However, the measured values reflect an increase of 351% (132 and 464 Wm−2 for the plain tube and the system with CPC, respectively). The difference between the simulated and the measured values is attributed to the fact that the theoretical value is obtained assuming ideal conditions in the concentrator construction and practice, and there are optical losses due to imperfections in the curvature of the concentrator surfaces. As expected, Fig. 2b shows that IAV, CPC had higher values during all the experiments than IAV, TUBE, showing a mean IAV, CPC = 2.45 W m−2 while the mean IAV, TUBE = 0.53 W m−2.
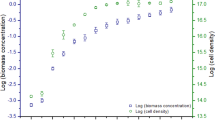
Distribution of the incident light on the circumferential angle of a culture tube with a compound parabolic concentrator (CPC) and plain tube system (a). Cell concentration of Chlorella vulgaris cultures in a tube with a compound parabolic concentrator (CPC: ▲) and plain tube system (●). Average light intensity calculated inside the C. vulgaris cultures (Iav, CPC: ∆) and plain tube system (Iav, TUBE: ○) (b)
Growth Rate and Cell Size
The cell concentration of C. vulgaris for both systems was similar for the first 28 h of culture, after which it increased in the tube until day 4 (22.33 × 106 cell ml−1). In contrast, the CPC culture reached a steady state after day 2, with mean values of 2.74 × 106 cell ml−1 (Fig. 2).
The growth rate was significantly higher (p < 0.05) in the tube system (1.14 divisions day−1) compared with the CPC system (0.98 divisions day−1) (Table 1). The generation time was significantly higher in the CPC (1.02 days) (p < 0.05) versus tube system (0.87 days) (Table 1).
The cells were 44% significantly larger (p < 0.05) in C. vulgaris cultures that were maintained in the CPC (11.23 μm) compared with the tube system (6.28 µm) (Table 1).
The CPC system delivered 351% more irradiance to C. vulgaris cells than the tube system. The similar cell concentrations in the 2 systems on the first day of the culture were attributed to the acclimation of the cells to the irradiance. In the CPC system, after 2 days of culture, the cell concentration of C. vulgaris was maintained because the cells adapted their growth and metabolism to survival at the high irradiance level (2134.4 μmol photons m−2 s−1, or 464 Wm−2) with regards to the tube system (607.2 μmol photons m−2 s−1, or 132 Wm−2), as evidenced by the lower growth rate and higher generation time.
The light intensity inside both culture systems was attenuated by absorption and shadow effect by others cells (Fig. 2b). The most important growth factor for phototrophic microalgae is light, which supplies the energy for microalgae biomass production [29, 30]. The light intensity that affected the cultures is measured inside; however, only a few works measured inside the cultures. The light intensity inside the culture is attenuated by absorption and shadowing by other cells [29]; this effect varies due to the effect of time of culture and the characteristics of the cells (size, pigments, concentration) and others related with the culture system (size, mixing).
The availability of light alters the growth rate, biomass production, and chemical composition in photoautotrophic microalgae cells [31, 32]. When C. vulgaris is maintained under various light regimens, high irradiance (100 μmol photons m−2 s−1) and the 16:8-h light:dark photoperiod at 25 °C elicit the maximum growth rate (1.13 divisions day−1) [33], similar to the values that were obtained with C. vulgaris in the tube in this study (1.14 divisions day−1) with an 11:13-h light photoperiod at 22 ± 3 °C. The growth of several microalgae species usually increases proportionally to the rise in irradiance levels until saturation intensity is measured, after which photoinhibition is observed. However, saturation intensity and photoinhibition are species-specific and depend on the light:dark photoperiod. For Desmodesmus sp., the biomass productivity increased with temperature and irradiance, and the optimal level of biomass productivity (0.76 g L−1 d−1) was obtained at 35 °C and 700 μmol photons m−2 s−1 [34]. The optimal light intensity (400–700 nm) for the most microalgae are among 10 to 250 μmol photons m−2 s−1, and direct sunlight yields up to 2000 μmol photons m−2 s−1 [35].
The increase in the size of C. vulgaris cells in the CPC system on day 4 of culture (79%) was due to a decline in growth in response to high irradiance levels compared with the tube system. The size of C. vulgaris cells can be modified by culture conditions, as reported by other groups [36, 37]. In previous studies on C. vulgaris cultures, an increase in cell size corresponded to a decrease in growth when the cells were maintained under yellow (16.29 μm) versus white light (14.84 μm) [36].
Biomass Production and Proximal Composition
The ODW of C. vulgaris on day 0 was the same for the CPC and tube systems (Fig. 3). In the CPC system, the ODW values were significantly higher after day 2 (p < 0.05). In contrast, for the tube system, the values were similar throughout the culture time after day 2 (Fig. 3). The TDW trended similarly as ODW (Fig. 3) for both systems. The ash content was significantly (p < 0.05) higher in the CPC system (Fig. 3).
Biomass production and proximate composition of Chlorella vulgaris cultures maintained in a tube with a compound parabolic concentrator (CPC) and plain tube system. Values with lowercase letters indicate significant differences between systems. Student’s t-test, α = 0.05: a > b. Mean values ± SD, n = 3
The rise in TDW, ODW, and ash content of C. vulgaris in the CPC system from day 1 to 4 was attributed to slower growth and, consequently, an increase in cell size; in the tube systems, the cells were smaller with low TWD, ODW, and ash content values, due to higher growth. TDW values for C. vulgaris in the CPC system were similar to reported values for this species, when maintained at a similar temperature with low irradiance (100 μmol photons m−2 s−1) and various culture media (Chu 10, WC, and LC) [38] or when used for food waste compost as an organic nutrient source [39]. However, the TDW for C. vulgaris cells in the tube system were lower due to the small cell size and increase in growth rate. The higher ash content of C. vulgaris cells in the CPC system was due to the rise in inorganic components in response to the low growth rate.
Until day 2 at 9:00 a.m., the protein content did not differ significantly (p > 0.05) between systems. However, from day 4 to day 5, the protein content increased rapidly in the CPC system (Fig. 3). The lipid content also differed significantly (p < 0.05) between systems—higher in the tube system until day 2 at 9:00 a.m., after which the CPC system had greater levels (Fig. 3). Carbohydrate content was significantly higher (p < 0.05) in the tube system until day 1, after which it rose quickly in the CPC system (Fig. 3).
The CPC system-generated C. vulgaris cells with elevated levels of all components of their proximate composition (proteins, carbohydrate, and lipids) from day 2, due to the higher irradiance in this system (2134.4 μmol photons m−2 s−1, or 464 Wm−2) versus the tube system (607.2 μmol photons m−2 s−1, or 132 Wm−2). The high protein, carbohydrate, and lipid content per cell for C. vulgaris in the CPC system were attributed to the cell concentration being maintained at similar levels throughout the culture, with a constant growth rate, and favoring the synthesis of basic components instead of division. C. vulgaris cells in the CPC system remained metabolically active despite the high irradiance in their culture.
The similar content of lipids in C. vulgaris cells in the CPC systems is possibly attributed to high stress due to the high irradiance, inducing and maintaining lipid synthesis throughout the culture. The higher lipid levels in cells in the CPC versus tube system correspond to the general trend that has been described for several microalgae strains that are maintained under high irradiance. In certain microalgae strains, light irradiance (350 to 700 μmol photons m−2 s−1) favors the overproduction of lipids, due to the excess energy that is used for storage products. For the cultures of Dunaliella viridis, total lipids per cell and acetone-mobile polar lipids decreased with light irradiance (35, 250, 700, 1500 μmol photons m−2 s−1), while the percentage of sterols and triglycerides increased with increasing irradiance used [40]. For C. vulgaris cultures, the increase in light intensity with red and white light LEDs lamps (130, 260, 390, and 520 μmol photons m−2 s−1) resulted in faster growth rates (0.36 day−1) and higher lipid content (up to 22%) [41]. Lipid synthesis requires an excess of ATP and NADPH, which are produced by photosynthesis, protecting microalgae cells from photochemical damage. The accumulation of lipids in microalgae cells occurs only when the availability of carbon exceeds the demand for starch synthesis [40].
When maintained at high levels of irradiance, various species of microalgae alter their metabolism, increasing their synthesis of lipids and fatty acids. The irradiance levels that are used to induce lipid synthesis in microalgae cultures are species-specific [42]. In C. vulgaris (University of Göettingen, Germany) that was maintained in basal medium, light intensity (solar irradiance) in September (598.7 μmol photons m−2 s−1, or 131.9 Wm−2 and between 28 and 32 °C) and June (851.4 μmol photons m−2 s−1, or 186.3 Wm−2 and between 30 and 36 °C) did not significantly affect the protein, fiber, moisture, and ash content of the cells. However, the nitrogen-free extract content decreased with higher light irradiance, whereas lipid content rose significantly. The increase in red light irradiance improved the growth rate and lipid content, whereas protein, fiber, ash, and moisture levels remained constant [41]. Chlorophytes usually produce significant amounts of lipids and carbohydrates, demonstrating their potential in the coproduction of biodiesel and bioethanol [43, 44]. The high irradiance in the CPC system increased lipid production by C. vulgaris cells.
After day 2, the biomass productivity (p < 0.05) and lipid productivity (p < 0.05) were significantly higher in the tube system throughout the culture (Fig. 4).
The lower BP and LP values of C. vulgaris in the CPC system resulted from the high irradiance, similar to cultures of C. sorokiniana that were maintained with M-8 medium and continuous illumination of 2100 μmol photons m−2 s−1 with red light diodes (LEDs). The light intensity that was used was similar to the maximum irradiance on a horizontal surface at latitudes below 37°. C. sorokiniana was not inhibited with the high light irradiance that was used, and biomass production increased with higher dilution rates [45]. The low values of BP and LP in the CPC system are due to the low growth produced after day 2, where the cells are maintained under high irradiance that produces inhibition of growth of C. vulgaris due to photoinhibition.
Photosynthetic Analysis and Pigment Content
Chlorophyll a (p < 0.05), b (p < 0.05), and c1 + c2 (p < 0.05) levels decreased significantly throughout the culture in both systems (Fig. 5), but chlorophyll a was significantly higher (p < 0.05) in the tube system (Fig. 5). In general, chlorophyll b (p < 0.05) and c1 + c2 (p < 0.05) levels were higher in the CPC system (Fig. 5). Carotenoid content decreased significantly (p < 0.05) until day 2 in both systems (Fig. 5), after which it was higher in the CPC system (Fig. 5).
Usually, cultures that are maintained under high irradiance have higher growth rates, but chlorophyll a content decreases due to the reduction in antenna units to harvest light. Algal species, cell size, geometry, and physiologically controlled pigment composition determine the optical properties of phytoplankton cells [46]. In C. vulgaris that was maintained under various irradiances (37.5 to 100 μmol photons m−2 s−1), the chlorophyll a content (μg ml−1) was lower at high irradiance, whereas β-carotene had an inverse pattern [33].
The effects of irradiance and temperature were studied in photobioreactor cultures of Chlorella sorokiniana (UTEX 2805)—free and immobilized with the bacteria Azospirillum brasilense—at 40 °C. As a result, the chlorophyll a content increased at high irradiance (2500 μmol photons m−2 s−1) for free cells and co-cultures (~ 45 ng cell−1 × 105) versus low irradiance (60 μmol photons m−2 s−1) (~ 20–30 ng cell−1 × 105). Chlorophyll b content trended similarly as chlorophyll a [47].
Microalgae cells usually require an adaptation period when they are transferred to different irradiance levels [48]. C. vulgaris cultures that were maintained in the CPC and tube systems were not acclimated to the irradiance that was used. When C. vulgaris was maintained at high irradiance, the chlorophyll content per cell rose in the CPC system, due to the increase in cell size in this system.
The increase in carotenoid content of C. vulgaris cells in the CPC system from day 2 to 4 was attributed to the synthesis of photo-regulating pigments that are photo-protective, dissipating excess light through a non-photochemical process called the xanthophyll cycle [48].
The photosynthetic efficiency (α) of the CPC system was significantly higher (p < 0.05) after day 2, except at the end of day 4 (Fig. 6). The maximum relative electron transport rate (ETRmax) did not show a clear pattern throughout the culture in either system (Fig. 6). Generally, the ETRmax increased in the CPC system (Fig. 6). The irradiance of saturation (Ik) was significantly higher (p < 0.05) in the tube system on days 2 and 3 of the culture. Fv/Fm was also significantly higher (p < 0.05) in the tube system (Fig. 6).
The maximum quantum efficiency of PSII (Fv/Fm) gives insights into the photosynthetic capacity of cells under various conditions and could be a marker of their physiological status and the impact of environmental conditions [49, 50]. Fv/Fm values of C. vulgaris cells in the CPC system were lower (0.25 to 0.45) from day 1 to 4, versus the tube system (0.65 to 0.69). With regard to the photobiochemical changes in Chlorella vulgaris during conversion from heterotrophic to phototrophic growth, after exposure to light, the cells experienced significant electron transport activity, with Fv/Fm values between 0.70 and 0.80 [49]. The Fv/Fm values for the tube system were similar to what was reported by Baker [49].
Many groups have stated that for several microalgae groups, Fv/Fm values of approximately 0.65 are indicative of cells without stress and that a decrease in Fv/Fm is indicative of stress [49, 50]. For 14 species of marine microalgae without stress condition, Fv/Fm varies from 0.43 to 0.72 and the sequence of different genus of algae was Chlorophyta (0.71) > Cryptophyta (0.62) > Bacillariophyta (0.60) > Xantophyceae (0.54) > Pyrrophyta (0.51) [50]. The Fv/Fm values for C. vulgaris in the tube system corresponded to those for Chlorophyta, but in the CPC system, lower values were obtained for this genus.
When C. vulgaris was grown in a biofilm reactor under mixotrophic conditions at 28 °C, with M8-a medium and 80 μmol photons m−2 s−1, CO2 was generated internally and reduced to carbohydrates or lipids. The values of photosynthetic parameters throughout the culture were as follows: Fv/Fm 0.64 to 0.69, ETRmax 53 to 77, α 0.19 to 0.27, and Ik 194 to 375. It was concluded that under mixotrophic growth conditions, autotrophy in C. vulgaris is not limited by glycerol and is fully complemented by heterotrophic metabolism through a cycle in which photosynthesis utilizes the CO2 that is released by respiration in the presence of glycerol and urea [51]. Compared with the photosynthetic parameters that were obtained by Rincon et al. [52], C. vulgaris had similar Fv/Fm values in the tube system and lower values in the CPC system, but Ik values were higher and ETRmax and α were lower in both systems. The lower Fv/Fm values in the CPC versus tube system indicated that the former is under stress conditions due to the higher light irradiance. The photosynthetic response of C. vulgaris to short-term UV-B radiation (280–315 nm) shows that with higher UV-B doses, Fv/Fm decreases from 0.65 to 0.20, and chlorophyll content declines from 1.28 to 1.05 mg m−3 [53]. Light intensity was used to improve lipid synthesis in Chlorella sp. HS2 for biodiesel production; this stress was induced by excessive irradiance from 50 to 900 μmol photons m−2 s−1. As a result, the ETRr increased from 16.65 to 32.2, and Fv/Fm values decreased from 0.68 to 0.54. In the exponential growth phase, lipid content correlated inversely with irradiance level, but during stationary growth, the lipid content level increased directly with the increase of irradiance level [54].
Only 1 study has examined a microalgae culture in a CPC system, measuring the effects of ultraviolet and visible radiation on Chlorella vulgaris in a new photobioreactor configuration. The maximum biomass production was 5 ± 1 × 10−3 g l−1 h−1, and the specific growth rate ranged from 1.1 × 10−2 to 2.0 × 10−2 h−1. Nutrient uptake of nitrogen and phosphorous increases with visible and ultraviolet irradiance (to 143 Wvis m−2 and Wuv m−2 for biomass production 143 Wvis m−2 and Wuv m−2 for nutrient removal) and then decreases due to photoinhibition. The authors examined the use of a CPC system for microalgae cultures and its potential use in countries with higher latitudes and lower solar irradiance levels [55]. The previous study did not measure the proximate composition or photosynthesis of C. vulgaris cells but constitutes the first use of CPC in outdoor microalgae cultures, necessitating further evaluation for application in aquaculture, biotechnology, pharmacology, and cosmeceuticals.
The assays for the CPC and tube systems were performed inside of a laboratory, with temperature-controlled by an air conditioner at 22 ± 3 °C. Temperature is one of the most important factors that affect the growth, proximate composition, and photosynthesis of microalgae cells. The optimal temperature for C. vulgaris is 30 °C, with which greater biomass production can be obtained [56]. When the cells of C. vulgaris were maintained at 35 °C, the growth decreased 17% compared with cultures at 30 °C. Temperatures above 38 °C cause C. vulgaris to die [57]. The use of CPC systems produced an increase in temperature due to the rise in irradiance [12,13,14,15]. This study used temperatures that permit the growth of C. vulgaris. But, if the CPC system is used for outdoor conditions, the temperature will increase and must be controlled with water to maintain the temperature in the appropriate range for the species that is used in the CPC system.
Conclusions
The use of the CPC system increases the irradiance level to the culture of C. vulgaris with regards to the plain tube system. The growth rate during the exponential growth of C. vulgaris was higher on the tube versus CPC system, but the cell size increased more in the latter. From day 2 to 4 of the culture, biomass production—in terms of organic dry weight and proximate composition (proteins, lipids, and carbohydrates)—was higher in the CPC versus tube system. Based on the photosynthetic parameters and maximum quantum efficiency of PSII (Fv/Fm), C. vulgaris cells that were maintained in the CPC system were under stress, due to the high irradiance that was used. From a practical perspective, these results emphasize the importance of controlling the irradiance in C. vulgaris cultures and demonstrate that CPC systems can be used for microalgae cultures to improve the distribution of the irradiance on the culture to induce stress by light. Further, this system can be used to modify the biochemical composition of microalgae cells for the production of biofuels and fine chemicals (e.g., pigments and lipids).
Abbreviations
- Symbol:
-
Description
- BPD:
-
Biomass productivity by day (g l−1 day−1)
- CPC:
-
Compound parabolic concentrator
- \({\text{F}}_{\text{m}}\) :
-
Maximal chlorophyll fluorescence yield when the reaction centers of PS II are closed
- \({\text{F}}_{\text{0}}\) :
-
Basic fluorescence yield recorded with low measuring light irradiances
- Fv/Fm:
-
The maximum quantum efficiency of PSII
- \({\text{F}}_{\text{v}}\) :
-
The maximum photochemical quantum yield of photosystem II
- L,M :
-
Limits of the parabola of compound parabolic concentrator
- K,L :
-
Limits of the involute of compound parabolic concentrator
- Log2 :
-
Logarithm base 2
- LPD:
-
Lipid productivity by day (g l−1 day−1)
- ODW:
-
Organic dry weight (pg cell−1)
- r:
-
Radius of the Pyrex® tube
- t:
-
Time of the experiment (days)
- TDW:
-
Total dry weight (pg cell−1)
- X,Y:
-
Coordinates in the Cartesian plane
- μ:
-
Growth rate (divisions day−1)
- φ :
-
Rim angle (degrees)
- \({\theta }_{\mathrm{m}\mathrm{a}\mathrm{x}}\) :
-
Acceptance half-angle of compound parabolic concentrator (degrees)
References
Zhu L (2015) Biorefinery as a promising approach to promote microalgae industry: an innovative framework. Renew Sustain Energy Rev 41:1376–1384. https://doi.org/10.1016/j.rser.2014.09.040
Yun CJ, Hwang KO, Han SS, Ri HG (2019) The effect of salinity stress on the biofuel production potential of freshwater microalgae Chlorella vulgaris YH703. Biomass Bioenerg 127:105277. https://doi.org/10.1016/j.biombioe.2019.105277
Wei C, Huang Y, Liao Q, Zhu X, Xia A, Zhu X (2020) Application of bubble carrying to Chlorella vulgaris flocculation with branched cationic starch: an efficient and economical harvesting method for biofuel production. Energ Convers Manag 213:112833. https://doi.org/10.1016/j.enconman.2020.112833
Christi T (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Chaundhary R, Khattar JIS, Singh DP (2014) Microalgae as feedstock for biofuel: biomass yield, lipid content and fatty acid composition as selection criteria. Int J Power a Renew Ener Syst 62–71
Farhad A, Kaveh S, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini M, Hadavand H, Mirzajanzadeh M, Malekzadeh S, Bakhtiari S (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2:258–267. https://doi.org/10.1016/j.algal.2013.04.003
Muller-Feuga A, Moal J, Kass J (2003) The microalgae of aquaculture. In: Støttup JG, McEvoy LA (eds) Life feeds in marine aquaculture, 1st edn. Blackwell Publishing Company, Oxford, pp 206–252
Molina E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–213. https://doi.org/10.1016/S0168-1656(01)00353-4
Perner-Nochta I, Posten C (2007) Simulations of light intensity variation in photobioreactors. J Biotechnol 131:276–285. https://doi.org/10.1016/j.jbiotec.2007.05.024
Gifuni I, Pollio A, Safi C, Marzocchella A, Olivieri G (2019) Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol 37(3):242–252. https://doi.org/10.1016/j.tibtech.2018.09.006
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433. https://doi.org/10.1016/j.algal.2019.101433
Khajepour F, Hosseini SA, Nasrabadi RG, Markou G (2015) Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl Biochem Biotechnol 6(8):2279–2289. https://doi.org/10.1007/s12010-015-1717-9
Cabrera A, Miralles S, Santos-Juanes L (2019) Solar water detoxification. In: Solar resources mapping. Springer, Cham, pp 341–351. https://doi.org/10.1007/978-3-319-97484-2_15
Fendrich M, Quaranta A, Orlandi M, Bettonte M, Miotello A (2019) Solar concentration for wastewaters remediation: a review of materials and technologies. Appl Sci 9(1):2–26. https://doi.org/10.3390/app9010118
Arunkumar T, Velraj R, Ahsan A, Khalifa AJN, Shams S, Denkenberger D, Sathyamurthy R (2016) Effect of parabolic solar energy collectors for water distillation. Desalin Water Treat 57(45):21234–21242. https://doi.org/10.1080/19443994.2015.1119746
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Khonkar HEI, Sayigh AAM (1995) Optimization of the tubular absorber using a compound parabolic concentrator. Renew Energy 6:17–21. https://doi.org/10.1016/0960-1481(94)00061-A
Evers EG (1991) A model for light-limited continuous cultures: growth, shading, and maintenance. Biotechnol Bioeng 38:254–259. https://doi.org/10.1177/019263658106544307
Paladino O, Neviani M (2019) Scale-up of photo-bioreactors for microalgae cultivation by π-theorem. Biochem Eng J 153:1–15. https://doi.org/10.1016/j.bej.2019.107398
Fogg GE, Thake B (1987) Cultures of limited volume. In: Fogg GE, Thake B (eds) Algal cultures and phytoplankton ecology, 3rd edn. University of Wisconsin Press, London, pp 12–42
Sorokin C (1973) Dry weight, packed cell volume and optical density. In: Stein JR (ed) Handbook of phycological methods and growth measured, 3rd edn. Cambridge University Press, New York, pp 321–343
Lowry OH, Rosebrough NJ, Faar AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Pande SV, Khan RP, Venkitasubramanian TA (1963) Microdetermination of lipids and serum total fatty acids. Anal Biochem 6:415–423. https://doi.org/10.1016/0003-2697(63)90094-0
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42(3–4):199–215. https://doi.org/10.1016/0304-3800(88)90057-9
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol 36(5):873–882. https://doi.org/10.1093/oxfordjournals.pcp.a078833
Parsons T, Maita Y, Lalli C (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Havlik I, Lindner P, Scheper T, Reardon KF (2013) On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol 31(7):406–414. https://doi.org/10.1016/j.tibtech.2013.04.005
Yun YS, Park JM (2003) Kinetic modeling of the ligh-dependent photosynthetic activity of the green microalga Chlorella vulgaris. Biotechnol Bioeng 83(3):303–311. https://doi.org/10.1002/bit.10669
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186. https://doi.org/10.1016/j.plipres.2006.01.001
Gonçalves CF, Menegol T, Rech R (2019) Biochemical composition of green microalgae Pseudoneochloris marina grown under different temperature and light conditions. Biocatal Agric Biotechnol 18:1–10. https://doi.org/10.1016/j.bcab.2019.101032
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23(4):721–726. https://doi.org/10.1007/s10811-010-9569-8
Ho SH, Chen CNN, Lai YY, Lu WB, Chang J (2014) Exploring the high lipid production potential of a thermotolerant microalga using statistical optimization and semi-continuous cultivation. Bioresour Technol 163:128–135. https://doi.org/10.1016/j.biortech.2014.04.028
Kromkamp JC, Beardall J, Sukenik A, Kopecký J, Masojídek J, Van Bergeijk S, Gabai S, Shaham E, Yamshon A (2009) Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems. Aquat Microbial Ecol 56(2–3):309–322. https://doi.org/10.3354/ame01318
Sánchez-Saavedra MP, Sauceda-Carbajal D, Castro-Ochoa FY, Molina-Cárdenas CA (2020) Use of light spectra to improve the growth and lipid content of Chlorella vulgaris for biofuels production. BioEnergy Res 13:487–498. https://doi.org/10.1007/s12155-019-10070-1
Kim DG, Lee C, Park SM, Choi YE (2014) Manipulation of light wavelength at appropriate growth stage to enhance biomass productivity and fatty acid methyl ester yield using Chlorella vulgaris. Bioresour Technol 159:240–248. https://doi.org/10.1016/j.biortech.2014.02.078
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Ciên 85(4):1427–1438. https://doi.org/10.1590/0001-3765201393312
Chew KW, Chia SR, Show PL, Ling TC, Arya SS, Chang JS (2018) Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresour Technol 267:356–362. https://doi.org/10.1016/j.biortech.2018.07.069
Gordillo FJL, Goutx M, Figueroa FL, Niell FX (1998) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10:135–144. https://doi.org/10.1023/A:1008067022973
Metsoviti MN, Papapolymerou G, Karapanagiotidis IT, Katsoulas N (2020) Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 9(1):1–17. https://doi.org/10.3390/plants9010031
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228. https://doi.org/10.1016/j.biortech.2015.05.021
Liu J, Yuan C, Hu G, Li F (2012) Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11–1 under nitrogen limitation. Appl Biochem Biotechnol 166(8):2127–2137. https://doi.org/10.1007/s12010-012-9639-2
Pandit PR, Fulekar MH, Karuna MSL (2017) Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ Sci Pollut Res 24(15):13437–13451. https://doi.org/10.1007/s11356-017-8875-y
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104(2):352–359. https://doi.org/10.1002/bit.22394
Dubinsky Z, Matsukawa R, Karube I (1995) Photobiological aspects of algal mass culture. J Mar Biotechnol 2:61–65
de-Bashan Trejo Huss Hernandez Bashan LEAVAJPY (2008) Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol 99(11):4980–4989. https://doi.org/10.1016/j.biortech.2007.09.065
Richardson K, Beardall J, Raven JA (1983) Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol 93(2):157–191. https://doi.org/10.1111/j.1469-8137.1983.tb03422.x
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Tan L, Xu W, He X, Wang J (2019) The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar Coast Shelf Sci 229:106411. https://doi.org/10.1016/j.ecss.2019.106411
Babaei A, Ranglová K, Malapascua JR, Torzillo G, Shayegan J, Benavides AMS, Masojídek J (2020) Photobiochemical changes in Chlorella G120 culture during trophic conversion (metabolic pathway shift) from heterotrophic to phototrophic growth regime. J Appl Phycol 32(5):2807–2818. https://doi.org/10.1007/s10811-020-02137-w
Rincon SM, Urrego NF, Avila KJ, Romero HM, Beyenal H (2019) Photosynthetic activity assessment in mixotrophically cultured Chlorella vulgaris biofilms at various developmental stages. Algal Res 38:101408. https://doi.org/10.1016/j.algal.2019.101408
Skórsk E, Murkowski A (2018) Photosynthetic responses of Chlorella vulgaris L. to short term UV-B radiation exposure. Acta Biol Crac Ser Bot 60(1):65–71. https://doi.org/10.24425/11804853
Kim DW, Shin WS, Sung MG, Lee B, Chang YK (2019) Light intensity control as a strategy to improve lipid productivity in Chlorella sp. HS2 for biodiesel production. Biomass Bioenergy 126:211–219. https://doi.org/10.1016/j.biombioe.2019.05.014
Lopes AP, Santos FM, Silva TF, Vilar VJ, Pires J (2020) Outdoor cultivation of the microalga Chlorella vulgaris in a new photobioreactor configuration: the effect of ultraviolet and visible radiation. Energies 13(8):1962. https://doi.org/10.3390/en13081962
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Proces: Proces Intensifi 48(6):1146–1151. https://doi.org/10.1016/j.cep.2009.03.006
Cassidy KO (2011) Evaluating algal growth at different temperatures. Theses of Master of Science in Biosystems and Agricultural Engineering. The University of Kentucky UKnowledge, pp 59
Funding
This work was funded by “Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE),” the Fund for Scientific Research and Technological Development of CICESE Call 2015 (Project: 623801).
Author information
Authors and Affiliations
Contributions
B.S.C. obtained the data and wrote the manuscript. M.P.S.S. wrote and organized the project, designed and supervised the research, and wrote the manuscript. D.S.C. wrote the project and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
All authors read and approved the final manuscript and agreed to this submission.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stephens-Camacho, B.E., Sauceda-Carvajal, D. & Sánchez-Saavedra, M.d. Growth, Proximate Composition, and Photosynthesis of Chlorella vulgaris Cultures Between a Photobioreactor Based in a Compound Parabolic Concentrator and a Plain Tubular System for a Biorefinery. Bioenerg. Res. 15, 230–241 (2022). https://doi.org/10.1007/s12155-021-10304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10304-1